Abstract
In this study, a simple, rapid, and efficient analytical method was proposed for the preconcentration and determination of trace amounts of cadmium and chromium in vegetable samples using magnetic solid-phase extraction (MSPE) coupled with electrothermal atomic absorption spectrometry (ETAAS) detection. Magnetic multi-walled carbon nanotubes (MWCNTs) as an effective adsorbent were synthesized and characterization was performed using scanning electron microscope, transmission electron microscopy, Fourier-transform infrared spectroscopy, X-ray diffraction, and vibrating-sample magnetometer. The experimental parameters were optimized using Box–Behnken design and maximum absorbance of Cd (II) and Cr (VI) was obtained at pH = 5, adsorbent dosage of 0.02 and 0.12 g, ligand concentration of 4 μg L−1, and extraction time of 22.5 min. The accuracy was assessed through proper recoveries in the range of 58.63–99.03% and 62.86–89.69% for Cd (II) and Cr (VI), respectively. In addition, precision was evaluated with a relative standard deviation (RSD) below 2.8% for both analytes. Linearity was in the range of 5–100 μg L−1 for both metal ions. The limit of detection and limit of quantification values were found below 0.3 μg L−1 and 0.3 μg L−1 using the ETAAS technique, respectively. The magnetic MWCNTs as a promising and low-cost adsorbent can be used in MSPE along with AAS approaches for the extraction and determination of heavy metal ions in several vegetable samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals can enter into the environment such as surface and groundwater, soil, and crop plants owing to human activities. Anthropogenic activities, including agriculture, steel and iron industry, mining, smelting processes, chemical manufacturing, domestic activities, tannery effluent sludge, compost waste, as well as fly ash are the main origins related to the heavy metals (Manzoor et al. 2018). Chromium (Cr), cadmium (Cd), copper (Cu), zinc (Zn), lead (Pb), mercury (Hg), manganese (Mn), and nickel (Ni) are known in the category of these contaminants (Arora 2019). Cadmium is tremendously hazardous to humans, which leads to serious problems in various organs such as lungs, kidneys, and liver. Its accumulation in tissues and organs can remain for many years. It is possible to excrete a small amount of cadmium from the body (Öztürk Er et al. 2018). Hexavalent chromium (VI) is very soluble and mobile, which damages the liver, respiratory organs, kidneys, and skin, causing different diseases like renal tubular necrosis, lung cancer, dermatitis, and perforation of the nasal septum (Zhong et al. 2016).
One of the major routes in which heavy metals enter the body is vegetables. The amount of heavy metal accumulation in vegetables depends on the agricultural soil and environmental conditions (Altunay and Katin 2020). Vegetables possess an essential role in the human diet owing to the presence of useful and necessary minerals and nutrients in their structure. Edible and non-edible parts of vegetables can uptake heavy metals higher than the permissible limit (Kumar et al. 2019). The maximum level of cadmium in vegetables and leafy vegetables has been reported 0.05 and 0.2 mg kg−1 wet weight, respectively (Altunay and Elik 2021; Commission Regulation 2011). Also, the allowable concentration of chromium in vegetable samples has been stated 2.3 mg kg−1 (Ashraf et al. 2021; Abrham and Gholap 2021). The growth of plants in soils contaminated with heavy metals and the consumption of these plants cause them to enter the food chain. Finally, their accumulation in living organisms is dangerous to human and animal health (Odobasic et al. 2017).
Numerous methods such as electrothermal atomic absorption spectrometry (ETAAS) (Oliveira Lopes and Rocha Chellini 2020), high-resolution continuum source graphite furnace atomic absorption spectrometry (HR-CS GF-AAS) (Pozzatti et al. 2017), inductively coupled plasma optical emission spectrometry (ICP-OES) (Feist and Mikula 2014), voltammetry (Palisoc et al. 2018; Locatelli and Melucci 2013), and flame atomic absorption spectrometry (FAAS) (Daşbaşı et al. 2015) have been used to determine heavy metals in different matrices.
Among these procedures, minimal sample preparation volume and the utilization of direct analysis without or minimum sample preparation are the advantages of the ETAAS approach. Also, the ETAAS possesses good sensitivity, low detection limit, and the possibility of automation. However, the lack of adequate sensitivity for low concentrations of metal ions and complex matrices is its drawback. Therefore, the preconcentration step is essential before determination (Arpa and Aridaşir 2019; Ling et al. 2021).
