Abstract
Small birds inhabiting regions with cold winter climates show seasonally flexible metabolic phenotypes, with the winter phenotype characterized by increments of summit metabolic rate (Msum) and cold tolerance. In the study reported here, we focused on variations in Msum as a metric of metabolic performance because it is positively correlated with cold tolerance in birds and positively related to overwinter survival in small mammals, although the latter has yet to be demonstrated in birds. Temperature appears to be a prominent driver of seasonal metabolic phenotypes in birds, as evidenced by the correlation between inter- and intra-seasonal variation in Msum and temperature variation, by recent temperature variables serving as better predictors of Msum variation than long-term climate variables, and by the induction of Msum variation by experimental cold exposure. In contrast, photoperiod and social status do not appear to be prominent drivers of metabolic flexibility in birds studied to date. Because skeletal muscle is the primary thermogenic tissue in birds, studies of the mechanistic underpinnings of metabolic flexibility have focused on skeletal muscles, particularly flight muscles. At the level of the skeletal muscle, two potential mechanisms exist for increasing thermogenic capacity, namely, muscle hypertrophy and elevated cellular metabolic intensity. Correlative studies suggest consistent winter increments in flight muscle size, with a potential regulatory role for the muscle growth inhibitor myostatin. Recent experimental studies in small birds, including modification of flight costs, cold acclimation, and exercise training, also suggest that muscle size is an important driver of metabolic flexibility in birds. Therefore, the focus of our study was on the seasonal regulation of muscle size and its contribution to metabolic flexibility. Future studies should address fitness consequences of Msum variation, the relative roles of muscle hypertrophy, and other factors (e.g., oxygen and substrate transport, cellular metabolic intensity) promoting Msum variation, as well as the molecular mechanisms underlying seasonal phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Seasonally flexible phenotypes in birds
Phenotypic plasticity allows organisms to better match their phenotypes, including morphology, physiology and behavior, to prevailing environmental or ecological conditions, and this matching may have positive fitness consequences (Pigliucci 2001). Phenotypic flexibility is a subset of phenotypic plasticity resulting in reversible phenotypic changes (Piersma and Drent 2003). It typically occurs in adult organisms encountering variable ecological or energetic demands throughout the annual cycle and/or inhabiting temporally or spatially variable environments (Piersma and Drent 2003; Piersma and van Gils 2011). Prominent drivers of flexible phenotypes in birds include all stages of the reproductive cycle (e.g., territory establishment, egg production, incubation, chick rearing), molt, migration, and cold winters (Swanson 2010; Vézina and Salvante 2010; Piersma and van Gils 2011). In this review, we have focused on cold winters as a selective force driving seasonal phenotypic flexibility in birds and on the flexibility of metabolic rates as an important element of these seasonal phenotypes.
Small birds, because of their relatively high surface area-to-volume ratios, experience high thermoregulatory demands in cold climates and therefore are expected to encounter greater seasonal variation in thermoregulatory costs compared to large birds (Dawson et al. 1983; Marsh and Dawson 1989). Indeed, for many small species wintering in cold climates, daily energy expenditure in winter may exceed that for all phases of the breeding season, including chick rearing (see Swanson 2010 for review). Winter acclimatization produces distinct seasonal phenotypes in these birds, with basal (BMR; minimum maintenance metabolism) and summit (Msum; maximum cold-induced metabolism) metabolic rates being higher in winter than in summer (Marsh and Dawson 1989; Swanson 2010). We focused in this review on Msum, rather than BMR, as a standardized measure of metabolic flexibility because it is more directly related to thermogenesis and resulting putative fitness consequences in cold climates than is BMR. BMR is not likely to be directly related to fitness except under conditions producing selection for reduced BMR (e.g., deserts, low-quality diets) (Tieleman et al. 2003; Bozinovic and Sabat 2010). Winter increments in the BMR of birds living in cold climates are thought to result from increased support costs for tissues devoted to energy provision and thermogenesis, rather than from direct selection for thermoregulation (Swanson 2010). Msum, in contrast, is positively correlated with cold tolerance and thermogenic endurance in birds (Swanson 1999; Swanson and Liknes 2006), and winter increments of Msum relative to summer of 10–50 % are common among small birds (Swanson 2010). High Msum is thus associated with improved thermoregulatory capacities and may thereby provide positive fitness benefits in cold winter environments by promoting survival (Swanson and Garland 2009). Indeed, Msum and overwinter survival are positively associated in small mammals (Hayes and O’Connor 1999; Sears et al. 2006; Clavijo-Baquet and Bozinovic 2012; Zub et al. 2014), but this relationship has yet to be directly tested in birds.
Cues for seasonally flexible metabolic phenotypes
Temperature and photoperiod vary on a seasonal basis in temperate-zone and arctic habitats. Thus, both temperature and photoperiod could serve as potential cues driving seasonally flexible metabolic phenotypes in birds, but surprisingly few studies have addressed the relative impacts of photoperiod and temperature on seasonal physiological performance (Carey and Dawson 1999; Swanson 2010). At high latitudes, photoperiod varies much more predictably over the year than does temperature, as winter temperature can vary substantially both among and within winters (e.g., Swanson and Olmstead 1999). Physiological responses to predictable (e.g., photoperiod) versus less predictable (e.g., temperature, precipitation) seasonal cues theoretically fall along a continuum from ultimate (i.e., an evolutionarily significant factor acting over the long term) to proximate (factors promoting short-term variation) responses (Fig. 1). Ultimate and proximate responses are not mutually exclusive, and a proximate response to short-term weather conditions superimposed on an ultimate response to seasonal climates might theoretically provide an optimally flexible phenotype (Fig. 1). Nevertheless, the two ends of the continuum generate distinct predictions for how physiological capacities should vary among and within winters. If birds are responding primarily in an ultimate manner to predictable seasonal changes in photoperiod, Msum should be stable among winters and should reach a maximum during the coldest average period of the winter. Moreover, Msum should be maintained at a level sufficient to withstand the most severe long-term climatic conditions for a given location. As a corollary prediction of the ultimate response scenario, Msum should be most strongly correlated with long-term mean climate variables and show little response to shorter term variations in temperature and weather. In contrast, if birds are responding primarily in a proximate manner to short-term weather variation, Msum should vary among winters, with higher levels in colder winters and peak levels at the coldest point of the particular winter season. This response would increase thermogenic capacity and provide better cold tolerance under colder conditions. A corollary prediction is that Msum should be correlated more strongly with shorter term (days to weeks) temperature variation than to long-term mean climate variables. These predictions can be tested, and a few studies have attempted to examine ultimate versus proximate responses in the metabolic capacities of birds to climate and weather variation.
