Abstract
Changes in flight muscle size are important mediators of phenotypic flexibility in birds, so the ability to track such changes over time in individual birds is a valuable tool for investigating phenotypic flexibility. Ultrasonography has been used to track changes in flight muscle size in shorebirds, but has not been previously used to track such changes in small birds, despite variation in flight muscle size being an important contributor to phenotypic flexibility in these birds. One prominent avian example of phenotypic flexibility is the seasonal phenotypes of small birds in response to climatic variation. The winter phenotype in these birds is characterized by increases in organismal metabolic rates and pectoralis muscle mass. We measured seasonal flight muscle size in House Sparrows (Passer domesticus, 25–30 g) using both ultrasonography and wet muscle mass and tested the correlation between ultrasonographic measures of breast muscle thickness and muscle mass. We further tested whether ultrasonographic measures of muscle thickness were sufficiently precise to detect seasonal variation in flight muscle mass. Muscle mass was significantly and positively associated with ultrasonographic measurements of breast muscle thickness for short-axis (SA), long-axis (LA), and combined SA and LA measurements. Breast muscle mass was significantly greater in winter than in summer (17.5 %) and muscle thickness also increased significantly in winter for both SA (9.1 %) and LA (7.5 %) measures. Thus, these data confirm that winter elevations of flight muscle mass consistently contribute to the winter phenotype in House Sparrows and that ultrasonography is effective in detecting seasonal changes in muscle mass in small birds.
Zusammenfassung
Jahreszeitliche phänotypische Flexibilität in der Größe der Flugmuskulatur bei kleinen Vögeln: Ein Vergleich von Maßen anhand von Ultraschall und Gewebegewicht
Veränderungen der Flugmuskulatur von Vögeln sind wichtige Mediatoren phänotypischer Flexibilität. Von daher ist die Möglichkeit, derartige zeitliche Veränderungen in individuellen Vögeln zu messen ein sehr hilfreich um phänotypische Flexibilität zu untersuchen. Mittels Ultraschalls können Veränderungen in der Flugmuskulatur von Seevögeln gemessen werden. Diese Methode wurde jedoch bis jetzt noch nicht in Singvögeln genutzt, und das obwohl Variation in der Größe von Flugmuskeln in diesen Arten ein wichtiger Indikator von phänotypischer Flexibilität ist. Ein bekanntes Beispiel von phänotypischer Flexibilität in der Ornithologie sind die saisonalen Veränderungen von Phänotypen kleiner Vögel als Reaktion auf Klimavariation. Die winterlichen Phänotypen dieser Vögel sind durch einen Anstieg physiologischer Umsatzraten und des Gewichtes des Pectoralis Muskels geprägt. Wir haben die saisonale Größe der Flugmuskulatur in Haussperlingen (Passer domesticus, 25–30 g) mittels Ultraschalls sowie durch Bestimmung der Dicke und des Nassgewichtes, sowie die Korrelation zwischen beiden bestimmt. Ferner haben wir untersucht ob die Ultraschallmessung der Dicke der Muskeln die saisonale Veränderung in der Masse der Flugmuskeln vorhersagen kann. Muskelmasse war signifikant positiv mit den Ultraschallmaßen short-axis (SA), long-axis (LA) und einer Kombination von SA und LA assoziiert. Die Masse des Brustmuskels war im Winter signifikant höher als im Sommer (17.5 %). Ebenso nahm die Dicke der Muskeln während des Winters zu (SA: 9.1 %, LS: 7.5 %). Unsere Daten unterstützen dass die Zunahme der Flugmuskulatur während des Winters konsistent zum Winterphänotyp von Haussperlingen beitragen, und ferner, dass Ultraschall ausreichend effektiv ist um saisonale Veränderungen in der Muskelmasse kleiner Vögel zu detektieren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flexible phenotypic responses permit birds to alter their morphology and physiology to better match changing energy demands throughout the annual cycle (Swanson 2010; Piersma and van Gils 2011). One well-known example of such reversible phenotypic flexibility are the seasonal phenotypes occurring in small birds in response to seasonal climatic variation (Swanson 2010). The winter phenotype in these birds is characterized by improved cold tolerance and enhanced capacities for thermogenesis (McKechnie and Swanson 2010; Swanson 2010). Winter increases in summit metabolic rates (M sum, maximal thermoregulatory metabolic rates) of 20–40 % relative to summer are common among small birds (Swanson 2010). Such increases in maximal thermogenic capacity are associated with improved shivering endurance at cold temperatures and heightened levels of cold tolerance (Marsh and Dawson 1989; Swanson 2001; Swanson and Liknes 2006).