Several techniques, including coprecipitation (Saçmacı and Kartal 2010; Ipeaiyeda and Ayoade 2017), membrane filtration (Divrikli et al. 2007), dispersive liquid–liquid microextraction (Sorouraddin et al. 2020), and cloud point extraction (Kamel et al. 2019; Karadaş 2015), have been reported for the preconcentration of metal ions. Time-consuming, excessive consumption of organic solvents, unfavorable enrichment factors, as well as secondary wastes are disadvantages of these methods (Roushani et al. 2017).
One of the most remarkable used procedures for the separation and preconcentration of metal ions is magnetic solid-phase extraction (MSPE) due to its simplicity, high yield, low-cost, and high selectivity (Ibarra et al. 2015). The basis of this method is the usage of magnetic adsorbent for the adsorption and desorption of analyte. The magnetic material maintains the target compound. Then, the separation of the magnetic particle (containing the analyte) from the sample solution is performed using an external magnetic field (Hagarov 2020). Low stability of magnetic nanoparticles (NPs) such as Fe3O4 and γ-Fe2O3 in solution, mainly in acidic solution, is known as their limitations, which results in the decomposition of materials and the loss of magnetic properties (Yilmaz and Soylak 2020). To overcome this problem, the modification with other materials such as carbon nanotubes (CNTs) is necessary. They have received a lot of attention due to their physical features and many extensive applications. Based on their constituent layers, CNTs are divided into single-walled (SWCNTs) and multi-walled (MWCNTs). Unlike SWCNTs, MWCNTs possess excellent properties like large surface area, prominent structural characteristics, as well as good thermal and mechanical stability (Zhaoa et al. 2021; Pashai Gatabi et al. 2016). Also, their affinity to target compounds can be controlled based on the surface functionalization via chemical or physical techniques (Barbosa et al. 2020).
The magnetic MWCNTs have been showed super operational stability and the maintenance great adsorption and magnetic features even after several cycles in the adsorption and desorption process (Chen et al. 2012).
The dithiocarbamate derivative ligands can be used along with an adsorbent for the adsorption of heavy metal ions. Dithiocarbamates have nitrogen and sulfur atoms in different hybridized states, which cause a powerful ability to form chelate toward metal ions. These ligands have a great ability to adsorb heavy metals because of electron sharing between nitrogen, sulfur atoms, as well as metal ions (Abu-El-Halawa and Zabin 2017).
In this study, synthesized magnetic MWCNTs as an efficient and simple adsorbent along with 8-dithiocarbamate ligand were used in the MSPE method for the preconcentration of Cd and Cr ions in various vegetable samples. The fabricated magnetic MWCNTs were characterized using a scanning electron microscope (SEM), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and vibrating-sample magnetometer (VSM). In addition, ETAAS were used to determine the values of the mentioned ions. The effective parameters in the extraction process, including pH, adsorbent dosage, ligand concentration, and time, were optimized through response surface methodology (RSM) based on Box–Behnken design (BBD). To the best of our knowledge, there are no reports for the extraction of Cd (II) and Cr (VI) ions from the vegetable samples using magnetic MWCNTs.
Experimental
Materials
The standard solutions of 1000 mg L−1 cadmium and chromium were provided from Chem-Lab (Belgium). Also, ferric chloride hexahydrate (FeCl3.6H2O), ferrous chloride tetrahydrate (FeCl2.4H2O), carbon nanotube (99%), methanol, ethanol, acetonitrile, and dimethylformamide (DMF) were purchased from Merck (German). Dried vegetable samples, including basil, pennyroyal, parsley, and mint, were procured from local supermarkets (Tehran, Iran).
Apparatus
The determination of cadmium and chromium concentrations was performed using PerkinElmer ETAAS (PinAAcle 900 T, USA). The operating parameters for the ETAAS analysis of mentioned heavy metals are given in Table 1. FE-SEM (KYKY Technology, China) was used to determine the surface morphology of the adsorbent. The functional groups of synthesized adsorbent were studied by FTIR (JACSO-FTIR-410, Japan) with a KBr pellet. The crystallographic structure related to the adsorbent was investigated using XRD (X'pert-MPD, Netherland). VSM (LBKFB model, Meghnatis Daghigh, Kavir Co., Iran) was utilized to study the magnetic property of Fe3O4-MWCNTs. TOPwave microwave (Analytik Jena, Germany) was utilized to digest the dried vegetable samples. Vacuum oven (BINDER, Germany) and ultrasonic bath (BANDELIN, 35 kHz, Germany) were also used for drying the samples and homogenizing the solutions, respectively. A pH meter (Sartorius, USA) was used to adjust the pH value of the solutions.
