Abstract
In this study, an optimized QuEChERS sample preparation method was developed to analyze residues of six parabens: methyl-, ethyl-, n-propyl-, isopropyl-, n-butyl-, and isobutylparaben in five fresh-cut vegetables (potato, broccoli, carrot, celery, and cabbage) with high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). Homogenized samples were extracted using acetonitrile, and the extracts were cleaned with the novel sorbent multi-walled carbon nanotubes (MWCNTs). MWCNTs provided 84–94% removal of chlorophyll and lower matrix effects (MEs) compared to commonly used primary-secondary-amine (PSA) sorbent. Selected parabens were separated by HPLC with isocratic elution using acetonitrile and 0.1% (v/v) formic acid solution and determined by triple quadrupole MS/MS. The method validation results showed that recoveries were at 70–120% with RSDs <20%. Calibration curves showed linear responses of six parabens with R 2 > 0.99. Fifty fresh-cut vegetable samples from different farmer markets in Beijing, China were collected to measure the paraben residues, and only one sample was tested positive with methylparaben concentration at 81 μg/kg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With rapid development of fast-food industry, consumer demand for fresh-cut vegetables has been soaring in the recent decades. According to the definition of the International Fresh-cut Produce Association (IFPA), “fresh-cut produce is any fresh fruit or vegetable or combination of thereof physically altered from its original form, but remaining in a fresh state. These fruits and vegetables have been trimmed, peeled, washed and cut into 100% usable product that is bagged or prepackaged to offer consumers high nutrition, convenience and value while still maintaining freshness” (http://www.creativew.com/sites/ifpa/about.html). Processing vegetables and fruits may result in degradation of color, texture, and flavor. In order to extend a practical shelf life, various preservatives, including parabens, are utilized.
Parabens are alkyl esters of p-hydroxybenzoic acid, which plays an important role as an antimicrobial preservative in foodstuff, among which methylparaben, ethylparaben, n-propylparaben, isopropylparaben, n-butylparaben, and isobutylparaben are the most commonly used compounds in the group. They are widely applied in food products such as meat and milk products, seafood, fresh-cut vegetables, and others due to their low cost, high water solubility, and broad spectrum antimicrobial properties. As a result, the consumption of parabens is considerable.
However, studies have shown that parabens may have adverse human health effects. For example, Byford and Zhang’s studies showed that parabens’ estrogenic effect was related to human breast cancer cells (Byford et al. 2002; Zhang et al. 2013). Meanwhile, other studies have confirmed the estrogenic effect of parabens and demonstrated altering hormone signaling and gene expression (Routledge et al. 1998; Terasaka et al. 2006; Vo et al. 2011; Wróbel and Gregoraszczuk 2014). Aker (Aker et al. 2016) also reported endocrine-disrupting effect of parabens on thyroid and reproductive hormones during pregnancy.
Because of these effects, researchers in recent years focused on detection of paraben compounds in foodstuff. One survey for cereal products, meat, seafood, eggs, dairy products, bean products, vegetables, fruits, etc. was conducted to evaluate occurrence of parabens and to determine their concentrations using high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) detection, and nearly all of the food samples contained at least one kind of paraben and the total concentrations (sum of methyl-, ethyl-, n-propyl-, n-butyl-, benzyl-, and heptylparaben) ranged from below the limit of quantification (LOQ) to 2530 ng/g fresh weight, with an overall mean value of 39 ng/g (Liao et al. 2013). In addition to analysis in foods, study on maternal and fetal exposure to parabens reported their occurrence in the human umbilical-cord blood in a multi-ethnic urban US population, where five parabens (methyl-, ethyl-, n-propyl-, n-butyl-, and benzylparaben) were detected at a concentration of 25 ng/L to <0.01 ng/L (Pycke et al. 2015). Therefore, despite regions and ethnicities, the exposure of parabens is a worldwide phenomenon. The US Food and Drug Administration (FDA) has approved methyl-, propyl-, and butylparaben as synthetic flavoring substances and adjuvants (21 CFR 172.515) in beverages, in amounts not to exceed 20 mg/kg, and this is the only regulation we found related to foods. Because of ubiquitous human exposure to parabens, more studies emerged on developing new methods for their analysis and monitoring their residues.