Because thermoregulation in the cold is primarily accomplished by muscular shivering in small birds, phenotypic flexibility should be most evident in skeletal muscle, especially the pectoralis, which is the principal thermogenic organ (Hohtola 1982; Marjoniemi and Hohtola 1999; Dawson and O’Connor 1996). Positive correlations between pectoralis mass, M sum and cold tolerance are common among birds (Vézina et al. 2006; Swanson 2010). In addition, pectoralis muscle hypertrophy is a regular component of the winter phenotype in small birds and changes in pectoralis muscle mass often rather closely parallel changes in M sum (Swanson 2010). Taken together, these findings suggest that variation in pectoralis muscle mass might mediate similar variation in M sum and cold tolerance in small birds, but such a relationship has not yet been investigated, principally because of technical difficulties in non-invasively measuring muscle size in small birds.
Most studies of variation in muscle and organ masses have been carried out on populations during different seasons or different stages of migration, rather than tracking muscle and organ masses in individual birds over time. However, several recent studies have used ultrasonography to demonstrate that muscle size in individual shorebirds varies over time in association with season, differing portions of the migratory period, and cold acclimation, and that changing muscle size impacts thermogenic capacity (Dietz et al. 1999; Lindström et al. 2000; Vézina et al. 2006, 2007, 2011).
Historically, tracking flight muscle mass changes in birds associated with phenotypic flexibility required euthanizing individuals and excising muscles. However, more recently, techniques have been developed to allow non-invasive, repeatable, measures of flight muscle size on individual birds, including muscle scoring (Bairlein 1995; Gosler and Harper 2000; Constantini et al. 2007), “muscle-meter” measurement (Bauchinger et al. 2011), magnetic resonance imaging (Köver et al. 1998), and ultrasonography (Newton 1993; Dietz et al. 1999). Published data for ultrasonographic measurement of flight muscle size are available for birds as small as Canary (Serinus canaria, ~25 g; Newton 1993), Tree Swallow (Tachycineta bicolor, ~22 g; Winkler and Allen 1995, 1996), Barn Swallow (Hirundo rustica, ~20 g; Ward 1996; Engstrand and Bryant 2002), Great Tit (Parus major, ~17 g; Carrascal et al. 1998; Senar et al. 1999, 2002), Pied Flycatcher (Ficedula hypoleuca, ~15 g; Potti et al. 1999) and Savannah Sparrow (Passerculus sandwichensis, ~19 g, Rae et al. 2009). The ultrasound technique has been fully validated, including documenting error values in predicting flight muscle mass from ultrasound measurements, for shorebirds down to the size of Red Knots (Calidris canutus) at approximately 120 g (Dietz et al. 1999). Newton (1993) calculated a muscle volume index (USVOL) from keel length and six ultrasound muscle measurements and compared accuracy of the ultrasound index through regressions of USVOL against lean dry mass of the flight muscle. Other studies on small birds, however, did not include validations of the accuracy of the ultrasound measurements. In addition, no studies to date have yet compared relative changes in ultrasonographic measures of muscle size and muscle mass for small birds between seasons to determine if ultrasonography is sufficiently precise to detect such seasonal changes.
The objectives of this study were to test in a small bird, the House Sparrow (Passer domesticus, approximately 28–30 g body mass), whether (1) winter increases in flight muscle mass detected in a previous study (Liknes and Swanson 2011) were repeatable in a subsequent winter, and (2) ultrasonography could be used to detect summer to winter variation in flight muscle size. House Sparrows from our study population in southeastern South Dakota, USA, are common permanent residents (Tallman et al. 2002). This species shows elevated M sum and cold tolerance in winter relative to summer (Arens and Cooper 2005; Swanson and Liknes 2006), and the higher thermogenic capacity is associated with seasonal variation in pectoral muscle mass (Liknes and Swanson 2011). Thus, House Sparrows serve as an excellent candidate to investigate whether ultrasound can detect seasonal differences in flight muscle size in small birds.
Methods
Bird capture and dissection
We captured House Sparrows near Vermillion, Clay County, South Dakota, USA (42°47′N, 96°55′W) in summer (8 June–22 August 2007, n = 13) and winter (6 November 2007–28 February 2008, n = 17). Summer birds consisted of both adults and hatching-year birds (aged by skull ossification) that had attained full adult body mass (Pyle 1997). At capture, we measured body mass (M b ) to the nearest 0.1 g, and wing chord and tarsus length to the nearest 0.1 mm. We captured birds in the morning and transferred birds to the laboratory for ultrasound measurements of flight muscle size on the same morning. Following ultrasound measurements, we euthanized birds by cervical dislocation, dissected out the left pectoralis and supracoracoideus muscles on ice, and determined wet masses of muscles to the nearest 0.01 g. We calculated flight muscle mass for subsequent comparisons with ultrasound measurements by adding left pectoralis and supracoracoideus masses.