Synthesis of Fe3O4 NPs
Five g of FeCl2·4H2O and 12.5 g of FeCl3·6H2O were dissolved into 65 mL deionized water. Then, 2 mL of concentrated HCl was added into the solution. Six hundred mL of NaOH solution (1.5 M) was poured into the round-bottom flask and under vigorous stirring at 70 °C was added drop wise into the previous solution for 1 h. The obtained black magnetic NPs were washed three times with deionized water and dried in an oven at a temperature of 60 °C for 24 h (Lü et al. 2016).
Synthesis of magnetic MWCNTs
The CNTs and Fe3O4 NPs were combined with each other with a ratio of 1:1 (w/w). In this procedure, dispersion of CNTs (2 g) in DMF (50 mL) was accomplished by sonication. Afterward, 2 g of Fe3O4 NPs was added, which were previously dispersed in 50 mL of DMF. Then, sonication was performed for 15 min, as well as for the facile separation, 300 mL distilled water was added. The collection of magnetic CNTs from the mixture was carried out using a nylon membrane (0.45 μm × 47 mm). Eventually, washing with water and drying (at 90 °C for 24 h) were conducted. In order to prevent oxidation, the magnetic CNTs were kept under a vacuum atmosphere (Mohamad Nasir et al. 2019).
Real sample preparation
0.4 g of each dried vegetable sample was accurately weighed. Then, the samples were separately digested with 6 mL of concentrated HNO3 at 170 °C in microwave digestion. The solutions were evaporated using a hot plate at a temperature of 200 °C and mixed with 1 mL of H2O2. Afterward, 100 mL of deionized water was added to the digested samples.
MSPE procedure
Samples containing 25 mL of chromium and cadmium with the concentration of 1 µg L−1 were added to the 4 μg L−1 of 8-dithiocarbamate as a ligand. Then, 0.02 g and 0.12 g of the magnetic CNTs was added to the chromium and cadmium solution, respectively with pH = 5. The mixture was stirred at 360 rpm for 22.5 min with constant speed at room temperature. Afterward, an external magnet was used to separate the adsorbent from the aqueous phase. Two mL of methanol–nitric acid (0.5 mol L−1) and acetonitrile–nitric acid (0.5 mol L−1) were added to the adsorbent and stirred for 22.5 min. The clear phase contains chromium and cadmium ions, which were measured by ETAAS.
Design of experiments (DOE)
One-factor-at-a-time (OFAT) approach is used to optimize the experimental parameters. In this method, one variable is changed and the other variables are constant. Its disadvantages include high laborious, high cost, and time-consuming. In order to overcome these drawbacks, response surface methodology (RSM) has been used. It is a statistical method that the interaction among the factors is evaluated and the optimum conditions are selected. Box–Behnken design (BBD) is known as one such RSM (Parmar et al. 2020).
The optimization of the effective variables, as well as the interaction between them, can be facilitated with the least number of experiments using BBD. It is an inexpensive alternative to central composite design (CCD) due to its fewer factor levels, as well as the lack of extremely high and low levels (Parmar et al. 2020; Polat and Sayan 2019).
In this study, Design-Expert 10.0 was used to implement the BBD method. The effect of experimental parameters, including pH, adsorbent dosage, ligand concentration, and time of extraction was assessed to achieve the optimal levels and the interactions between the factors. The ranges of the variables (lower limit and higher limit) related to each factor were specified (Table 2).
The number of runs in the BBD is obtained by Eq. (1).
In this equation, the number of variables and the repeat number of runs at a center point are indicated by K and C0, respectively (Rais et al. 2021). In this study, 36 runs with 12 replications were performed.
The quadratic response model was considered as the best model, which can be described via Eq. (2).
Herein, βi and βii denote the linear effect of factor Xi and the square effect of factor Xii, respectively. βij is linear by the linear effect of the factors Xi and Xj. The unknown constant error vector is represented by Ɛ (Rais et al. 2021).
Results and discussion
Characterization
SEM analysis
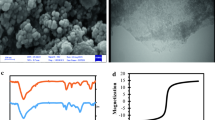
Figure 1 exhibits the SEM image of magnetic MWCNTs. As shown, the MWCNTs are similar to the thin strands that a large number of spherical particles are distributed on their surface. The interaction between the carboxyl functional groups and the Fe3O4 NPs leads to this linkage.
TEM analysis
The spherical Fe3O4 particles are located on the tubular surface of MWCNTs without obvious aggregation (Fig. 2). Complexes containing metal ions and carboxylic acid can be formed owing to numerous number of coordinating functional groups of carboxylic acid (Sadegh et al. 2014). The average size of nanocomposite is 7.75 nm.