For example, the residues of four parabens (methyl-, ethyl-, n-propyl-, and n-butylparaben) in seafood were analyzed using matrix solid-phase dispersion (MSPD) combined with gas chromatography–mass spectrometry (GC–MS) (Djatmika et al. 2016) and pressurized liquid extraction (PLE) combined with liquid chromatography quadrupole linear ion trap-tandem mass spectrometry (LC-QqLIT-MS/MS) (Han et al. 2016a). The determination of parabens in serum (Tahan et al. 2016) and human milk (Rodriguez-Gomez et al. 2015) was reported using liquid–liquid extraction and ultrasound-assisted extraction combined with HPLC–MS/MS detection. The residue analysis of parabens in pharmaceuticals (Moreta et al. 2015), cosmetics, and foods (Zhang et al. 2005) has been developed as well. Zhang’s study employed HPLC coupled with chemiluminescence detection and tested processed food like orange juice, soy sauce, vinegar, coca soda, and others with long shelf lives which contained far low concentration of methyl- and ethylparaben in soy sauce (Zhang et al. 2005).
Recently, Zhou (Zhou et al. 2015) reported a residue analysis method of 18 preservatives including four parabens (methyl-, ethyl-, n-propyl-, and n-butylparaben) in some vegetables (radish, tomato, cabbage, cowpea, and cucumber). The samples were extracted with 20 mL hexane–ethyl acetate (1:2, v/v) and 4 mL 2 mol/L ammonium acetate solution, and dispersive solid phase extraction (d-SPE) cleanup with primary secondary amine (PSA) and anhydrous magnesium sulfate (MgSO4) was conducted after the supernatant was concentrated by rotary vacuum evaporator, following by the final-extract analysis with UHPLC–MS/MS. This method achieved high analytical sensitivity, but it was time-consuming due to the concentration steps during the sample preparation.
QuEChERS (quick, easy, cheap, effective, rugged, and safe) method, which was first reported in 2003 (Anastassiades et al. 2003), has become the most prevalent method for determination of chemical residues in fruits and vegetables (Anastassiades et al. 2003; González-Curbelo et al. 2015; Schenck and Hobbs 2004). In the QuEChERS approach, different kinds of sorbents, such as PSA, C18, graphitized carbon black (GCB), alumina, cyanopropyl (Olsson et al. 2014), and aminopropyl (Mezcua et al. 2009), are used in the d-SPE cleanup procedure to remove co-extractive interferences (González-Curbelo et al. 2015). PSA effectively removes polar interferences such as fatty acids, organic acids, pigments etc., while GCB helps to retain pigments yet may result in high matrix effects (MEs) for some compounds (González-Curbelo et al. 2011; Wang et al. 2009; Wilkowska and Biziuk 2011).
Carbon nanotubes (CNTs) are a type of novel carbonaceous material first reported by Iijima in 1991 (Iijima 1991), and MWCNTs (multi-walled carbon nanotubes) are a kind of the nanotube material which has an excellent absorbing capacity due to its varying porosity and big surface areas. MWCNTs have been successfully applied in the QuEChERS procedure to remove interferences in vegetable matrices for pesticide-residue analysis (Zhao et al. 2012; Fan et al. 2014; Han et al. 2016c; Han et al. 2015; Pyrzynska 2011; Qin et al. 2015).
The purpose of this study was to develop a fast, easy, and efficient residue-analysis method for six parabens: methyl-, ethyl-, n-propyl-, isopropyl-, n-butyl-, and isobutylparaben in representative fresh-cut vegetables. The QuEChERS method and the novel sorbent MWCNTs were tested in sample preparation, and cleanup with MWCNTs was investigated. Additionally, two parabens (isopropylparaben and isobutylparaben) that were not investigated before were included in method development.
Materials and Methods
Materials
Ultra-pure water was prepared with a Milli-Q water purification system from Millipore, USA. HPLC-grade acetonitrile and PSA were both obtained from CNW Technologies GmbH (Germany). Analytical reagent grade sodium chloride (NaCl) was obtained from Sinopharm Chemical Reagent (Beijing, China). MgSO4 was obtained from Xilong Chemical Co. Ltd. (Beijing, China). MWCNTs (average diameter of 10–20 nm) were provided by Tianjin Bonna-Agela (Tianjin, China). An ultraviolet spectrophotometer (TU-1900) was used for detection of ultraviolet absorption.