Ultrasound measurements
We used a Visual Sonics Vevo 770 High-Resolution Imaging system with a Model 710B scan head for ultrasound measurements of muscle thickness. We used a frequency of 25 MHz and a focal length of 15 mm for all measurements. We conducted ultrasound measurements on unanesthetized birds with a cloth bag placed over their head to calm them yet allow breathing. Two people were involved in all ultrasound measurements, one to hold the bird and one to operate the scan head. To conduct the ultrasound measurements, we moistened the plumage over the breast with warm water and pushed aside the feathers to expose the skin in the region overlying the flight muscles. After the skin was exposed, we added water-soluble recording gel to the surface of the skin and placed the scan head into the recording gel so that it just touched the skin surface for measurement of muscle thickness. We recorded muscle thickness measurements to the nearest 0.01 mm along both the short and long axis of the flight muscles (Dietz et al. 1999), and we repeated both measurements three times on each individual bird for calculation of repeatabilities according to Lessels and Boag (1987). For subsequent analyses, we used the average of these three measures, although in a few cases we excluded obvious outlier values (>20 % different from the average of the other two values) from computing the average muscle thickness.
For short-axis (SA; “transversal” in Dietz et al. 1999) measurements, we standardized the position of measurements by placing a metal ruler across the bottom of the furculum and sliding the scan head forward along the left breast musculature so that it was just touching the base of the ruler. At this position, the scan head revealed both the sternal keel and the horizontal portion of the sternum. We measured flight muscle thickness along a right angle to the horizontal sternum at its junction with the sternal keel (Fig. 1a). For long-axis (LA; “horizontal” in Dietz et al. 1999) measurements, we rotated the scan head 90° from the SA position and moved the scan head so that the heart was at the extreme lower left region of the field of view and the horizontally oriented sternum was clearly visible. We measured LA muscle thickness as the distance between the sternum and the muscle surface at the point where the horizontal portion of the sternum began to angle downward on the ultrasound image (Fig. 1b).
Statistics
We present data as means ± SD. For seasonal comparisons of muscle mass and thickness, we used Student’s t test, or the Mann–Whitney test if parametric assumptions were not met. We used least squares regressions of SA, LA, and combined [(SA + LA)/2] ultrasound measures of flight muscle thickness against wet flight muscle mass. We verified that all data were normally distributed (Shapiro–Wilk test) before regression analyses. We calculated repeatability of ultrasound measurements from individual birds as the intraclass correlation coefficient according to Lessels and Boag (1987). We accepted significance at P < 0.05.
To validate the ability of the regression models to predict flight muscle mass, we used a data exclusion procedure similar to those of Dietz et al. (1999) and Bauchinger et al. (2011). We randomly excluded data for 6 of 30 birds (20 %) and calculated the regression equation using data from the remaining 24 birds. Following Bauchinger et al. (2011), we always included the most extreme values for muscle thickness in the data used to calculate the regression equations to ensure that the full range of variation was included in the regression models. We then used the regression equations to predict flight muscle mass for the excluded individuals. We compared predicted values with actual values, calculating absolute (|predicted mass − actual mass|) and relative [|(predicted mass/actual mass) − 1|] discrepancies. We repeated the exclusion procedure 1,000 times and averaged discrepancies over all replicates to produce an overall estimate of the predictive power of the regression equation. We conducted this validation procedure independently for short-axis, long-axis and combined short- and long-axis [(SA + LA)/2] measurements.
Results
Ultrasound calibration, validation and repeatability
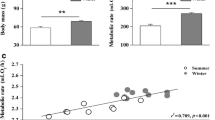
Least squares regression revealed significant positive correlations between wet breast muscle mass (combined left pectoralis and supracoracoideus masses, in grams) and ultrasound measurements of muscle thickness (mm) for short-axis, long-axis, and combined [(short-axis + long-axis)/2] measures (Fig. 2). The regression equations (n = 30 birds) were:
Repeatability, calculated as the intra-class correlation coefficient according to Lessels and Boag (1987), was 0.765 for short-axis measurements and 0.490 for long-axis measurements. Short-axis and long-axis measurements of muscle thickness were also significantly and positively correlated (r = 0.725, P < 0.001).