FTIR analysis
As shown in Fig. 3a and b, the absorption peaks at 3941 and 3424 cm−1 are related to the O–H stretching vibration. The peaks at 2931 and 2923 cm−1 are attributed to the stretching or bending modes of –CH2. The peak appearing at 1747 cm−1 corresponded to the stretch mode of carboxylic groups (Wang et al. 2021; Soni et al. 2020). The band located at 1465 cm−1 can be related to the O–H bending mode of the COOH group (Pashai Gatabi et al. 2016). The band at 1639 cm−1 represents the C=O stretching of the COOH group. The observed peak at 1380 cm−1 is assigned to the C–OH stretching. The existence of peaks at 1106 and 1118 cm−1 is due to the stretching modes of C–O (Soni et al. 2020). The characteristic peak at 578 cm−1 belongs to the Fe−O bending vibration (Lu et al. 2017).
XRD analysis
The XRD pattern of magnetic MWCNTs is shown in Fig. 4. The diffraction peaks at 2θ = 26.27° and 43.56° are dedicated to (002) and (100) planes, which represent the hexagonal graphite structure. The main peaks are characterized at 2θ = 18.66°, 30.69°, 35.97°, 57.45°, and 62.89°, which assigned to the (111) (220), (311), (511), and (440) crystal planes, respectively. This pattern is in accordance with the inverse spinel crystalline structure of maghemite (JCPDS file, No. 39–1346) (Pashai Gatabi et al. 2016; Sadegh et al. 2014).
VSM analysis
The magnetic intensity of MWCNTs is shown in Fig. 5. The saturation magnetization (σs) value of MWCNTs is 23.81 emu g−1. According to the articles, the magnetic property of the Fe3O4-MWCNTs is less than the bare Fe3O4 NPs, which is due to the coating Fe3O4 NPs with the MWCNTs.
Selecting the best solvent
The solvent type has a significant effect on the yield (Recovery %) (Onyebuchi and Kavaz 2020). In order to preconcentration of Cd (II) and Cr (VI) ions, two types of solvents, including methanol/acetonitrile (50:50 v/v) and ethanol/acetonitrile (50:50 v/v), were used. Between these solvents, methanol/acetonitrile with a recovery percentage of 99.85 was selected as the best solvent (Fig. 6a). Afterward, different ratios of methanol/acetonitrile (25:75 v/v, 75:25 v/v, 50:50 v/v) were examined. The maximum recovery (98%) was related to the methanol/acetonitrile (50:50 v/v) (Fig. 6b).
One of the main parameters for evaluating the MSPE performance is the preconcentration factor (PF), which is calculated using Eq. (1).
where Cr and Cs denote the analyte concentration in the eluent phase and the initial concentration of the analyte in the sample solution, respectively (Khodadadi et al. 2021). In this study, the preconcentration factor was found to be 100.
Box–Behnken design
According to the results of Table 3, experiments were accomplished and the obtained results are the absorption of Cd and Cr ions. The absorbance was in the range of 0.5087–0.9647 and 0.0202 to 0.0897 for the Cd and Cr, respectively. The maximum absorbance of Cd (0.9647) was related to the pH = 5, adsorbent dosage of 0.02 g, ligand concentration of 4 μg L−1, and extraction time of 22.5 min. Also, the highest absorbance (0.0968) of Cr was obtained at the pH = 5, the adsorbent dosage of 0.12 g, ligand concentration of 4 μg L−1, and extraction time of 22.5 min.
Analysis of variance (ANOVA)
The significance and adequacy of the model were studied using ANOVA (Tables 4 and 5). The coefficients obtained from the ANOVA tables related to both ions were replaced in Eq. (2), which are described using Eqs. (4) and (5) for Cd and Cr ions, respectively.
In addition, the probability values (p values) were used to determine the significance of the model, factors, as well as their interactions. According to the p values of Cd ion (p < 0.05), it could be concluded that linear coefficients except for B, interactive coefficients except for AD, and all quadratic coefficients were significant. The F value model (88.44) represented that the model was significant with a p value lower than 0.0001. Due to the p value corresponding to the lack of fit (LOF) (0.9845), this parameter is not significant, indicating that the error can be ignored and the model describes all data well. The predicted coefficient of determination (R2) of 0.9615 has a proper agreement with the adjusted R2 of 0.9738. Furthermore, a high value of the R2 equal to 0.9849 revealed a good correlation between the experimental and predicted values.
The model, all factors, their interactions (AB, BC, BD), as well as A2, B2, C2, and D2 related to the Cr ion are significant because their p value is less than 0.05. The p value of LOF is 0.9275, indicating that the model is consistent with the experimental responses owing to the larger p value than the α level (0.05). The value of R2 is equal to 0.9858. Also, the high adjusted R2 value (0.9754) confirms that the model is highly significant. The obtained model can well predict the experimental results because the value of the predicted R2 is high (0.9557).