Paraben standards (methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl) were purchased from Sinopharm Chemical Reagent with purity of 98.5%. Individual standard stock solutions were prepared at 1000 mg/L in a 20-mL flask in HPLC-grade acetonitrile. The standard mixture stock solution of the six target analytes was prepared at 100 mg/L in MeCN and was used for the preparation of matrix-matched standard solution and also for fortification. All of them were stored at −20 °C.
Sample Collection and Pre-treatment
Fresh-cut vegetables, including cabbage, carrot, celery, and potato, as well as broccoli, were purchased from different supermarkets located in three different districts (Haidian, Xicheng, and Dongcheng) in Beijing, China. Ten samples were collected for each kind of vegetable. These fresh-cut vegetables were homogenized using a MJ-BL25B2 mill (Media Ltd., Beijing, China), and the test portion was taken for determination of parabens’ residues. All of the samples were stored at −20 °C until analysis.
Sample Preparation
Sample preparation was based on the QuEChERS method. The homogenized sample (10.0 g) was weighed into a 50-mL centrifuge tube. For the fortification experiments, the samples were spiked with mixture solution of the six parabens (methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl) and allowed to stand at room temperature for 15 min. Then, 10 mL of acetonitrile (with 1% formic acid) was added, and the tubes were vigorously vortexed for 1 min using a Targin VX-III Multi-Tube Vortexer. After vortexing, 1.0 g NaCl as well as 4 g anhydrous MgSO4 were added into the tube successively, which was cooled in icy water bath immediately. Once the tubes were cooled down (for about 3 min), they were vortexed for 1 min and centrifuged at 3800 rpm (2260 rcf) for 5 min. Then, 1 mL organic phase (upper layer) was transferred into a 2-mL micro-centrifuge tube with previously weighed 5 mg MWCNTs as well as 150 mg anhydrous MgSO4. The mixture was vortexed for 30 s and then centrifuged at 10,000 rpm (4472 rcf) for 1 min. The upper layer was filtered through a 0.22-μm nylon filter membrane into an LC autosampler vial for HPLC–MS/MS analysis.
HPLC–MS/MS Conditions
An Agilent 6410B Triple Quadrupole HPLC–MS/MS (Agilent Technologies, USA) was employed, equipped with a HPLC reverse-phase C18 column (50 mm× 2.1 mm × 3.5 μm particle size). The flow rate was 0.2 mL/min. Isocratic elution was performed with acetonitrile as mobile phase A and 0.1% formic acid in ultrapure water as mobile phase B by a ratio of 40:60 in a 9-min run. The HPLC column was washed using gradient washing program after each sequence of about 30 injections to avoid the possible occurrence of ghost peaks. Mass spectrometry was carried out in multiple reaction monitoring (MRM) mode with negative electrospray ionization (ESI-). All the parameters for MRM transitions and collision energies (listed in Table 1) were optimized in order to obtain highest sensitivity and selectivity.
Method Validation
To evaluate trueness and precision of the method, a fortified recovery study of the six parabens in the five representative vegetables (cabbage, carrot, celery, potato, and broccoli) was conducted at four spiking levels: 0.05, 0.1, 0.5, and 1.0 mg/kg, and with five replicates at each level. The samples were spiked before the sample preparation. Matrix-matched calibration curves were prepared and applied for accurate quantification of the six parabens in different vegetable samples. The fortified recoveries and relative standard deviation (RSD) were calculated for each analyte, and the results were assessed in compliance with the European Union Guidelines SANTE/11945/2015, which requires the average recoveries at 70–120% with RSD ≤20%. The LOQ was set at the lowest spiking concentration that had satisfactory recovery and precision.
The linearity of calibration curves for each analyte was studied by HPLC–MS/MS analysis of five calibration solutions at concentrations of 0.05, 0.1, 0.5, 1.0, and 2.0 mg/L both in pure solvent (MeCN) and in matrix extracts. MEs were calculated as slope ratios using the following equation: %ME = 100% × (slopematrix/slopeMeCN − 1) (Han et al. 2015).