Ultrasound measurements of breast muscle thickness ranged from 4.2 to 6.7 mm for short-axis measurements and from 5.0 to 7.3 mm for long-axis measurements. Breast muscle mass (left side only) ranged from 1.92 to 3.21 g. Mean absolute discrepancies for the predictive regression equations for muscle mass calculated from the validation studies of ultrasound measurements (Fig. 2) were 0.13 ± 0.08 g for short-axis measurements, 0.14 ± 0.10 g for long-axis measurements, and 0.11 ± 0.08 g for combined short- and long-axis measurements. Mean relative discrepancies were 4.9 ± 3.1, 5.6 ± 4.4, and 4.0 ± 2.9 %, for short-axis, long-axis, and combined measurements, respectively.
Seasonal variation in mass and morphometrics
Body mass (M b ) was significantly greater (9.5 %, t 28 = 4.653, P < 0.001) in winter than in summer for House Sparrows in this study (Table 1). Mean wing chord did not differ significantly (t 28 = 1.265, P = 0.216) between summer (73.6 ± 2.2 mm, n = 13) and winter (74.7 ± 2.2 mm, n = 17) birds, but mean tarsus length was significantly greater (t 28 = 2.059, P = 0.049) in winter (22.4 ± 0.7 mm) than in summer (21.9 ± 0.7 mm), although the difference amounted to only 2.3 %. Mean pectoralis wet mass also increased significantly in winter (t 28 = 5.056, P < 0.001), with winter values exceeding summer values by 18.6 % (Table 1). Similarly, mean winter supracoracoideus wet mass significantly exceeded (U = 169.5, P = 0.013) summer values by 9.4 % (Table 1). Mean combined breast muscle wet mass (pectoralis + supracoracoideus) was also significantly greater in winter than in summer (17.5 %, t 28 = 4.984, P < 0.001; Fig. 3). The supracoracoideus/pectoralis ratio was 0.121 ± 0.007 (n = 17) in winter and 0.131 ± 0.009 (n = 13) in summer; these values differed significantly (8.3 %, t 28 = 3.349, P = 0.002).
Seasonal variation in mean ± SD breast muscle mass and ultrasonographic measures of muscle thickness in House Sparrows. Significant seasonal differences are noted by asterisks. Ultrasonographic measures of muscle thickness include SA short-axis measurements; LA long-axis measurements, and (SA + LA)/2 = the sum of short- and long-axis measures divided by 2
Ultrasound measurements of muscle thickness also showed significant seasonal differences for short-axis, long-axis, and combined measures. Mean short-axis muscle thickness was 9.1 % greater in winter than in summer (Fig. 3; t 28 = 3.063, P = 0.005). Mean long-axis muscle thickness was 7.5 % greater in winter than in summer (Fig. 3; t 28 = 2.766, P = 0.010). Finally, combined short- and long-axis measures of muscle thickness [(SA + LA)/2] also showed mean values significantly greater in winter than in summer (Fig. 3; U = 170.0, P = 0.013).
Discussion
Ultrasonographic measurements of flight muscle size in House Sparrows were significantly and positively correlated with flight muscle mass. The percentage of variation in ultrasonographic measures of flight muscle size explained by variation in flight muscle mass (from R 2 values from regressions) was 74.3 % for short-axis measurements and 65.6 % for long-axis measurements. These values are similar to those from Red Knots; 78.1–83.5 % for short-axis measurements and 69.6–70.1 % for long-axis measurements (Dietz et al. 1999). R 2 values for White-throated Dippers, Cinclus cinclus (0.927, n = 11) and Canaries (0.964, n = 7) were higher (Newton 1993), but these measurements used dead birds and an ultrasound index including six ultrasonic measurements of flight muscle mass along with keel length, rather than one or two ultrasound measurements, as in this study.