The normal plot of residuals (normal % probability versus internally studentized residuals) is illustrated in Fig. 7a and b. The normally distribution is observed for both ions because the points are satisfactorily close to the straight line and there is no variance deviation. The relationship between the variables and the response was effectually increased by this model. In addition, the high correlation between predicted and actual values related to the Cd and Cr ions is observed that the values are near to a straight line. The externally studentized residuals against the run number were plotted. The satisfying fit of the model can be expressed, as all the data points were obtained within the proper range (\(-\) 3.74 to 3.74 for Cd and \(-\) 3.68 to 3.68 for Cr).
Three-dimensional response surface plots
Figures 8 and 9 depict the three-dimensional response surface plots of both metal ions. The interaction between the parameters and determination of the optimum level related to each factor can be investigated using these figures. These kinds of plots display the effect of two variables on the response at the central point of other variables.
Figure 8a represents the interactive effect of pH and adsorbent dosage on the response surfaces (absorbance). At this step, ligand concentration and time were kept constant at 2.25 μg L−1 and 22.50 min, respectively. The absorbance was enhanced with increasing pH up to 5. By increasing pH, the H+ is diminished in the solution, leading to a decrease in the protonation effect of the COOH group on the adsorbent surface, and the number of adsorption sites on the adsorbent surface is increased. Hence, the adsorption is improved. With a further increase of pH, the solution continuously becomes alkaline, and metal ions begin to precipitate (Rais et al. 2021). The maximum Cd absorbance of 0.9647 was obtained at 0.02 g of adsorbent dosage.
Figure 8b demonstrates the interactive effect of ligand concentration and adsorbent dosage where pH and time were kept constant at 5 and 22.5 min, respectively. With an increase in ligand concentration of 0.5 to 4 μg L−1, the absorbance increased. The highest absorbance was achieved at a ligand concentration of 4 μg L−1.
The effect of time and adsorbent dosage on absorbance is shown in Fig. 8c where pH and ligand concentration were kept constant at 5 and 2.25 μg L−1, respectively. The Cd absorbance was found to be enhanced up to 22.5 min with an increase in adsorbent dosage from 0.02 to 0.12 g. After a contact time of 22.5 min, diminish in the remaining number of active sites on the adsorbent surface occurs.
Figure 8d reveals the effects of ligand concentration and pH when adsorbent dosage and time were kept constant at 0.07 g and 22.5 min, respectively. It was observed that with an increase in ligand concentration from 0.5 to 4, an increase in absorbance was observed. Also, when the pH was enhanced from 3 to 5, the absorbance was found to be increased.
The effect of time and ligand concentration on absorbance is depicted in Fig. 8e, where the pH and adsorbent dosage were considered 5 and 0.07 g, respectively. With an increase in the contact time from 5 to 22.5 min, the absorbance was found to be enhanced. Furthermore, an increase in ligand concentration showed an increase in response.
In the case of Cr absorbance, Fig. 9a exhibits the effects of adsorbent dosage and pH on Cr absorbance when ligand concentration and time were kept constant at 2.25 μg L−1 and 22.5 min, respectively. It can be seen that with an increase in adsorbent dosage from 0.02 to 0.12 g, an increase in absorbance occurs. When the pH was increased from 3 to 5, the Cr absorbance was found to be enhanced. It has already been stated that higher pH may cause metal precipitation.
Figure 9b shows the interactive effect of ligand concentration and adsorbent dosage on the absorbance of Cr where pH and time values were kept constant at 5 and 22.5 min, respectively. An increase in ligand concentration led to increase in absorbance.
By increasing contact time up to 22.5 min, as well as increasing the adsorbent dosage from 0.02 to 0.12 g the absorbance was enhanced (Fig. 9c). By elevating the adsorbent dosage, the available active sites of the adsorbent surface were increased and more metal ions are adsorbed on the adsorbent surface.
Determination of Cd and Cr ions in real samples
The efficiency of the suggested technique for the preconcentration and extraction of the Cd, and Cr ions from real samples including basil, pennyroyal, parsley, mint, and dill was evaluated. Two various concentrations containing 10 and 20 μg L−1 were used for the spike solutions. In order to determine the values of Cd and Cr ions, ETAAS method was utilized. The accuracy was represented via the recovery percentage, which calculated for each sample (Tables 6). The recovery related to the ETAAS approach was 58.63% to 99.03% and 62.86% to 89.69% for the Cd (II) and Cr (VI), respectively. In addition, the precision was studied using relative standard deviation (RSD) values, which were found lower than 2.8% for the ETAAS, respectively. It can be concluded that the ETAAS had a significant performance. According to the results, the proposed adsorbent possesses a high potential for the effective extraction of the Cd and Cr ions from the real samples.
