Abstract
Comparative analyses of avian energetics often involve the implicit assumption that basal metabolic rate (BMR) is a fixed, taxon-specific trait. However, in most species that have been investigated, BMR exhibits phenotypic flexibility and can be reversibly adjusted over short time scales. Many non-migrants adjust BMR seasonally, with the winter BMR usually higher than the summer BMR. The data that are currently available do not, however, support the idea that the magnitude and direction of these adjustments varies consistently with body mass. Long-distance migrants often exhibit large intra-annual changes in BMR, reflecting the physiological adjustments associated with different stages of their migratory cycles. Phenotypic flexibility in BMR also represents an important component of short-term thermal acclimation under laboratory conditions, with captive birds increasing BMR when acclimated to low air temperatures and vice versa. The emerging view of avian BMR is of a highly flexible physiological trait that is continually adjusted in response to environmental factors such as temperature. The within-individual variation observed in avian BMR demands a critical re-examination of approaches used for comparisons across taxa. Several key questions concerning the shapes and other properties of avian BMR reaction norms urgently need to be addressed, and hypotheses concerning metabolic adaptation should explicitly account for phenotypic flexibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenotypic flexibility, one category of phenotypic plasticity, involves reversible within-individual phenotypic adjustments (Piersma and Drent 2003). Birds exhibit phenotypic flexibility in numerous physiological traits, including digestive enzyme activities (Afik et al. 1995; Karasov 1996; Levey et al. 1999; Martinez del Rio et al. 1995), cutaneous evaporation rates (Haugen et al. 2003; Hoffman and Walsberg 1999; Marder and Arieli 1988; McKechnie and Wolf 2004b), and the upper and lower limits of metabolic power (Battley et al. 2001; McKechnie et al. 2007; Piersma et al. 1995; Swanson 1990; Swanson (2007); Tieleman et al. 2003). Flexibility in physiological phenotypes has far-reaching implications for ecological and evolutionary physiologists, since many of the traits that are compared among taxa are not fixed, but fluctuate through time within individuals. The inter- and/or intraspecific variation observed in avian physiological traits thus reflects a number of sources of phenotypic variation, including body mass, phylogenetic inertia and genotypic adaptation, as well as phenotypic flexibility associated with acclimation/acclimatization history.
Basal metabolic rate (BMR) is the minimum maintenance metabolism of a normothermic resting endotherm, measured at thermoneutral environmental temperatures and in the absence of thermoregulatory, digestive, circadian or other increments in metabolic heat production (Dawson and Whittow 2000; McNab 1997). Whereas measurements of field metabolic rate (FMR; total daily energy expenditure) allow researchers to quantify total energy requirements and elucidate patterns of energy allocation in free-ranging animals (e.g., Nagy 1987; Tieleman et al. 2004; Weathers and Sullivan 1993), BMR is a standardized, baseline parameter whose long history of empirical measurements and comparative analyses has made it an extremely useful trait for comparing metabolic power output among avian taxa (Bennett and Harvey 1987; Lasiewski and Dawson 1967; McKechnie et al. 2006; McNab 1988; Tieleman and Williams 2000; Weathers 1979; White et al. 2007). Recent analyses have shown that, once body mass (M b) effects are removed, residual interspecific variation in BMR is correlated with summit metabolism (M sum, the maximum resting rate of metabolic thermogenesis) in birds (Rezende et al. 2002) and with M sum and FMR in mammals (White and Seymour 2004), although Ricklefs et al. (1996) found no correlation between avian BMR and FMR. These M b-independent correlations between BMR and ecologically-relevant measures of energy expenditure, when taken together with a number of studies reporting significant repeatabilities for avian BMR (e.g., Bech et al. 1999; Rønning et al. 2005; Tieleman et al. 2003), emphasize the validity of using BMR as a comparative metabolic parameter.
An increasing number of studies reveal considerable phenotypic flexibility in avian BMR, a finding that calls for new approaches to comparative analyses, and a fundamental change in how physiologists view the ecological and evolutionary significance of intra- and interspecific BMR variation. In this paper, I review published data on phenotypic flexibility in avian BMR, and discuss the implications of within-individual variation for comparative analyses seeking to infer metabolic adaptation. I also identify key questions that need to be addressed before phenotypic flexibility in avian BMR can be conceptually integrated with other sources of metabolic variation.
Patterns of phenotypic flexibility in avian BMR
Within-individual adjustments in avian BMR have typically been examined in one of three contexts: seasonal acclimatization, the physiological adjustments associated with long-distance migration, or short-term thermal acclimation in artificial environments. I follow Piersma and Drent (2003) and numerous other authors in using the term “acclimation” to refer to responses to artificial environments and “acclimatization” to refer to responses to natural environments, although this distinction can be blurred, for instance when the aviaries that captive birds are housed in are enclosed during winter but not summer (e.g., Riddle et al. 1934).
Seasonal acclimatization
Phenotypic flexibility in BMR represents an important component of seasonal acclimatization for many Holarctic species (Table 1, also reviewed in Dawson 2003 and Swanson 2007). Seasonal metabolic adjustments have been investigated in approximately 20 species representing seven orders (Table 1). With few exceptions (Dawson et al. 1985; Pohl 1971; Rising and Hudson 1974), significant BMR adjustments occurred between seasons, ranging in magnitude from <10 to 64% for total BMR, and from <10 to 40% for mass-specific BMR (Table 1). The largest fractional seasonal adjustment in total BMR was recorded in house sparrows (Passer domesticus; Arens and Cooper 2005), whereas the largest mass-specific adjustment was observed in downy woodpeckers (Picoides pubescens; Liknes and Swanson 1996). Piersma et al. (1995) reported even larger adjustments (110% of total BMR and 55% of mass-specific BMR) in captive red knots (Calidris canutus islandica) that had been housed in outdoor aviaries for 2–3 years. However, it is likely that these large adjustments were at least partly associated with the birds’ annual migratory cycle, rather than solely representing seasonal acclimatization (Piersma et al. 1995).