Diagram of potential relationships among environmental and ecological cues, ultimate genetic and proximate phenotypically flexible adaptive responses, and measures of performance (cold tolerance and summit metabolism) or fitness (overwinter survival) for birds in cold winter climates. Ultimate and proximate responses may interact to optimally match phenotypes to environmental or ecological demands and thereby influence performance and fitness. It is also important to note that optimal phenotypes are subject to change depending on current environmental or ecological demands
Swanson and Olmstead (1999) studied three species of small resident passerines, namely, the Black-capped Chickadee (Poecile atricapillus), Dark-eyed Junco (Junco hyemalis), and American Tree Sparrow (Spizella americana), overwintering in the cold winter climate of South Dakota, USA. All three species showed differences in Msum among winters, with higher Msum during colder winters. These authors also found that all three species responded more strongly to short-term (0–7 days prior to metabolic measurements) and medium-term (14–30 days prior to metabolic measurements) temperature variation than to long-term mean temperatures or to the date of measure, suggesting a proximate response to temperature by these species. Some evidence for long-term temperature variables influencing Msum was also apparent for all three species, although cumulative partial R 2 values for long-term temperature variables were generally <0.1, whereas those for short- and medium-term temperature variables ranged from 0.15 to 0.55 (Swanson and Olmstead 1999). These findings therefore support a proximate role for temperature in explaining Msum variation.
Interestingly, Swanson and Olmstead (1999) also found that, of the three species, temperature variables explained the least amount of variation in Msum for chickadees. However, recent evidence indicates that this may be due to the particular pattern of metabolic response to temperature in this species. Petit et al. (2013) examined monthly changes in Msum of Black-capped Chickadees from Quebec, Canada, over two successive years, with measurements ranging from October to March, with further measurements in August as a summer reference. These authors found different patterns of variation over the two winters. Msum peaked in February in the first year but increased steadily through March in the second year. Temperatures were colder earlier in the first year and moderated by March, whereas in the second year, early winter temperatures were warmer, but cold temperatures persisted later in the winter (Petit et al. 2013). These data are, therefore, consistent with a proximate response to temperature.
Petit et al. (2013) studied monthly variation in Msum relative to its seasonal amplitude (i.e., the difference between measures in August and February) and observed that by October, the time at which temperatures at the study location in Quebec begin to fall below freezing, chickadees already had a Msum that was 22 % higher than that measured in summer. Similarly, Liknes et al. (2002) noted that the Msum of American Goldfinches from South Dakota was 21 % higher in April than in the summer but 14 % lower than that in the winter. April is the month when mean minimum temperatures at this location begin to exceed freezing. These findings suggest that Msum tracks seasonal changes in temperature in small birds. They also show that the birds begin to upregulate their thermogenic capacity before the onset of winter and that it remains upregulated as the temperature slowly warms in the spring. These data do not, however, describe the precise response of Msum to changes in temperature (i.e., reaction norm).
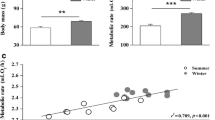
The reaction norm of Msum was addressed by Petit and Vézina (2014a), who used Msum data to investigate how free-living chickadees adjust their metabolic performance to variations in minimal temperature, maximal wind speed, minimal absolute humidity, and minimal atmospheric pressure measured within the hour of capture. Controlling for the effect of body mass and sex on Msum, these authors observed that minimal ambient temperature was the main driver of Msum variation (Fig. 2), with a lesser effect of absolute humidity. The relationship between Msum and temperature followed an inverse sigmoid shape (Fig. 2) with Msum plateauing at temperatures of less than −10 °C and greater than 24 °C and varying negatively and linearly with temperatures between these extremes. The birds therefore reached their highest winter Msum when mean minimal temperatures were around −10 °C (i.e., in January and February) and maintained this metabolic phenotype until temperatures warmed in the spring. Petit and Vézina (2014a) argued that the upper plateau of Msum could be due to physiological or morphological limits on heat production capacity, such as pectoral muscles reaching their maximal size. This would theoretically force birds to use strategies during the coldest days to reduce heat loss, such as selecting microhabitats sheltered from wind but exposed to solar radiation or using facultative hypothermia. Recent evidence suggests that actively foraging Black-capped Chickadees do use mild hypothermia during the day as a means of saving energy (Lewden et al. 2014) and that the depth of hypothermia increases during the coldest days of winter. Lewden et al. (2014) also showed that the depth of daytime hypothermia is not related to Msum or to the amount of body lipids; in other words, daytime hypothermia is not exclusively used by birds showing poor thermogenic capacity or low energy reserves but rather by all birds in response to cold ambient temperature. In theory, this strategy could potentially reduce the need for increasing Msum further during the coldest days of winter and contribute to the observed plateau. Although a more precise definition of the influence of daytime hypothermia on the Msum reaction norm in chickadees and determination of whether this hypothermic response occurs in species other than chickadees will require more attention, it appears that the upper plateau of Msum provides sufficient reserve capacity to face transient events of much colder temperatures (e.g. <−20 °C) typical of the peak of winter.
Reaction norm of maximum cold-induced metabolism (M sum ) during the seasonal variation in minimal ambient temperature measured in free-living Black-capped Chickadees from eastern Canada within 1 h of capture. Residuals correct for the effect of sex, body mass, and minimal absolute humidity on Msum. Data are from Petit and Vézina (2014a)
Assuming that the pattern of Msum variation found by Petit and Vézina (2014a) is representative of the species rather than a single population, these findings suggest that chickadees are quite responsive to a mean minimal temperature within the −10 to 24 °C range. Below −10 °C, the birds appear to maintain a stable winter phenotype, with the Msum being much less influenced by temperature but likely adjusted to conditions of a particular winter (Swanson and Olmstead 1999). Therefore, the weak effect of temperature on Msum found by Swanson and Olmstead (1999) for chickadees could be due to the fact that four of the five winters during which they conducted their observations had mean minimal ambient temperatures of less than −10 °C. Collectively, these data suggest that chickadees show a pattern of metabolic flexibility which combines both ultimate and proximate responses, although the pattern could also be explained as a proximate response with an upper ceiling on Msum flexibility, with the ceiling also potentially varying among winters. Documentation of whether similar Msum reaction norms exist for other populations or species and definition of the limits to metabolic flexibility in small birds will require further research.