On the other hand, recovery percentage and RSD% related to the intra-day and inter-day were investigated with five replicate (Table 7). In the ETAAS method, this value was < 2.5 and < 2 for intra-day and inter-day, respectively. Hence, satisfactory precision was observed in this approach. Furthermore, the calculation of recovery values was accomplished and the results revealed that the recovery was higher than 97%.
Tolerance of other ions on the proposed method
The interference experiment was accomplished to evaluate the selectivity of the suggested approach through the determination of Cd and Cr ions along with other ions (Table 8). Therefore, different cations (Na+, Li+, Ca2+, Fe3+, Zn2+, Ni2+, Cu2+, Mn2+, Ba2+) and anions (Cl−, F−, Br−, Co3−) were added to the solution containing Cd(II) and Cr(VI) ions and the recovery percentage was determined for each interfering ion. The results indicated that the presence of large amounts of species in real samples had no effect on recovery of Cd and Cr ions.
Comparison with literature
The suggested approach was compared with other analytical methods described for the determination of Cd (II), Cr (VI), Cu (II), and Zn (II) in various samples in terms of LOD, LOQ, RSD, and recovery (Table 9). Compared to other extraction methods, which may have been more difficult and time-consuming to prepare the adsorbent, the proposed method is simpler and the adsorbent preparation is easier. The RSDs achieved via the present technique are satisfactory and comparable with those of the other approaches. The LOD values of both metal ions related to the ETAAS are lower than LODs of the other methods. The LOQs of the proposed technique for Cd (II) and Cr (VI) are lower than the previously reported methods. Also, the obtained recoveries were in the acceptable ranges.
LOD and LOQ are determined as LOD = 3 × SD/m and LOQ = 10 × SD/m where SD is the standard deviation of the lowest concentration in the calibration plot and m is the slope of the calibration plot (Öztürk Er et al. 2018).
Conclusion
In this study, the MSPE procedure using magnetic MWCNTs as an adsorbent was presented for the extraction and preconcentration of Cd and Cr ions from different vegetable samples prior to ETAAS detection. To optimize the magnetic extraction parameters such as pH, adsorbent dosage, ligand concentration, and extraction time, BBD was implemented. The recovery percentage and RSD values obtained from spike results proved the acceptable accuracy and precision of the technique to the selected samples. The low LODs and LOQs, as well as a good linear range related to the ETAAS for both ions were provided. The magnetic adsorbent carrying the metal ion could be simply separated from the aqueous solution using an external magnetic field without filtration or centrifugation process. The MSPE approach is fast, simple, efficient, and inexpensive, which is convenient to complicated matrices such as vegetable samples with satisfactory recovery.
References
Abrham F, Gholap AV (2021) Analysis of heavy metal concentration in some vegetables using atomic absorption spectroscopy. Pollution 7:205–216
Abu-El-Halawa R, Zabin SA (2017) Removal efficiency of Pb, Cd, Cu and Zn from polluted water using dithiocarbamate ligands. J Taibah Univ Sci 11:57–65
Altunay N, Elik A (2021) Ultrasound-assisted alkanol-based nanostructured supramolecular solvent for extraction and determination of cadmium in food and environmental samples: Experimental design methodology. Microchem J 164:105958
Altunay N, Katin KP (2020) Ultrasonic-assisted supramolecular solvent liquid-liquid microextraction for determination of manganese and zinc at trace levels in vegetables: experimental and theoretical studies. J Mol Liq 310:113192
Arora R (2019) Adsorption of heavy metals: a review. Mater Today Proc 18:4745–4750
Arpa C, Aridaşir I (2019) Ultrasound assisted ion pair based surfactant-enhanced liquid–liquid microextraction with solidification of floating organic drop combined with flame atomic absorption spectrometry for preconcentration and determination of nickel and cobalt ions in vegetable and herb samples. Food Chem 284:16–22
Ashraf I, Ahmad F, Sharif A, Raza Altaf A, Teng H (2021) Heavy metals assessment in water, soil, vegetables and their associated health risks via consumption of vegetables District Kasur, Pakistan. SN Appl Sci 3:552