Repeatabilities of flight muscle thickness measurements in this study, 0.765 for short-axis and 0.49 for long-axis, were generally similar to those for flight muscle measurements in Red Knots (0.68–0.83 for short-axis and 0.51–0.52 for long-axis; Dietz et al. 1999), although Vézina et al. (2006) report a higher repeatability of 0.97 for short-axis measurement of muscle thickness in Red Knots. Carrascal et al. (1998) completed ultrasound measurements of muscle width on individual wild, free-living Great Tits captured multiple times in late autumn and early winter in Spain and calculated a repeatability of 0.66 for these birds, which is slightly lower than our value for repeatability during a single measurement period. Mean relative discrepancies of actual muscle masses from predicted values generated by the regression equation relating ultrasonographic measures of pectoralis muscle width to muscle mass in our study ranged from 4.0 (combined short- and long-axis measures) to 5.6 % (long-axis measurement). These values are smaller than discrepancies for birds from other ultrasonographic studies, which range from 18.9 to 23.7 % for short-axis measurements and from 22.4 to 24.9 % for long-axis measurements for Red Knots (approximately 120 g body mass), and from 8.6 % for long-axis measurements to 9.3 % for short-axis measurements for Eurasian Golden Plovers (Pluvialis apricaria, approximately 211 g body mass) (Dietz et al. 1999). The greater predictive ability in our study, despite the much smaller body size of House Sparrows compared to the shorebirds previously measured by Dietz et al. (1999), is likely due to the use of a higher frequency echo in our study (25 vs. 10 MHz). This suggests that higher frequencies provide greater resolution for ultrasound measurements of muscle size in small birds. The predictive ability of the regression equation for ultrasound measurements of muscle mass in our study also compares favorably with that of the recently developed muscle meter technique (Bauchinger et al. 2011), where mean discrepancies from predictive equations were 4.4 ± 3.0 % for Eurasian Starlings (Sturnus vulgaris, M b approximately 70 g) and 9.1 ± 6.5 % for Garden Warblers (Sylvia borin, M b approximately 20 g).
These data suggest that ultrasound is an effective method for detecting muscle size variation in response to changing ecological or energetic demands on small birds generally. Higher ultrasound frequencies provide higher resolution of visual details but shorter image depths (Dietz et al. 1999). We used a frequency of 25 MHz in this study, whereas previous ultrasound validation studies have used frequencies ranging from 7.5 to 10 MHz (Newton 1993; Dietz et al. 1999). The higher frequency in this study is beneficial for detecting visual details on muscles of smaller birds, yet the focal length was still 15 mm, which was sufficient for measuring muscle size in House Sparrows, where muscle thickness ranged from 4.2 to 6.7 mm for short-axis measurements and from 5.0 to 7.3 mm for long-axis measurements. For comparison, muscle thickness in Red Knots ranged from 7 to 17 mm for short-axis measurements (Dietz et al. 1999; Lindström et al. 2000), so the focal length in this study would likely be insufficient for muscle size measurement in the larger Red Knot. We suggest that the higher frequency is preferred for flight muscle measurements of small birds because the higher image resolution is beneficial for precise measurements of their smaller muscles.
We found that short-axis measurements provided more precise (higher R 2 for regressions against muscle mass and lower discrepancies) and more repeatable measures of flight muscle size than long-axis measurements and were more effective in detecting seasonal differences in muscle size (higher P values for seasonal comparisons than long-axis measurements). Compared to short-axis measurements, combining short- and long-axis measurements of flight muscle size into a single muscle size index produced a slightly higher R 2 for regressions against muscle mass and slightly lower discrepancies from predicted values than short-axis measurements alone, but comparable P values for seasonal comparisons. Because long-axis measurements were more difficult to standardize than short-axis measurements, usually took longer to conduct than short-axis measurements, and added little to the precision of measurements and to the overall ability to detect differences in muscle size, we suggest that short-axis measurements are sufficient for most studies using this method in small birds. This is similar to results from shorebirds, where short-axis measurements provided higher precision and repeatability than long-axis measurements (Dietz et al. 1999). Indeed, most of the recent studies using this method for muscle size measurement on shorebirds use only short-axis measures (e.g., Vézina et al. 2006, 2007, 2011).