Results and Discussion
Optimization of LC-MS/MS Conditions
Since the six parabens have similar chemical structures, especially two pair of isomers (n-/iso-propylparabens and the n-/iso-butylparabens), they share the same precursor ions and similar retention times as shown in Table 1. In order to get accurate identification and quantification of these analytes, the LC conditions were optimized. Both gradient and isocratic elution conditions for the LC separation of the six parabens were investigated, and the elution results at different volume ratio of mobile phase A/B at isocratic 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, and 30:70 were compared. It was found that in most cases, the two pairs of isomers (n-/iso-propyl and n-/iso-butyl) eluted at the same retention time, which made it impossible to identify them by retention time only. Also, we did not achieve better separation even when the gradient elution programs were used, whereas the running time was more than 15 min and even longer if the equilibrium time was considered. When the isocratic elution method at A/B = 40:60 was used, the best separation for the two pairs of isomers was achieved with the retention time difference of about 0.2 min and the LC run time of 9 min. Considering that the isocratic mobile phase does not elute all the matrix components in the same chromatogram, in order to avoid the potential influence of ghost peaks in the chromatogram, the HPLC column was washed using a gradient washing program after each sequence of about 30 injections of each matrix.
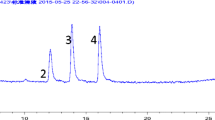
Optimization of MS/MS conditions for each analyte involved selecting precursor ions in full scan spectra and product ions with the highest responses at optimized collision energy voltages. Figure 1 shows a typical MRM chromatogram of the six parabens at the optimized HPLC–MS/MS conditions. In all previous publications on parabens’ analysis (Djatmika et al. 2016; Han et al. 2016a; Rodriguez-Gomez et al. 2015; Zhang et al. 2005; Zhou et al. 2015), only four parabens, methyl-, ethyl-, n-propyl-, and n-butylparabens, were analyzed, but isopropyl- and isobutylparabens were not included. Because these isomers have similar MRM transitions and can co-elute at most of LC separation settings, LC conditions allowing their separation are critical to avoid misidentification.
Sample Preparation
With the purpose of developing fast, easy, and reliable residue-analysis method of six selected parabens in vegetables, the QuEChERS approach was applied in this study. As described in the sample preparation section, 10 g of sample was extracted with 10 mL of MeCN and 1.0 g NaCl as well as 4 g anhydrous MgSO4 were used for the salting-out step. The recoveries were found to be satisfactory for all six parabens in five representative vegetables.
For dispersive-SPE cleanup procedure, an appropriate sorbent is expected to adsorb co-extractive interferences from the matrix extracts, but not influence the targeted analytes. It was shown that 50 mg PSA and 150 mg anhydrous MgSO4 was efficient enough for the cleanup of vegetables (Anastassiades et al. 2003; Cherta et al. 2013). In previous studies, 5 mg of novel sorbent MWCNTs was successfully applied for pesticide-residue analysis in spinach and cauliflower (Fan et al. 2014), organic spinach (Qin et al. 2016), and cowpea (Han et al. 2016c) with satisfactory recoveries for most analytes (pesticides) as well as removal of pigments from colored vegetables. Therefore, in this study, 50 mg PSA and 5 mg MWCNTs were both compared along with 150 mg MgSO4 for 1 mL QuEChERS extracts in d-SPE cleanup. For the assessment of cleanup of the two different sorbents, the recoveries, MEs and the removal of pigments, were evaluated in five representative vegetables.
Among all co-extracted interferences that are affecting the trueness of the analysis, pigments are visible interferences and include chlorophyll, carotenoids, and others. Figure 2 shows extract colors before and after cleanup with PSA and MWCNTs. It can be clearly seen that MWCNTs achieved better color removal than PSA, especially for carrot and broccoli, for which the color was significantly lighter after cleanup with MWCNTs vs. PSA. The removal of chlorophyll and carotenoids was determined by measuring the differences of the UV absorbance of the sample extracts at the maximum absorbance wavelength for chlorophyll (680 nm) and carotenoids (450 nm) (Han et al. 2016b; Ha et al. 2010). The removal rate was defined as the differences of the absorbance before and after cleanup compared to the value before the cleanup.