Shifting the upper and lower limits of reaction norms could be adaptive under various environmental conditions. For example, raising the metabolic ceiling could be advantageous for species inhabiting cold climates (relative to species inhabiting warm climates) or for cold climate resident species during especially cold winters (relative to warmer winters). Such a strategy would allow birds to maintain a similar fraction of Msum for thermoregulation under colder conditions (Liknes et al. 2002), rather than approaching the Msum more closely. Maintaining a stable, rather than increasing, fraction of Msum under cold conditions could be adaptive because exercise (and presumably shivering) intensity is positively related to potentially detrimental influences on muscle function, such as increased oxidative damage (Johnson et al. 2012; Parker et al. 2014; Jenni-Eiermann et al. 2014), tissue injury (Carmeli et al. 2005), and elevated cell/tissue turnover (Bauchinger et al. 2010). Reduction of the lower limit of reaction norms should be adaptive largely in the context of energy conservation strategies where activity levels are reduced (Tieleman et al. 2006; Steiger et al. 2009; Wagner et al. 2013; Barske et al. 2014) and where maintaining the metabolic machinery necessary for the support of high levels of aerobic metabolism is not necessary (e.g., warm deserts, stable tropical climates; Wiersma et al. 2012). For species inhabiting highly variable environments, a greater overall width of reaction norms (e.g., greater phenotypic flexibility) might also be selectively favored and endow these species with a greater capacity to respond to heterogeneous environments.
Experimental studies simultaneously examining the relative influences of photoperiod and temperature on metabolic rates in birds are scarce, although several studies have examined the independent influence of these factors on metabolic rates, principally the BMR (reviewed by Carey and Dawson 1999; McKechnie 2008; McKechnie and Swanson 2010; Swanson 2010). Experimental cold exposure of captive birds generally results inan elevated BMR relative to that of non-cold-exposed groups (McKechnie 2008). Cold exposure in captivity, however, may result in different patterns of BMR change relative to that in wild birds exposed to seasonally cold temperatures, perhaps due to the inability to employ the full suite of behavioral responses to cold available to wild birds (Maldonado et al. 2009) or to differing conditions between captive and free-living birds (e.g., constantly available food in captivity). The effect of photoperiod on BMR in birds has been less studied than the effect of cold exposure, but short photoperiods may also stimulate an increase in BMR (Carey and Dawson 1999).
Experimental cold exposure may also increase Msum in birds (Vézina et al. 2006, 2011; van de Ven et al. 2013), but the role of photoperiod in mediating variation in Msum has been less studied. Vézina et al. (2011) conducted a 1-year-long experiment with indoor captive Red Knots (Calidris canutus) kept in constant cold (5 °C) and thermoneutral (25 °C) conditions while being exposed to the natural photoperiod. Over the year, cold-acclimated birds maintained a 10 % heavier body mass than individuals kept at thermoneutrality. As body mass variations are correlated with changes in pectoral muscle size in this species (Vézina et al. 2007, 2011), this gain of mass led to a 13 % higher mean Msum in the cold-acclimated birds (Vézina et al. 2011; see below for the effect of muscle size on Msum). However, birds from both temperature treatments also increased their body mass during the winter months (by 17–18 %, relative to summer months) as a result of an endogenous circannual clock fine-tuned to photoperiod (Cadée et al. 1996; Piersma 2002; Vézina et al. 2011). Consequently, the authors interpreted the seasonal changes in body mass of the Red Knots, and the correlated effects on thermogenic capacity, to be the result of a combination of preprogrammed seasonal adjustments and temperature-dependent responses. More recently, Swanson et al. (2014a) used a more direct approach to experimentally address the relative roles of temperature and photoperiod on Msum variation in Dark-eyed Juncos. These authors exposed birds collected in the early winter to a 2 × 2 experimental acclimation design for 6 weeks, with cold (3 °C) and warm (24 °C) temperatures and short [8:16 h, light (L):dark (D), respectively; short day (SD)] and long [16:8 h, L:D, respectively; long day (LD)] photoperiods. Msum increased over the acclimation period by 16–19 % in both cold-exposed groups, but remained unchanged in warm-exposed birds irrespective of the photoperiod (SD or LD), despite birds in the LD arm of the study being photosensitive, as indicated by gonad growth under the LD but not the SD condition. The data of Swanson et al. (2014a) therefore suggest that temperature, rather than photoperiod, is the primary driver of Msum variation in juncos, which is consistent with previous data on wild birds showing that metabolic rates in juncos respond most strongly to temperature variables in the 14- to 30-day period prior to metabolic measurements (Swanson and Olmstead 1999).
Ecological factors, in addition to environmental variation, can also affect Msum in birds (e.g., Vézina et al. 2007; Swanson 2010), and one such factor potentially important to winter Msum variation is social dominance. Several passerine species wintering at northern latitudes form flocks in winter. This offers significant advantages, but living in a group also involves social constraints, with individual social rank potentially affecting the resting or BMR (Roskaft et al. 1986; Hogstad 1987; Bryant and Newton 1994; but see Vézina and Thomas 2000 and Lewden et al. 2012). For example, Roskaft et al. (1986) found that social status explained 59 % of variation in mass-specific resting metabolic rate (RMR; daytime resting energy consumption of non-fasted birds measured at 10 °C) in wintering Great Tits (Parus major). Dominant birds not only had a higher RMR, but also had larger hearts relative to their body mass (Roskaft et al. 1986). Only one study, however, has considered the potential effect of social interaction on maximal thermogenic capacity. Working with six flocks of Black-capped Chickadees followed over 2 years, Lewden et al. (2012) predicted higher Msum and better cold tolerance in dominant individuals under the expectation that increased aggressive behavior in dominant birds would require support from larger flight muscles and hearts (Roskaft et al. 1986; Ekman 1990; Otter et al. 1997). These authors found that dominant individuals were structurally larger and heavier for their size and indeed tended to carry larger pectoral muscles and fat reserves than subordinate birds. However, the Msum was not affected by social rank. Therefore, low ranking individuals tolerated cold as well as dominant birds, presumably because all birds faced similar thermostatic costs and winter survival required an upregulation of Msum to levels that were independent of social rank.