Barbosa MO, Ribeiro RS, Ribeiro ARL, Pereira FR, M, Silva AMT, (2020) Solid-phase extraction cartridges with multi-walled carbon nanotubes and effect of the oxygen functionalities on the recovery efficiency of organic micropollutants. Sci Rep 10:22304
Chen B, Wang S, Zhang Q, Huang Y (2012) Highly stable magnetic multiwalled carbon nanotube composites for solid-phase extraction of linear alkylbenzene sulfonates in environmental water samples prior to high-performance liquid chromatography analysis. Analyst 137:1232–1240
Commission Regulation (EU) EC (2011) Amending regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 111(2011):3–6
Daşbaşı T, Saçmacı S, Ülgen A, Kartal S (2015) A solid phase extraction procedure for the determination of Cd(II) and Pb(II) ions in food and water samples by flame atomic absorption spectrometry. Food Chem 174:591–596
de Oliveira M, Lopes A, Rocha Chellini P, Arromba de Sousa R (2020) Cadmium and chromium determination in herbal tinctures employing direct analysis by graphite furnace atomic absorption spectrometry (GF-AAS). Anal Lett 53:2096–2110
Dias FS, Guarino ME, Costa Pereira AL, Pedra PP, Bezerra MA, Gustavo Marchetti S (2019) Optimization of magnetic solid phase microextraction with CoFe2O4 nanoparticles for preconcentration of cadmium in environmental samples by flame atomic absorption spectrometry. Microchem J 146:1095–1101
Divrikli U, Arslan Kartal A, Soylak M, Elci L (2007) Preconcentration of Pb(II), Cr(III), Cu(II), Ni(II) and Cd(II) ions in environmental samples by membrane filtration prior to their flame atomic absorption spectrometric determinations. J Hazard Mater 145:459–464
Feist B, Mikula B (2014) Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem 147:302–306
Hagarov I (2020) Magnetic solid phase extraction as a promising technique for fast separation of metallic nanoparticles and their ionic species: a review of recent advances. J Anal Methods Chem 2020:8847565
Ibarra IS, Rodriguez JA, Galán-Vidal CA, Cepeda A, Miranda JM (2015) Magnetic solid phase extraction applied to food analysis. J Chem 8:1–13
Kamel HA, El-Galil E, Amr A, Al-Omar MA, Elsayed EA (2019) Pre-concentration based on cloud point extraction for ultra-trace monitoring of Lead (II) using flame atomic absorption spectrometry. Appl Sci 9:4752
Karadaş C, (2015) A novel cloud point extraction method for separation and preconcentration of cadmium and copper from natural waters and their determination by flame atomic absorption spectrometry. Water Qual Res J 52:178–186
Khodadadi S, Konoz E, Ezabadi A, Niazi A (2021) Magnetic solid-phase extraction using ionic liquid-modified magnetic nanoparticles for the simultaneous extraction of cadmium and lead in milk samples; evaluation of measurement uncertainty. J Mex Chem Soc 65:457–468
Kumar S, Prasad Sh, Kumar Yadav K, Shrivastava M, Gupta N, Nagar Sh, Bach QV, Kamyab H, Khan ShA, Yadav S, Chand Malav L (2019) Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environ Res 179:108792
Ling Y, Luo F, Zhu Sh (2021) A simple and fast sample preparation method based on ionic liquid treatment for determination of Cd and Pb in dried solid agricultural products by graphite furnace atomic absorption spectrometry. LWT-Food Sci Technol 142:111077
Locatelli C, Melucci D (2013) Voltammetric method for ultra-trace determination of total mercury and toxic metals in vegetables. Comparison with spectroscopy. Cent Eur J Chem 11:790–800
Lü HY, Yang SH, Deng J, Zhang ZH (2016) Magnetic Fe3O4 Nanoparticles as New, Efficient, and Reusable Catalysts for the Synthesis of Quinoxalines in Water. Aust J Chem 63:1290–1296
Lu W, Li J, Sheng Y, Zhang X, You J, Chen L (2017) One-pot synthesis of magnetic iron oxide nanoparticle-multiwalled carbon nanotube composites for enhanced removal of Cr(VI) from aqueous solution. J Colloid Interface Sci 505:1134–1146
Manzoor J, Sharma M, Ahmad Wani KH (2018) Heavy metals in vegetables and their impact on the nutrient quality of vegetables: a review. J Plant Nutr 41:1–20
Mohamad Nasir AN, Yahaya N, Nadhirah Mohamad Zain N, Lim V, Kamaruzaman S, Saadc B, Nishiyama N, Yoshida N, Hirota Y (2019) Thiol-functionalized magnetic carbon nanotubes for magnetic micro-solid phase extraction of sulfonamide antibiotics from milks and commercial chicken meat products. Food Chem 276:458–466
Mohammad Sorouraddin S, Ali Farajzadeh M, Dastoori H (2020) Development of a dispersive liquid-liquid microextraction method based on a ternary deep eutectic solvent as chelating agent and extraction solvent for preconcentration of heavy metals from milk samples. Talanta 208:120485