The removal rates in Table 2 indicated that 84–94% of chlorophyll in the extracts was removed by MWCNTs in broccoli, celery, and cabbage, while only 3–55% was removed by PSA. Chlorophyll content in potato and carrot were very low (the absorbance at 680 nm was only about 0.001–0.002), and not much difference was observed after cleanup using the two sorbents. Carrot extracts contain high amount of carotenoids, and the results showed that MWCNTs removed 98% of carotenoids in carrots, while the removal rates were only 17% by PSA cleanup. MWCNTs showed good removal capability for colored contents in the extracts of the five vegetables.
After the cleanup, remaining interferences in the final extracts can influence ionization of analytes in the ion source and also may affect analyte responses. In order to compare cleanup efficiency, MEs of the method after cleanup with two studied sorbents were compared. Table 3 lists MEs of six parabens in the five matrices. Usually, −20% < ME <20% was considered to be insignificant (Zhang et al. 2016).
The results showed that for the five analytes eluting after 2 min (including ethyl, n-propyl, isopropyl, n-butyl, and isobutyl), almost all of the MEs in the five matrices were between −20 and 20%, except the ME of ethylparaben in celery was 27% using PSA and was improved to 9% using MWCNTs. But for methylparaben (t R = 1.8 min), MEs were relatively high. The MEs varied from 9 to −50% in the five vegetables when PSA was used for cleanup, and the use of MWCNTs reduced MEs to 5 to −24%. Also, the MEs for each analyte showed either enhancement or suppression which not only depended on the matrix, but also was related to the cleanup sorbents. For example, the ME of methylparaben in potato showed low enhancement (9%) when PSA was used for cleanup while it changed to suppression (−11%) when MWCNTs were used. But overall, MWCNTs gave better cleanup than PSA for the detection of six parabens in the five matrices. These results also indicate that for HPLC–MS/MS, the pigment (color) in the final extracts might not be the main factor which causes MEs, especially for late-eluting analytes. In another study (Zhou et al. 2015), PSA and MgSO4 were applied for the cleanup of vegetable extracts for the analysis of the four parabens, and the absolute value of MEs were 4 to 78%, which is mostly higher than in our study.
Method Validation Results
To access the reliability of the method, linearity and fortified recoveries were investigated. Calibration curves of methyl-, ethyl-, n-propyl-, isopropyl-, n-butyl-, and isobutylparaben indicated linear correlations of responses vs. concentrations within the range of 0.05 to 2.0 mg/L, and the coefficient of determination (R 2) were all greater than 0.99. Fortified recovery study was conducted at four concentration levels in five replicates, and the recoveries ranged from 81 to 113% with RSDs lower than 11% (listed in Table 4). The LOQ of the method was 0.05 mg/kg in all five matrices. The validation results are acceptable by SANTE/11945/2015 for the residue-analysis methods in foods.
Application to Real Samples
To demonstrate the applicability of the established method to real samples, 50 fresh-cut vegetable samples, including cabbage, carrot, celery, and potato, as well as broccoli, were collected from the farmer markets in different districts in Beijing. The developed method was applied to analyze the residues of the six parabens in real samples. The results showed that only methylparaben was detected in celery sample with a concentration of 81 μg/kg. All the other analytes were below LOQ. The developed method was fast, easy, effective, and suitable for the determination of the residue of six parabens in the fresh-cut vegetables, which could be used in the routine food-quality monitoring of the fresh-cut foods.
Conclusions
In this study, modified QuEChERS method with HPLC–MS/MS detection was developed and evaluated to analyze the residual quantities of six parabens in five fresh-cut vegetables (cabbage, carrot, celery, potato, and broccoli). The results indicated that MWCNTs can be used as an effective d-SPE sorbent in the QuEChERS method and it demonstrated high removal rates for chlorophyll and carotenoids in the extracts of different matrices, especially colored vegetables. The method was fast, easy, and reliable, and ultimately, the method was applied to detect the six parabens residues in real samples, and 81 μg/kg methylparaben was detected in celery. This demonstrates that the developed method is suitable to monitor residues of the six parabens in fresh-cut vegetables. The novel sorbent MWCNTs provide a suitable alternative material for the cleanup procedure in residue analysis of the parabens in vegetables.