In summary, data collected to date suggest that temperature is the primary cue driving summer-to-winter, as well as within-winter, metabolic flexibility. More experimental and field studies are nevertheless needed to refine our understanding of flexible metabolic responses of birds wintering in cold climates. For example, the fact that Swanson and Olmstead (1999) observed stronger effects of ambient temperature on the Msum of juncos and tree sparrows relative to that of chickadees measured at the same time suggests that the shape of the Msum versus temperature reaction norms differs among species (McKechnie 2008). Future studies should therefore include multiple models, with comparisons including populations and species differing in their natural wintering thermal environments. More experimental data, including repeated measures of metabolic capacity over short time periods, are also needed. This will allow the assessment of the rate of adjustment of metabolic phenotypes in response to changes in thermal conditions, the degree to which metabolic phenotypes are reversible, and the potential limits or constraints on metabolic flexibility. In addition, the assumed but untested link between individual winter Msum and fitness (Swanson and Garland 2009) should also be investigated.
Some direct experimental evidence exists for an effect of photoperiod on BMR (Carey and Dawson 1999), but effects on Msum are either indirect (e.g., circannual cycle of body mass in Red Knots; Vézina et al. 2011) or absent (e.g., juncos; Swanson et al. 2014a) for birds wintering in temperate-zone climates. The potential for a seasonally flexible metabolic response, including Msum, driven by photoperiod, with a superimposed response to acute temperature conditions remains possible (Vézina et al. 2011), and additional studies which experimentally address temperature and photoperiod as concurrent cues for seasonal metabolic flexibility are needed. Concrete generalizations on the relative roles of temperature and photoperiod as drivers of seasonal metabolic flexibility will require further experimental research on a wider variety of birds wintering in cold climates. Dominance status does not appear to influence winter Msum and, thereby, seasonal metabolic flexibility, in Black-capped Chickadees, which to our knowledge is the only species studied to date in this context. However, additional studies on other species with socially organized flocks are necessary before firmer conclusions on the impact of social status on metabolic flexibility can be drawn.
Skeletal muscle mass as a driver of seasonal Msum variation
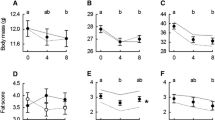
Because Msum in birds is largely the result of active shivering by skeletal muscles, the flight muscles (especially the pectoralis since it is the largest muscle in the body) are considered to be the primary thermogenic organ (Marsh and Dawson 1989). Seasonal flexibility of Msum should thus be rooted in seasonal flexibility of the pectoralis. An important mechanism for increasing organismal thermogenic capacity in winter is indeed to increase the size of the pectoralis. Such increases in pectoralis mass are consistent contributors to the winter phenotype of small birds in cold climates (Fig. 3; Swanson 2010; Liknes and Swanson 2011; Swanson and Merkord 2013). Moreover, flight muscle and/or pectoralis mass are positively correlated with Msum in individual birds for most species studied to date (Vézina et al. 2006, 2007, 2011; Swanson et al. 2013; Petit and Vézina 2014b). However, such correlations are apparently not universal (Swanson et al. 2014b), but in cases where body mass-independent residuals of pectoralis mass and Msum are not correlated, raw values may nevertheless be related (Fig. 4). A positive relationship between raw pectoralis mass and Msum, but not for residuals, suggests that one mechanism for improving organismal thermogenic capacity and cold tolerance is to increase body mass. This would involve the hypertrophy of overall skeletal muscle masses, including pectoralis, within the body (Vézina et al. 2011; Swanson et al. 2014b). Supporting this assertion, Petit et al. (2014) found a significant effect of the combined mass of all skeletal muscles on Msum in Black-capped Chickadees.
Relative summer-to-winter increases in Msum and wet pectoralis muscle mass for a variety of small bird species for which both seasonal variation in Msum and pectoralis muscle mass have been measured. Data are from Swanson (2010), Sgueo et al. (2012), and Petit et al. (2014). DEJU Dark-eyed Junco, Junco hyemalis, HOFI House Finch, Haemorhous mexicanus, MOCH Mountain Chickadee, Poecile gambeli, JUTI Juniper Titmouse, Baeolophus ridgwayi, BCCH Black-capped Chickadee, Poecile atricapillus [two populations: one from South Dakota (-SD), and one from Quebec (-Que)], WBNU White-breasted Nuthatch, Sitta carolinensis, HOSP House Sparrow, Passer domesticus, NOCA Northern Cardinal, Cardinalis cardinalis
Correlations between log10-transformed raw and residual pectoralis muscle mass versus Msum for Black-capped Chickadee, Poecile atricapillus (BCCH; data from Petit et al. 2014), American Goldfinch, Spinus tristis (AMGO; Swanson et al. 2013), House Sparrow, Passer domesticus (HOSP), and Dark-eyed Junco, Junco hyemalis (DEJU; Swanson et al. 2014b). Residuals control for the effect of body mass (minus pectoralis mass). Significant correlations are denoted by regression lines and were detected for all species for raw values, but only for goldfinches for residuals
Experimental modification of pectoralis muscle mass is also accompanied by corresponding changes in Msum (Petit and Vézina 2014a, b; Zhang and Swanson 2014). Similarly, hematocrit (the fraction of red blood cells in whole blood) and heart mass also increase in winter for most birds wintering in cold climates (Swanson 1990; Liknes and Swanson 2011; Swanson et al. 2014a), and both are positively correlated with Msum in individual birds (Petit et al. 2014; Petit and Vézina 2014b; Swanson et al. 2014b). These findings suggest that increasing the capacity for oxygen transport to shivering muscles is also an important component of the winter phenotype in small birds. Thus, winter increases in pectoralis muscle and heart mass appear to be prominent mechanistic drivers of seasonal metabolic flexibility, which brings up the question of how these mass increases are regulated.
One potential candidate for the regulation of seasonal phenotypic flexibility in muscle and heart masses is the muscle growth inhibitor myostatin. Myostatin is a member of the transforming growth factor-beta family of growth factors and acts to inhibit the incorporation of satellite cells into the muscle fiber, thereby facilitating a decrease in muscle size (McCroskery et al. 2003). Myostatin acts in an autocrine/paracrine manner, so it is produced by and acts on skeletal muscle (Lee 2004) and is also expressed in the heart (Callis et al. 2009; Bish et al. 2010; Swanson et al. 2014c). Myostatin is initially synthesized and released in a latent form (containing a prodomain bound to the mature myostatin) that is incapable of binding to the activin IIB receptor and consequently initiating a cellular response (Lee 2004). Cleavage of the prodomain by metalloproteinases, including the tolloid-like proteases TLL-1 and TLL-2, is required to activate myostatin (Huet et al. 2001; Wolfman et al. 2003). Thus, both the expression of myostatin and its activation by the metalloproteinases are potential steps regulating myostatin function upstream of activin IIB receptor binding (Lee 2004, 2008).