While several studies have used ultrasonography to measure flight muscle size variation in response to cold acclimation, migration, reproduction, diet manipulation, or seasonal cycles (e.g., Ward 1996; Winkler and Allen 1996; Carrascal et al. 1998; Dietz et al. 1999; Lindström et al. 2000; Engstrand and Bryant 2002; Vézina et al. 2006, 2007, 2011), no studies have examined the effects of changing energy demands on muscle size by measuring relative changes in both flight muscle thickness (determined by ultrasonography) and mass. Thus, whether ultrasound measurements are sufficiently precise to detect such changes remains an open question. Dietz et al. (1999) documented that flight muscle thickness increased by 29 % in Red Knots after 3 months on a captive diet. Vézina et al. (2006) were unable to document significant effects of cold acclimation on flight muscle thickness in Red Knots, despite cold-induced increases in M sum and BMR of 13 and 17–26 %, respectively. However, pectoralis muscle thickness in Red Knots was positively associated with performance measures in the cold (i.e., increased thermogenic capacity and cold tolerance) (Vézina et al. 2006, 2007). Lindström et al. (2000) documented that flight muscle thickness in Red Knots varied by 15–18 % during flight, fasting, and refueling stages of migratory stopover. For comparison, pectoralis muscle lean dry mass of Red Knots was approximately 29 % higher during the peak spring migratory period (late May) than during the early part of fall migration (July) (Piersma et al. 1999). Similarly, muscle thickness varied by 7–15 % for captive Red Knots from different temperature treatments between May and July (Vézina et al. 2011), suggesting coincident changes in flight muscle mass and muscle thickness in Red Knots. None of these studies, however, documented relative changes in flight muscle size by both ultrasonography and dissection (i.e., muscle mass), so whether the magnitude of variation is similar between the two measurement methods is unknown. We found that flight muscle mass in House Sparrows increased by 17.5 % from summer to winter and flight muscle thickness (from the same individual birds) showed winter increases of 9.1 % for short-axis and 7.5 % for long-axis ultrasound measurements. Thus, ultrasound measurements of muscle thickness underestimated seasonal changes in muscle mass in House Sparrows. Whether such underestimation is typical for ultrasonographic measures of muscle size will require further study, but because ultrasound measurements of muscle thickness are linear measures of a three-dimensional structure, underestimation of variation in muscle mass from a linear measure might be expected. Nevertheless, winter increases in both flight muscle mass and flight muscle thickness were significant, so both methods effectively detect this magnitude of variation in muscle size on birds as small as House Sparrows.
Significant winter increases in pectoralis muscle mass have been previously documented for House Sparrows (8 % wet mass, 17 % dry mass; Liknes and Swanson 2011), so the 18.6 % winter increase in wet pectoralis muscle mass in this study confirms that such increases are a consistent component of the winter phenotype in House Sparrows from cold winter climates. Interestingly, supracoracoideus mass also increased significantly for winter House Sparrows in this study, but not significantly for sparrows in Liknes and Swanson (2011), although the percent increase in supracoracoideus mass in this study (9.4 %) was smaller than that for pectoralis and similar to the 5.4 % winter elevation in supracoracoideus mass in Liknes and Swanson (2011). This magnitude of seasonal variation in flight muscle mass closely approximates the winter increase in M sum documented for this population of House Sparrows (11 %, Swanson and Liknes 2006), although the winter elevation of M sum for the South Dakota population, at least for the years when we measured M sum, is lower than that for House Sparrows from Wisconsin (30 %; Arens and Cooper 2005) and Ontario (43 %; Hart 1962). Pectoralis and supracoracoideus muscle masses of South Dakota House Sparrows were similar between this study and Liknes and Swanson (2011), with all values from both summer and winter being within 10 % of each other (Table 1).
The supracoracoideus mass to pectoralis mass ratio (s/p ratio) decreased in winter House Sparrows in this study as a result of relatively greater winter increases in pectoralis than supracoracoideus mass. The marked seasonal phenotypic flexibility in the s/p ratio of Red Knots is likely principally a function of maneuverability and energetic requirements for flight throughout the annual cycle (Piersma and Dietz 2007). Such an explanation does not seem to apply to the summer to winter variation in the s/p ratio for House Sparrows documented in this study, as flight requirements during daily activities are not obviously different between the two seasons. However, the capacity for shivering heat production does vary seasonally in House Sparrows, increasing during the winter (Arens and Cooper 2005; Swanson and Liknes 2006), so it seems likely that seasonal differences in thermogenic capacity might be driving the seasonal variation in s/p ratios. Marsh and Dawson (1989) suggested that one potential limit for thermogenic capacity in birds is that isometric shivering is limited by power production of the smaller muscle of the antagonistic muscle pair; in the case of the flight muscles, the supracoracoideus. If seasonal variation in thermogenic capacity were limited by this mechanism, then supracoracoideus mass should be preferentially elevated in winter relative to pectoralis mass to support higher thermogenic capacity. This was not the case in this study, as the opposite pattern occurred. Thus, these data confirm the conclusion of Liknes and Swanson (2011) that disproportionate increases in supracoracoideus mass do not contribute to winter increases in thermogenic capacity in small birds, and suggest that morphological mechanisms locking the shoulder joint into position for shivering occur during shivering thermogenesis in birds, thereby allowing the winter hypertrophy of the pectoralis muscle to contribute fully to winter increases in shivering heat production.