References
Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD (2016) Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res 151:30–37
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Byford JR, Shaw LE, Drew MGB, Pope GS, Sauer MJ, Darbre PD (2002) Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 12:49–60
Cherta L, Beltran J, López F, Hernández F (2013) Application of fast gas chromatography–mass spectrometry in combination with the QuEChERS method for the determination of pesticide residues in fruits and vegetables. Food Anal Method 6:1170–1187
Djatmika R, Hsieh CC, Chen JM, Ding WH (2016) Determination of paraben preservatives in seafood using matrix solid-phase dispersion and on-line acetylation gas chromatography-mass spectrometry. J Chromatogr B 1036-1037:93–99
Fan SF, Zhao PY, Yu CS, Pan CP, Li XS (2014) Simultaneous determination of 36 pesticide residues in spinach and cauliflower by LC-MS/MS using multi-walled carbon nanotubes-based dispersive solid-phase clean-up. Food Addit Contam 31:73–82
González-Curbelo MÁ, Hernándezborges J, Ravelopérez LM, Rodríguezdelgado MÁ (2011) Insecticides extraction from banana leaves using a modified QuEChERS method. Food Chem 125:1083–1090
González-Curbelo MÁ, Socas-Rodríguez B, Herrera-Herrera AV, González-Sálamo J, Hernández-Borges J, Rodríguez-Delgado MÁ (2015) Evolution and applications of the QuEChERS method. Trends Anal Chem 71:169–185
Ha J, Shim YS, Seo HY, Nam HJ, Ito M, Nakagawa H (2010) Rapid method for determination of β-carotene in foods using ultra high performance liquid chromatography. Food Sci Biotechnol 19:1199–1204
Han YT, Zou N, Song L, Li Y, Qin Y, Liu S, Li X, Pan CP (2015) Simultaneous determination of 70 pesticide residues in leek, leaf lettuce and garland chrysanthemum using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J Chromatogr B 1005:56–64
Han C, Xia BQ, Chen XZ, Shen JC, Miao Q, Shen Y (2016a) Determination of four paraben-type preservatives and three benzophenone-type ultraviolet light filters in seafoods by LC-QqLIT-MS/MS. Food Chem 194:1199–1207
Han LJ, Matarrita J, Sapozhnikova Y, Lehotay SJ (2016b) Evaluation of a recent product to remove lipids and other matrix co-extractives in the analysis of pesticide residues and environmental contaminants in foods. J Chromatogr A 1449:17–29
Han YT, Song L, Zou N, Chen RH, Qin YH, Pan CP (2016c) Multi-residue determination of 171 pesticides in cowpea using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J Chromatogr B 1031:99–108
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Liao C, Chen LX, Kannan K (2013) Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ Int 57-58:68–74
Mezcua M, Martínezuroz MA, Wylie PL, Fernándezalba AR (2009) Simultaneous screening and target analytical approach by gas chromatography-quadrupole-mass spectrometry for pesticide residues in fruits and vegetables. J AOAC Int 92:1790–1806
Moreta C, Tena MT, Kannan K (2015) Analytical method for the determination and a survey of parabens and their derivatives in pharmaceuticals. Environ Res 142:452–460
Olsson P, Holmbäck J, Nilsson U, Herslöf B (2014) Separation and identification of lipid classes by normal phase LC-ESI/MS/MS on a cyanopropyl column. Eur J Lipid Sci Technol 116:653–658
Pycke BF, Geer LA, Dalloul M, Abulafia O, Halden RU (2015) Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ Int 84:193–200
Pyrzynska K (2011) Carbon nanotubes as sorbents in the analysis of pesticides. Chemosphere 83:1407–1413
Qin YH, Zhao PY, Fan SF, Han YT, Li YJ, Zou N, Song SY, Zhang Y, Li FB, Li XS (2015) The comparison of dispersive solid phase extraction and multi-plug filtration cleanup method based on multi-walled carbon nanotubes for pesticides multi-residue analysis by liquid chromatography tandem mass spectrometry. J Chromatogr A 1385:1–11