Most research on myostatin in birds has centered on domestic species, with a particular focus on embryonic and post-hatch chick development. These studies have demonstrated that myostatin is negatively correlated with myogenic satellite cell proliferation and differentiation, hyperplasia, and/or hypertrophy of muscle cells in cell cultures (Kocamis et al. 2001; McFarland et al. 2006, 2007). Myostatin expression is also downregulated during embryonic development (Amthor et al. 2002; Huang et al. 2011) and during post-hatch chick growth and development. In the latter case, myostatin expression decreases markedly immediately after hatching and is accompanied by rapid muscle growth (Mott and Ivarie 2002; Guernec et al. 2003). Pertinent to the topic of this review, several studies have examined myostatin mRNA expression during exposure of chicken embryos or chicks (Gallus domesticus) to cool or cold temperatures. Gabriel et al. (2003) exposed chick embryos to 36 °C or 44 °C and found myostatin expression was decreased in those exposed to 44 °C but that exposure to 36 °C had no effect on myostatin expression. For post-hatch chicks capable of thermoregulation, however, exposure to cold (4 °C) for 24–48 h resulted in reduced myostatin expression and increased leg muscle mass (Ijiri et al. 2009). In this same study, the percentage of slow oxidative muscle fibers also increased in the muscles of chicks aged <7 days which had not fully developed their thermoregulatory capacities. For chicks aged >8 days, where full thermoregulatory capacities were present, no changes in myostatin expression or leg muscle growth and fiber composition were induced by cold exposure (Ijiri et al. 2009). These studies of development and growth periods in birds suggest a role for myostatin reduction as a driver of increases in muscle mass in juvenile birds. However, fewer studies have addressed the potential role for myostatin in muscle remodeling in adult birds, despite its function in muscle (Whittemore et al. 2003; Mosher et al. 2007; Welle et al. 2007; LeBrasseur et al. 2009) and heart (Morissette et al. 2006; Callis et al. 2009; Bish et al. 2010) remodeling in adult mammals.
If myostatin were to be involved in regulating the winter increments in pectoralis muscle mass commonly observed in wintering birds, patterns of mRNA or protein expression for myostatin and its metalloproteinase activators in pectoralis muscle and heart should be consistent with winter hypertrophy of these tissues (i.e., winter reductions in expression). In fact, patterns of mRNA expression for myostatin and/or its metalloproteinase activators consistent with this prediction have been documented for the pectoralis muscle and/or heart of the House Sparrow and American Goldfinch (Swanson et al. 2009, 2014c). In addition, in one study where Black-capped Chickadees failed to show winter hypertrophy of the pectoralis muscle or heart relative to the summer, minimal seasonal changes in mRNA expression or protein levels of myostatin and the TLLs in the pectoralis muscle and heart were detected (Swanson et al. 2014c). Moreover, in another study, cold-exposed Dark-eyed Juncos were found to exhibit elevated Msum relative to control birds, but this was not accompanied by cold-induced increases in pectoralis muscle mass or myostatin mRNA expression (Stager et al. 2015). Thus, patterns of summer-to-winter or cold-induced variation in the myostatin system have been found to be largely consistent with seasonal changes in muscle and heart masses and with a regulatory role for myostatin in the seasonal modulation of thermogenic capacity and cold tolerance (Fig. 5).
Summer-to-winter variation [winter/summer (W/S) percentage change] in average pectoralis mass (Pec mass), mRNA expression for myostatin (Myo RNA), and protein levels of tolloid-like metalloproteinases (TLL-1 mRNA, TLL-2 mRNA) and myostatin (Myo protein; not measured for the House Sparrow) for the three species measured to date: House Sparrow, Passer domesticus (Swanson et al. 2009), American Goldfinch, Spinus tristis and Black-capped Chickadee, Poecile atricapillus (Swanson et al. 2014c). Note that pectoralis mass generally increases in the winter and expression of the myostatin system is generally reduced in the winter
In contrast, the patterns of myostatin and tolloid-like metalloproteinase (TLL-1, -2) mRNA expression in the pectoralis muscle are not consistent with a role in the regulation of flight muscle hypertrophy associated with migration in birds (Price et al. 2011; King et al. 2015)—although the pattern of myostatin protein levels were consistent with just such a role (King et al. 2015). These data suggest that post-transcriptional modifications of myostatin may be important to its function in regulating muscle size, at least during migration, and that the myostatin system may not function in identical manners in regulating muscle or heart hypertrophy during migration and winter acclimatization in birds. Firm conclusions regarding the differential involvement of the myostatin system in the regulation of muscle or heart hypertrophy during winter acclimatization and migration in birds will require further study of the expression patterns of myostatin and tolloid-like metalloproteinase (TLL-1, -2) mRNA and protein patterns from a wider range of bird species.
Expression patterns and protein levels of myostatin and the TLLs should also be determined for other stages of the annual cycle of birds where variation in muscle or heart size occurs (e.g., nestling provisioning, molt, migration staging) to determine whether myostatin might function as a general mediator of muscle and heart remodeling throughout the annual cycle. Regulation of the myostatin signaling system downstream of the activin RIIB receptor via the SMAD or other signaling pathways (Heldin et al. 1997; Miyazawa et al. 2002; Morrissette 2006; Rodgers and Garikipati 2008; Bish et al. 2010) during periods of muscle and heart remodeling in the annual cycle of birds should also be a target of future research. Research addressing the patterns of expression and functional roles of other muscle growth regulators (Price et al. 2011; Stager et al. 2015) in mediating seasonal variation in muscle mass and Msum would also be profitable.