The magnitude of seasonal variation in flight muscle size that we documented for House Sparrows in this study is within the range of that induced by seasonal acclimatization and migration in other small birds. Winter increases in pectoralis muscle mass relative to summer in other small birds range from 8 to 13 % in European Starlings (Vézina and Williams 2003, 2005) to 33 % in House Finches Carpodacus mexicanus (O’Connor 1995) and Mountain Chickadees Poecile gambeli (Cooper 2002) (Table 2). Increases in pectoralis muscle mass resulting from migratory hypertrophy in small birds cover a similar range, from 9 % in Sanderlings Calidris alba (Evans et al. 1992) to 35 % in Gray Catbirds Dumetella carolinensis (Marsh 1984; Table 2). Thus, hypertrophy of flight muscles, particularly the pectoralis, is a regular component of phenotypically flexible responses of small birds to changing energy demands (Swanson 2010). Given that we successfully detected seasonal changes of flight muscle masses of 17.5 % by ultrasonography in House Sparrows in this study, we expect that ultrasonography will be a useful method for non-invasive detection of cold- and migration-induced flight muscle hypertrophy in individuals of other small birds, given appropriate considerations of frequency and focal distance in ultrasound measurements.
References
Arens JR, Cooper SJ (2005) Metabolic and ventilatory acclimatization to cold stress in house sparrows (Passer domesticus). Physiol Biochem Zool 78:579–589
Baggott GK (1975) Moult, flight muscle “hypertrophy” and premigratory lipid deposition of the juvenile Willow warbler, Phylloscopus trochilus. J Zool 175:299–314
Bairlein F (1995) Manual of field methods ESF (http://www.ifv-vogelwarte.de/ESF/manual.pdf)
Bauchinger U, McWilliams SR, Kolb H, Popenko VM, Price ER, Biebach H (2011) Flight muscle shape reliably predicts flight muscle mass of migratory songbirds: a new tool for field ornithologists. J Ornithol 152:507–514
Carrascal LM, Senar JC, Mozetich I, Uribe F, Domènech J (1998) Interactions among environmental stress, body condition, nutritional status, and dominance in great tits. Auk 115:727–738
Constantini D, Cardinale M, Carere C (2007) Oxidative damage and anti-oxidant capacity in two migratory bird species at a stop-over site. Comp Biochem Physiol C 144:363–371
Cooper SJ (2002) Seasonal metabolic acclimatization in mountain chickadees and juniper titmice. Physiol Biochem Zool 75:386–395
Dawson WR, O’Connor TP (1996) Energetic features of avian thermoregulatory responses. In: Carey C (ed) Avian energetics and nutritional ecology. Chapman & Hall, New York, pp 85–124
Dietz MW, Dekinga A, Piersma T, Verhulst S (1999) Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiol Biochem Zool 72:28–37
Engstrand SM, Bryant DM (2002) A trade-off between clutch size and incubation efficiency in the barn swallow Hirundo rustica. Funct Ecol 16:782–791
Evans PR, Davidson NC, Uttley JD, Evans RD (1992) Premigratory hypertrophy of flight muscles: an ultrastructural study. Ornis Scand 23:238–243
Gosler AG, Harper DGC (2000) Assessing the heritability of body condition in birds: a challenge exemplified by the great tit, Parus major L. (Aves). Biol J Linn Soc 71:103–117
Guglielmo CG, Williams TD (2003) Phenotypic flexibility of body composition in relation to migratory state, age and sex in the western sandpiper. Physiol Biochem Zool 76:84–98
Hart JS (1962) Seasonal acclimatization in four species of small wild birds. Physiol Zool 35:224–236
Hohtola E (1982) Thermal and electromyographic correlates of shivering thermogenesis in the pigeon. Comp Biochem Physiol 73A:159–166
Köver G, Romvari R, Horn P, Berenyi E, Jensen JF, Sørensen P (1998) In vivo assessment of breast muscle, abdominal fat and total fat volume in meat-type chickens by magnetic resonance imaging. Acta Vet Hungar 46:135–144
Landys-Ciannelli MM, Piersma T, Jukema J (2003) Strategic size changes of internal organs and muscle tissue in the Bar-tailed Godwit during fat storage on a spring stopover site. Funct Ecol 17:151–159
Lessels CM, Boag PT (1987) Unreapeatable repeatabilities: a common mistake. Auk 104:116–121
Liknes ET, Swanson DL (2011) Phenotypic flexibility of body composition associated with seasonal acclimatization of passerine birds. J Therm Biol 36:363–370
Lindström Å, Kvist A, Piersma T, Dekinga A, Dietz M (2000) Avian pectoral muscle size rapidly tracks body mass changes during flight, fasting and fuelling. J Exp Biol 203:913–919