Qin YH, Huang BY, Zhang JR, Han YT, Li YJ, Zou N, Yang JG, Pan CP (2016) Analytical method for 44 pesticide residues in spinach using multi-plug-filtration cleanup based on multiwalled carbon nanotubes with liquid chromatography and tandem mass spectrometry detection. J Sep Sci 39:1757–1765
Rodriguez-Gomez R, Dorival-Garcia N, Zafra-Gomez A, Camino-Sanchez FJ, Ballesteros O, Navalon A (2015) New method for the determination of parabens and bisphenol A in human milk samples using ultrasound-assisted extraction and clean-up with dispersive sorbents prior to UHPLC-MS/MS analysis. J Chromatogr B 992:47–55
Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP (1998) Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol 153:12–19
Schenck FJ, Hobbs JE (2004) Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. Bull Environ Contam Toxicol 73:24–30
Tahan GP, Santos NK, Albuquerque AC, Martins I (2016) Determination of parabens in serum by liquid chromatography-tandem mass spectrometry: correlation with lipstick use. Regul Toxicol Pharmacol 79:42–48
Terasaka S, Inoue A, Tanji M, Kiyama R (2006) Expression profiling of estrogen-responsive genes in breast cancer cells treated with alkylphenols, chlorinated phenols, parabens, or bis- and benzoylphenols for evaluation of estrogenic activity. Toxicol Lett 163:130–141
Vo TT, Jung EM, Choi KC, Frank HY, Jeung EB (2011) Estrogen receptor α is involved in the induction of Calbindin-D 9k and progesterone receptor by parabens in GH3 cells: a biomarker gene for screening xenoestrogens. Steroids 76:675–681
Wang X, Li Q, Xie J, Jin Z, Wang J, Li Y, Jiang K, Fan S (2009) Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett 9:3137–3141
Wilkowska A, Biziuk M (2011) Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem 125:803–812
Wróbel AM, Gregoraszczuk EŁ (2014) Actions of methyl-, propyl- and butylparaben on estrogen receptor-α and-β and the progesterone receptor in MCF-7 cancer cells and non-cancerous MCF-10A cells. Toxicol Lett 230:375–381
Zhang QL, Lian M, Liu LJ, Cui H (2005) High-performance liquid chromatographic assay of parabens in wash-off cosmetic products and foods using chemiluminescence detection. Anal Chim Acta 537:31–39
Zhang ZB, Sun LB, Hu Y, Jiao J, Hu JY (2013) Inverse antagonist activities of parabens on human oestrogen-related receptor gamma (ERRgamma): in vitro and in silico studies. Toxicol Appl Pharmacol 270:16–22
Zhang ZH, Li MH, Feng MY, Zhu KC, Han LJ (2016) Dissipation dynamics and final residues of cloransulam-methyl in soybean and soil. Environ Monit Assess 188:168–178
Zhao PY, Wang L, Zhou L, Zhang FZ, Kang S, Pan CP (2012) Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J Chromatogr A 1225:17–25
Zhou X, Cao SR, Li XL, Tang BB, Ding XW, Xi CX, Hu JT, Chen ZQ (2015) Simultaneous determination of 18 preservative residues in vegetables by ultra high performance liquid chromatography coupled with triple quadrupole/linear ion trap mass spectrometry using a dispersive-SPE procedure. J Chromatogr B 989:21–26
Acknowledgments
The authors would like to thank the National Agricultural Product Quality and Safety Risk Assessment Project Plan for 2015 (XHTC-FW-2015-262).
Author information
Authors and Affiliations
Contributions
Both Lijun Han and Canping Pan are corresponding authors, and they contributed equally to the work.
Corresponding authors
Ethics declarations
Ethical Approval
This work does not involve the use of any human participants or animals.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The mention of brand or firm name does not constitute an endorsement by the US Department of Agriculture above others of a similar nature not mentioned. USDA is an equal-opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Song, S., Zhang, Z., Zou, N. et al. Determination of Six Paraben Residues in Fresh-cut Vegetables Using QuEChERS with Multi-walled Carbon Nanotubes and High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Food Anal. Methods 10, 3972–3979 (2017). https://doi.org/10.1007/s12161-017-0970-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0970-7