Odobasic A, Sestan I, Bratovcic A (2017) The extraction of heavy metals from vegetable samples. ingredients extraction by physicochemical methods in food. Academic Press, pp 253–273
Onyebuchi C, Kavaz D (2020) Effect of extraction temperature and solvent type on the bioactive potential of Ocimum gratissimum L. extracts. Sci Rep. https://doi.org/10.1038/s41598-020-78847-5
Öztürk Er E, Maltepe E, Bakirdere S (2018) A novel analytical method for the determination of cadmium in sorrel and rocket plants at ultratrace levels: magnetic chitosan hydrogels based solid phase microextraction-slotted quartz tube flame atomic absorption spectrophotometry. Microchem J 143:393–399
Palisoc Sh, Cedrickd Estioko L, Natividad M (2018) Voltammetric determination of lead and cadmium in vegetables by graphene paste electrode modified with activated carbon from coconut husk. Mater Res Express 5:085035
Parmar P, Shukla A, Goswami D, Patel B, Saraf M (2020) Optimization of cadmium and lead biosorption onto marine Vibrio alginolyticus PBR1 employing a Box-Behnken design. Chem Eng J Adv 4:100043
Pashai Gatabi M, Milani Moghaddam H, Ghorbani M (2016) Efficient removal of cadmium using magnetic multiwalled carbon nanotube nanoadsorbents: equilibrium, kinetic, and thermodynamic study. J Nanopart Res 18:189
Polat S, Sayan P (2019) Application of response surface methodology with a Box-Behnken design for struvite precipitation. Adv Powder Technol 30:2396–2407
Pozzatti MS, Borges AR, Dessuy MB, Vale MR, Welz B (2017) Determination of cadmium, chromium and copper in vegetables of the Solanaceae family using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Anal Methods 9:329–337
Rais S, Islam A, Ahmad I, Kumar S, Chauhan A, Javed H (2021) Preparation of a new magnetic ion-imprinted polymer and optimization using Box-Behnken design for selective removal and determination of Cu(II) in food and wastewater samples. Food Chem 334:127563
Rotimi Ipeaiyeda A, Ruth Ayoade A (2017) Flame atomic absorption spectrometric determination of heavy metals in aqueous solution and surface water preceded by co-precipitation procedure with copper(II) 8-hydroxyquinoline. Appl Water Sci 7:4449–4459
Roushani M, Mmohammad Baghelani Y, Abbasi Sh, Mavaei M, Zia Mohammadi S (2017) Flame atomic absorption spectrometric determination of cadmium in vegetable and water samples after preconcentration using magnetic solid-phase extraction. Int J Veg Sci 23:304–320
Saçmacı S, Kartal S (2010) Determination of some trace metal ions in various samples by FAAS after separation/preconcentration by copper(II)-BPHA coprecipitation method. Microchim Acta 170:75–82
Sadegh H, Shahryari-ghoshekandi R, Kazemi M (2014) Study in synthesis and characterization of carbon nanotubes decorated by magnetic iron oxide nanoparticles. Int Nano Lett 4:129–135
Soni G, Jain K, Soni P, Kumari Jangir R, Vijay YK (2020) Synthesis of multiwall carbon nanotubes in presence of magnetic field using underwater arc discharge system. MaterToday Proc 30:225–228
Wang Z, Xu W, Jie F, Zhao Z, Zhou K, Liu H (2021) The selective adsorption performance and mechanism of multiwall magnetic carbon nanotubes for heavy metals in wastewater. Sci Rep 11:16878
Yilmaz E, Soylak M. Type of new generation separation and preconcentration methods. InNew Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species 2020 Jan 1 (pp. 75-148). Elsevier.
Zhaoa N, Biana Y, Dong X, Gao X, Zhao L (2021) Magnetic solid-phase extraction based on multi-walled carbon nanotubes combined ferroferric oxide nanoparticles for the determination of five heavy metal ions in water samples by inductively coupled plasma mass spectrometry. Water Sci Technol 84:1417–1424
Zhong WS, Ren T, Zhao LJ (2016) Determination of Pb (lead), Cd (cadmium), Cr (chromium), Cu (copper), and Ni (nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal 24:46–55
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khodadadi, S., Konoz, E., Niazi, A. et al. Preconcentration of heavy metal ions on magnetic multi-walled carbon nanotubes using magnetic solid-phase extraction and determination in vegetable samples by electrothermal atomic absorption spectrometry: Box–Behnken design. Chem. Pap. 76, 6735–6751 (2022). https://doi.org/10.1007/s11696-022-02330-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02330-w