If the myostatin system functions as a driver of muscle mass variation in birds, then blocking myostatin should induce muscle growth. Several studies have demonstrated just such effects in domestic bird species. Kim et al. (2006) produced a monoclonal antibody against the active form of myostatin and injected this antibody into eggs; at 35 days of life, the chicks from the injected eggs had a larger body (4 %) and muscle (5.5 %) mass than those hatched from vehicle-injected control eggs. Kim et al. (2012) performed an in vitro reporter gene assay in rhabdomyosarcoma cell culture. These authors found that the solubilized extracellular domain of the activin RIIB receptor derived from chicken reduced myostatin activity, indicating successful myostatin blocking activity amenable to future in vivo studies. Follistatin is another myostatin blocker, and its injection into leg muscle of 4-week old ducklings (Anas platyrhynchos) was found to increase muscle fiber hypertrophy and satellite cell activation frequency (Liu et al. 2012). These studies demonstrate that the effects of myostatin blocking activity on muscles have, to date, dealt with embryonic and chick growth. Future studies should attempt to block myostatin and examine its impact on muscle (and heart) remodeling in adult birds to determine whether functional effects of myostatin are consistent with patterns of expression suggesting a role in mediating seasonal changes in muscle mass and thermogenic or exercise capacities.
Summary and conclusions
Summit metabolic rate in small birds is a highly flexible trait and typically increases in response to increasing energy demands, such as thermoregulation in cold winters and increased flight demands (Swanson 2010; McKechnie and Swanson 2010; Petit and Vézina 2014b). Increases in Msum confer enhanced cold tolerance (Swanson 2001; Swanson and Liknes 2006), and high Msum presumably promotes increases in overwinter survival and fitness (Hayes and O’Connor 1999; Swanson and Garland 2009), although evidence supporting this connection is just beginning to accumulate for birds (Vézina et al., unpublished data). Ecological factors, such as social dominance, appear to play little role in mediating Msum variation in birds (Lewden et al. 2012). Among environmental factors, temperature appears to be the primary driver of seasonal Msum variation, with a lesser role for humidity (Swanson et al. 2014a; Petit and Vézina 2014a). Temperature also appears to act predominately in an proximate manner, producing flexible responses over periods of days to weeks (Swanson and Olmstead 1999; Swanson et al. 2014a), but some evidence also supports a longer-term, or ultimate response to seasonal temperature variation, although the magnitude of this ultimate response may vary among species (Petit and Vézina 2014a). Defining the shape of reaction norms of Msum to temperature within species, both between seasons and across distributional ranges, and among species inhabiting different climates, will assist in more precisely defining the role of temperature in mediating Msum variation and the time frame over which this mediation occurs. Such studies will provide a fruitful avenue for future research.
Winter increments of Msum in birds are generally accompanied by increases in skeletal muscle (especially pectoralis) and heart masses (Vézina et al. 2007, 2011; Liknes and Swanson 2011; Petit et al. 2013). Seasonal variation of the muscle growth inhibitor myostatin and its tolloid-like metalloproteinase activators in pectoralis muscle and heart are generally, although not without exception, consistent with a winter downregulation of the myostatin system contributing to the seasonal variation in pectoralis and heart masses (Swanson 2009; Swanson et al. 2014a, b c). Interestingly, seasonal correlations between muscle and heart masses and the tolloid-like metalloproteinases are sometimes stronger than those for myostatin itself (Swanson et al. 2014c). It is likely that other muscle growth factors are also involved in regulating seasonal flexibility of muscle and heart masses (e.g., Price et al. 2011), therefore future research should investigate seasonal variation in expression and functional consequences not only for myostatin (including the pathway downstream from the activing IIB receptor) but also for other muscle growth regulators.
References
Amthor H, Huang R, McKinnell I, Christ B, Kambadur R, Sharma M, Patel K (2002) The regulation and action of myostatin as a negative regulator of muscle development during avian embryogenesis. Dev Biol 251:241–257
Barske J, Fusani L, Wikelski M, Feng NY, Santos M, Schlinger B (2014) Energetics of the acrobatic courtship in male golden-collared manakins (Manacus vitellinus). Proc Roy Soc B 281:20132482
Bauchinger U, Keil J, McKinney RA, Starck JM, McWilliams SR (2010) Exposure to cold but not exercise increases carbon turnover rates in specific tissues of a passerine. J Exp Biol 213:526–534
Bish LT, Morine KJ, Sleeper MM, Sweeney HL (2010) Myostatin is upregulated following stress in an Erk-dependent manner and negatively regulates cardiomyocyte growth in culture and in a mouse model. PLoS One 5:e10230
Bozinovic F, Sabat P (2010) On the intraspecific variability in basal metabolism and the food habits hypothesis in birds. Curr Zool 56:759–766
Bryant DM, Newton AV (1994) Metabolic costs of dominance in dippers Cinclus cinclus. Anim Behav 48:447–455
Cadée N, Piersma T, Daan S (1996) Endogenous circannual rhythmicity in a non-passerine migrant, the knot Calidris canutus. Ardea 84:75–84
Callis TE, Pandya K, Seok HY, Tang R-H, Tatsuguchi M, Huang Z-P, Chen J-F, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang D-Z (2009) MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119:2772–2786
Carey C, Dawson WR (1999) A search for environmental cues used by birds in survival of cold winters. Curr Ornithol 15:1–31
Carmeli E, Moas M, Lennon S, Powers SK (2005) High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibers. Exp Physiol 90(4):613–619
Clavijo-Baquet S, Bozinovic F (2012) Testing the fitness consequences of the thermoregulatory and parental care models for the origin of endothermy. PLoS One 7:e37069
Dawson WR, Marsh RL, Yacoe ME (1983) Metabolic adjustments of small passerine birds for migration and cold. Am J Physiol 245:R755–R767
Ekman J (1990) Alliance in winter flocks of willow tits - effects of rank on survival and reproductive success in male-female associations. Behav Ecol 26:239–245
Gabriel JE, Alvares LE, Gobet MC, de Paz CCP, Packer IU, Macari M, Coutinho LL (2003) Expression of MyoD, myogenin, myostatin and Hsp70 transcripts in chicken embryos submitted to mild heat or cold. J Therm Biol 28:261–269
Guernec A, Berri C, Chevalier B, Wacrenier-Cere N, Le Bihan-Duval E, Duclos MJ (2003) Muscle development, insulin-like growth factor-I and myostatin mRNA levels in chickens selected for increased breast muscle yield. Growth Horm IGF Res 13:8–18