Marjoniemi K, Hohtola E (1999) Shivering thermogenesis in leg and breast muscles of Galliform chicks and nestlings of the domestic pigeon. Physiol Biochem Zool 72:484–492
Marsh RL (1984) Adaptations of the Gray Catbird Dumetella carolinensis to long-distance migration: flight muscle hypertrophy associated with elevated body mass. Physiol Zool 57:105–117
Marsh RL, Dawson WR (1989) Avian adjustments to cold. In: Wang LCH (ed) Advances in comparative and environmental physiology 4: animal adaptation to cold. Springer, New York, pp 205–253
McKechnie AE, Swanson DL (2010) Sources and significance of variation in basal, summit and maximal metabolic rates in birds. Curr Zool 56:741–758
Newton SF (1993) Body condition of a small passerine bird: ultrasonic assessment and significance in overwinter survival. J Zool Lond 229:561–580
O’Connor TP (1995) Metabolic characteristics and body composition in house finches: effects of seasonal acclimatization. J Comp Physiol B 165:298–305
Piersma T, Dietz MW (2007) Twofold seasonal variation in the supposedly constant, species-specific ratio of upstroke to downstroke flight muscles in red knots Calidris canutus. J Avian Biol 38:536–540
Piersma T, van Gils JA (2011) The flexible phenotype: a body-centred integration of ecology, physiology, and behavior. Oxford University Press, Oxford
Piersma T, Gudmundsson GA, Lilliendahl K (1999) Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Physiol Biochem Zool 72:405–415
Potti J, Moreno J, Merino S, Frias O, Rodriquez R (1999) Environmental and genetic variation in the haematocrit of fledgling pied flycatchers Ficedula hypoleuca. Oecologia 120:1–8
Pyle P (1997) Identification guide to North American birds. Slate Creek Press, Bolinas, California, USA, Part I. Columbidae to Ploceidae
Rae LR, Mitchell GW, Mauck RA, Guglielmo CG, Norris DR (2009) Radio transmitters do not affect the body condition of savannah sparrows during the fall premigratory period. J Field Ornithol 80:419–426
Senar JC, Conroy MJ, Carrascal LM, Domènech J, Mozetich I, Uribe F (1999) Identifying sources of heterogeneity in capture probabilities: an example using the Great Tit Parus major. Bird Study 46(suppl.):S248–S252
Senar JC, Domènech J, Uribe F (2002) Great Tits (Parus major) reduce body mass in response to wing area reduction: a field experiment. Behav Ecol 13:725-727
Swanson DL (1991) Substrate metabolism under cold stress in seasonally acclimatized dark-eyed juncos. Physiol Zool 64:1578–1592
Swanson DL (2001) Are summit metabolism and thermogenic endurance correlated in winter acclimatized passerine birds? J Comp Physiol B 171:475–481
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Bio. 209:466–474
Tallman DT, Swanson DL, Palmer JS (2002) Birds of South Dakota, 3rd edn. South Dakota Ornithologists’ Union, Aberdeen
Vézina F, Williams TD (2003) Plasticity in body composition in breeding birds: what drives the metabolic costs of egg production? Physiol Biochem Zool 76:716–773
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2007) Thermogenic side effects to migratory disposition in shorebirds. Am J Physiol Regul Integr Comp Physiol 292:1287–1297
Vézina F, Dekinga A, Piersma T (2011) Shorebirds’ seasonal adjustments in thermogenic capacity are reflected by changes in body mass: how preprogrammed and instantaneous acclimation work together. Integr Comp Biol 51:394–408
Ward S (1996) Energy expenditure of female barn swallows Hirundo rustica during egg formation. Physiol Zool 69:930–951
Winkler DW, Allen PE (1995) Effects of handicapping on female condition and reproduction in tree swallows (Tachycineta bicolor). Auk 112:737–747
Winkler DW, Allen PE (1996) The seasonal decline in tree swallow clutch size: physiological constraint or strategic adjustment? Ecology 77:922–932
Acknowledgments
We thank Amy Stephenson for help with conducting the ultrasound measurements. We also thank Greg Mitchell and an anonymous reviewer for their helpful comments on an earlier version of this manuscript. David Swanson was supported by NSF IOS 1021218.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Rights and permissions
About this article
Cite this article
Swanson, D.L., Merkord, C. Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements. J Ornithol 154, 119–127 (2013). https://doi.org/10.1007/s10336-012-0877-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0877-4