Hayes JP, O’Connor CS (1999) Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53:1280–1287
Heldin C-H, Miyazono K, ten Dijke P (1997) TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471
Hogstad O (1987) It is expensive to be dominant. Auk 104:333–336
Huang K-L, Wang J-W, Han C-C, Liu H-H, Li L, Dai F, Pan Z, Xu F, He H, Xu H (2011) Developmental expression and alternative splicing of the duck myostatin gene. Comp Biochem Physiol Part D 6:238–243
Huet C, Li Z-F, Liu H-Z, Black RA, Galliano M-F, Engvall E (2001) Skeletal muscle cell hypertrophy induced by inhibitors of metalloproteinases; myostatin as a potential mediator. Am J Physiol 281:C1624–C1634
Ijiri D, Miura M, Kanai Y, Hirabayashi M (2009) Increased mass of slow-type skeletal muscles and depressed myostatin gene expression in cold-tolerant chicks. Zool Sci 26:277–283
Jenni-Eiermann S, Jenni L, Smith S, Constantini D (2014) Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLoS One 9:e97650
Johnson BD, Padilla J, Wallace JP (2012) The exercise dose affects oxidative stress and brachial artery flow-mediated dilation in trained men. Eur J Appl Physiol 112:33–42
Kim YS, Bobbili NK, Paek KS, Jin HJ (2006) Production of a monoclonal anti-myostatin antibody and the effects of in ovo administration of the antibody on posthatch broiler growth and muscle mass. Poultry Sci 85:1062–1071
Kim YS, Kim KH, Kim CJ (2012) Production of bioactive extracellular domain of pig and chicken activin type IIB receptors in Pichia pastoris. Process Biochem 47:139–146
King MO, Zhang Y, Carter T, Johnson J, Harmon E, Swanson DL (2015) Phenotypic flexibility of skeletal muscle and heart masses and expression of myostatin and tolloid–like proteinases in migrating passerine birds. J Comp Physiol B 185. doi:10.1007/s00360-015-0887-7
Kocamis H, McFarland D, Killefer J (2001) Temporal expression of growth factor genes during myogenesis in satellite cells derived from biceps femoris and pectoralis major muscles of the chicken. J Cell Physiol 186:146–152
LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA (2009) Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol 64A:940–948
Lee S-J (2004) Regulation of muscle mass by myostatin. Annual Rev Cell Dev Biol 20:61–86
Lee S-J (2008) Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS One 3:e1628
Lewden A, Petit A, Vézina F (2012) Dominant black-capped chickadees pay no maintenance energy costs for their wintering status and are not better at enduring cold than subordinate individuals. J Comp Physiol B 182:381–392
Lewden A, Petit M, Milbergue M, Orio S, Vézina F (2014) Evidence of facultative daytime hypothermia in a small passerine wintering at northern latitudes. Ibis 156:321–329
Liknes ET, Swanson DL (2011) Phenotypic flexibility of body composition associated with seasonal acclimatization of passerine birds. J Therm Biol 36:363–370
Liknes ET, Scott SM, Swanson DL (2002) Seasonal acclimatization in the American goldfinch revisited: to what extent to metabolic rates vary seasonally? Condor 104:548–557
Liu H-H, Wang J-W, Yu H-Y, Zhang R-P, Chen X, Jin H-B, Dai F, Li L, Xu F (2012) Injection of duck recombinant follistatin fusion protein into duck muscle tissues stimulates satellite cell proliferation and muscle fiber hypertrophy. Appl Microbiol Biotechnol 94:1255–1263
Maldonado K, Cavieres G, Veloso C, Canals M, Sabat P (2009) Physiological responses in rufous-collared sparrows to thermal acclimation and seasonal acclimatization. J Comp Physiol B 179:335–343
Marsh RL, Dawson WR (1989) Avian adjustments to cold. In: Wang LCH (ed) Advances in comparative and environmental physiology 4: animal adaptation to cold. Springer, New York, pp 205–253
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147
McFarland DC, Velleman SG, Pesall JE, Liu C (2006) Effect of myostatin on turkey myogenic satellite cells and embryonic myoblasts. Comp Biochem Physiol Part A 144:501–508
McFarland DC, Velleman SG, Pesall JE, Liu C (2007) The role of myostatin in chicken (Gallus domesticus) myogenic satellite cell proliferation and differentiation. Gen Comp Endocr 151:351–357
McKechnie AE (2008) Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J Comp Physiol B 178:235–247
McKechnie AE, Swanson DL (2010) Sources and significance of variation in basal, summit and maximal metabolic rates in birds. Curr Zool 56:741–758
Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K (2002) Two major Smad pathways in TGF-β superfamily signaling. Genes Cells 7:1191–1204
Morissette MR, Cook SA, Foo SY, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A (2006) Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99:15–24
Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh C, Parker HG, Ostrander EA (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 5:e79
Mott I, Ivarie R (2002) Expression of myostatin is not altered in lines of poultry exhibiting myofiber hyper- and hypoplasia. Poult Sci 81:799–804
Otter K, Chruszcz B, Ratcliffe L (1997) Honest advertisement and song output during the dawn chorus of black-capped chickadees. Behav Ecol 8:167–173
Parker L, McGuckin TA, Leicht AS (2014) Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin Physiol Funct Imaging 34:377–383
Petit M, Vézina F (2014a) Reaction norms in natural conditions: how does metabolic performance respond to weather variations in a small endotherm facing cold environments? PLoS One 9:e113617
Petit M, Vézina F (2014b) Phenotype manipulations confirm the role of pectoral muscles and haematocrit in avian maximal thermogenic capacity. J Exp Biol 217:824–830
Petit M, Lewden A, Vézina F (2013) Intra-seasonal flexibility in avian metabolic performance highlights the uncoupling of basal metabolic rate and thermogenic capacity. PLoS One 8:e68292
Petit M, Lewden A, Vézina F (2014) How does flexibility in body composition relate to seasonal changes in metabolic performance in a small passerine wintering at a northern latitude? Physiol Biochem Zool 87:539–549
Piersma T (2002) When a year takes 18 months: evidence for a strong circannual clock in a shorebird. Naturwissenschaften 89:278–279
Piersma T, Drent J (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18:228–233
Piersma T, van Gils J (2011) The flexible phenotype: a body-centred integration of ecology, physiology, and behavior. Oxford University Press, Oxford
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. Johns Hopkins University Press, Baltimore
Price ER, Bauchinger U, Zajac DM, Cerasale DJ, McFarlan JT, Gerson AR, McWilliams SR, Guglielmo CG (2011) Migration- and exercise-induced changes to flight muscle size in migratory birds and association with IGF1 and myostatin mRNA expression. J Exp Biol 214:2823–2831
Rodgers BD, Garikipati DK (2008) Clinical, agricultural and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534
Roskaft E, Jarvi T, Bakken M, Bech C, Reinertsen RE (1986) The relationship between social status and resting metabolic rate in great tits (Parus major) and pied flycatchers (Ficedula hypoleuca). Anim Behav 34:838–842
Sears MW, Hayes JP, O’Connor CS, Geluso K, Sedinger JS (2006) Individual variation in thermogenic capacity affects above-ground activity of high-altitude deer mice. Funct Ecol 20:97–104
Sgueo C, Wells ME, Russell DE, Schaeffer PJ (2012) Acclimatization of seasonal energetics in northern cardinals (Cardinalis cardinalis) through plasticity of metabolic rates and ceilings. J Exp Biol 215:2418–2424
Stager M, Swanson DL, Cheviron Z (2015) Regulatory mechanisms of metabolic flexibility in the dark–eyed junco (Junco hyemalis). J Exp Biol 218 (in press). doi:10.1242/jeb.113472
Steiger SS, Kelley JP, Cochran WW, Wikelski M (2009) Low metabolism and inactive lifestyle of a tropical rain forest bird investigated via heart-rate telemetry. Physiol Biochem Zool 82:580–589
Swanson DL (1990) Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the dark-eyed junco (Junco hyemalis). Auk 107:561–566
Swanson DL (2001) Are summit metabolism and thermogenic endurance correlated in winter-acclimatized passerine birds. J Comp Physiol B 171:475–481
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Biol 209:466-474
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129
Swanson DL, Garland T Jr (2009) The evolution of high summit metabolism and cold tolerance in birds and its impact on present-day distributions. Evolution 63:184–194
Swanson DL, Merkord CL (2013) Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements. J Ornithol 154:119–127
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperature on metabolism in passerine birds. Physiol Biochem Zool 72:566–575
Swanson DL, Sabirzhanov B, VandeZande A, Clark TG (2009) Seasonal variation of myostatin gene expression in pectoralis muscle of house sparrows (Passer domesticus) is consistent with a role in regulating thermogenic capacity and cold tolerance. Physiol Biochem Zool 82:121–128
Swanson DL, Zhang Y, King MO (2013) Individual variation in thermogenic capacity is correlated with flight muscle size but not cellular metabolic capacity in American goldfinches, Spinus tristis. Physiol Biochem Zool 86:421–431
Swanson DL, Zhang Y, Liu J-S, Merkord CL, King MO (2014a) Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. J Exp Biol 217:866–875
Swanson DL, Zhang Y, King MO (2014b) Mechanistic drivers of flexibility in summit metabolic rates of small birds. PLoS One 9:e101577
Swanson DL, King MO, Harmon E (2014c) Seasonal variation in pectoralis muscle and heart myostatin and tolloid-like proteins in small birds: a regulatory role for seasonal phenotypic flexibility? J Comp Physiol B 184:249–258
Tieleman BI, Williams JB, Bloomer P (2003) Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc Roy Soc Lond B 270:207–214
Tieleman BI, Dijkstra TH, Lasky JR, Mauck RA, Visser GH, Williams JB (2006) Physiological and behavioural correlates of life-history variation: a comparison between tropical and temperate zone House Wrens. Funct Ecol 20:491–499
van de Ven TMFN, Mzilikazi M, McKechnie AE (2013) Phenotypic flexibility in body mass, basal metabolic rate and summit metabolism in southern red bishops (Euplectes orix): responses to short term thermal acclimation. Comp Biochem Physiol Part A 165:319–327
Vézina F, Salvante K (2010) Behavioral and physiological flexibility are used by birds to manage energy and support investment in the early stages of reproduction. Cur Zool 56:767–792
Vézina F, Thomas DW (2000) Social status does not affect resting metabolic rate in wintering dark-eyed junco (Junco hyemalis). Physiol Biochem Zool 73:231–236
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2007) Thermogenic side effects to migratory disposition in shorebirds. Am J Physiol Regul Integr Comp Physiol 292:1287–1297
Vézina F, Dekinga A, Piersma T (2011) Shorebirds’ seasonal adjustments in thermogenic capacity are reflected by changes in body mass: how preprogrammed and instantaneous acclimation work together. Integr Comp Biol 51:394–408
Wagner DN, Mineo PM, Sgueo C, Wikelski M, Schaeffer PJ (2013) Does low daily energy expenditure drive low metabolic capacity in the tropical robin, Turdus grayi? J Comp Physiol B 183:833–841
Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA (2007) Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab 292:E985–E991
Whittemore L-A, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O’Hara D, Pearson A, Quazi A, Ryerson S, Tan X-Y, Tomlinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM (2003) Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Comm 300:965–971
Wiersma P, Nowak B, Williams JB (2012) Small organ size contributes to the slow pace of life in tropical birds. J Exp Biol 215:1662–1669
Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomlinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee S-J (2003) Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 100:15842–15846
Zhang Y, Swanson DL (2014) Cold and exercise training produce similar Increases in maximal metabolic output in house sparrows. Integr Comp Biol 54[Suppl 1]:e233
Zub K, Borowski Z, Szafranska PA, Wieczorek M, Kanoarzewski M (2014) Lower body mass and higher metabolic rate enhance winter survival in root voles, Microtus oeconomus. Biol J Linn Soc 113:297–309
Acknowledgments
DLS was supported by the U.S. National Science Foundation IOS-1021218 and the University of South Dakota. FV was supported by a Discovery grant 9045333 from the Natural Sciences and Engineering Research Council of Canada as well as a Nouveaux Chercheurs grant 132032 from the Fonds de Recherche du Québec sur la nature et les technologies. We thank the many postdoctoral fellows, graduate students, and undergraduate students who contributed substantially to this work, and M. Petit for providing Black-capped Chickadee data for Figs. 2, 3 and 4. We also thank two anonymous reviewers for the constructive comments on an earlier version of the manuscript.
Compliance with ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. DLS collected birds for these studies under active State and Federal (United States Fish and Wildlife Service, MB758442) scientific collecting permits, and all studies were approved by the University of South Dakota Institutional Animal Care and Use Committee. All bird manipulations by FV were approved by the animal care committee of the Université du Québec à Rimouski and have been conducted under scientific and banding permits from Environment Canada—Canadian wildlife service.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Matthysen.
Rights and permissions
About this article
Cite this article
Swanson, D.L., Vézina, F. Environmental, ecological and mechanistic drivers of avian seasonal metabolic flexibility in response to cold winters. J Ornithol 156 (Suppl 1), 377–388 (2015). https://doi.org/10.1007/s10336-015-1192-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1192-7