Recent evidence suggests that the winter BMR of species resident in highly seasonal environments reflects the prevailing conditions immediately before metabolic measurements. In black-capped chickadees (Poecile atricapillus), dark-eyed juncos (Junco hyemalis) and American tree sparrows (Spizella arborea), BMR was most strongly correlated with short- (0–7 days preceding measurements) and medium-term (14–30 days preceding measurements) climatic variables (Swanson and Olmstead 1999). Similarly, the best multivariate model for variation in the winter BMR of great tits (Parus major) included average minimum air temperature (T a) during the week preceding measurements as a predictor variable (Broggi et al. 2007).
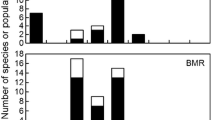
Most studies of seasonal metabolic adjustments have involved Holarctic species and, with few exceptions, have found that BMR is up-regulated during winter (Table 1, Fig. 1b). In contrast, Maddocks and Geiser (2000) found that mass-specific BMR in Australian silvereyes (Zosterops lateralis) was 25% higher in summer, directionally opposite to the typical pattern for similarly sized Holarctic species. This observation highlights the need for more data on seasonal BMR adjustments in species from tropical and subtropical latitudes.
Relationships between body mass and ratio of winter/summer metabolic rate (MR) in birds. The upper graph (a) shows the data analyzed by Weathers and Caccamise (1978), with data that meet the criteria for basal metabolic rate (BMR) shown as solid circles, and data that do not meet the criteria for BMR or for which conditions were not fully specified shown as triangles. Three data for which I was unable to obtain the original references are indicated with crosses. The lower graph (b) shows the corresponding relationship in species for which seasonal BMR data are currently available (Table 1 )
The direction and magnitude of seasonal BMR adjustments have been widely accepted as varying with M b (e.g., Hayworth and Weathers 1984; Swanson 2007; Swanson and Weinacht 1997) on the basis of an analysis presented for “fasting metabolic rate” by Weathers and Caccamise (1978). In the latter study, data on seasonal metabolic adjustments in 20 species revealed a negative relationship between M b and the ratio of winter to summer metabolic rate, with species smaller than 200 g generally increasing their metabolic rate during winter, but with the opposite pattern in larger species (Fig. 1a). Closer examination of the data used by these authors, however, reveals a number of potentially confounding factors. First, the Weathers and Caccamise (1978) analysis included metabolic data irrespective of whether or not they met the criteria for BMR (Table 2). Of the 22 data included, 7 met the criteria for BMR, whereas 9 did not (Table 2). The measurement conditions for a further three species were not specified in sufficient detail to determine whether BMR criteria were met (Table 2). Weathers and Caccamise’s (1978) own data for monk parakeets (Myiopsitta monachus) reveal the problems that can arise if BMR and non-BMR data are analyzed together. In M. monachus, metabolic rates that met the criteria for BMR were 28% higher in summer compared to winter, but measurements during the active phase (i.e., daytime) and included in the analysis discussed above were almost identical across seasons (Weathers and Caccamise 1978). There are also several inconsistencies between Table 3 and Fig. 5 of Weathers and Caccamise (1978), with some of the winter/summer ratios calculated from the metabolic rates provided in Table 3 not matching any of the ratios plotted in Fig. 5. The slightly different regression equation in Fig. 1a of the present paper compared with Fig. 5 of Weathers and Caccamise (1978) reflects these differences.
Assessment of the M b-dependence of the direction and magnitude of seasonal BMR adjustments is further complicated by variation among studies in terms of the origins of experimental populations and/or housing conditions prior to metabolic measurements. The available data comprise a combination of measurements from free-ranging birds, in which BMR was measured shortly (typically within 24 h) after capture (e.g., Arens and Cooper 2005; Cooper 2000; Liknes and Swanson 1996), birds that had spent periods of weeks to months in captivity (e.g., Maddocks and Geiser 2000; Rising and Hudson 1974; Sharbaugh 2001), and captive-raised birds that were acclimatized to natural patterns of temperature and photoperiod in outdoor enclosures (Swanson and Weinacht 1997). Moreover, the data set analyzed by Weathers and Caccamise (1978) included BMR measurements for individuals that were acclimatized to natural temperature and photoperiod conditions (e.g., Dawson and Carey 1976), as well as individuals that were acclimated to artificial conditions indoors (e.g., Riddle et al. 1934; Wallgren 1954). Avian BMR scales differently in captive-raised and wild-caught birds (McKechnie et al. 2006), and reductions in pectoral muscle oxidative capacity (Saarela and Hohtola 2003) and BMR (McKechnie et al. 2007) have been linked to reduced activity levels in small cages. Collectively, these observations call into question whether seasonal metabolic adjustments are directly comparable among populations that differ in acclimation/acclimatization history and/or housing conditions. Although seasonal metabolic adjustments have been measured shortly after capture in wild populations of a number of species (Table 1), the range of M b is too small to rigorously examine the M b-dependence of seasonal adjustments. A pooled data set combining seasonal BMR adjustments from wild populations, populations maintained in captivity for weeks to months prior to measurements, and captive-raised populations does not reveal any significant relationship with M b (Table 1, Fig. 1b), although too few data for species with M b> 30 g are currently available to draw any conclusions regarding the M b-dependence of seasonal BMR adjustments.
Migratory cycles
Long-distance avian migration involves cyclical physiological and morphological adjustments, primarily driven by changes in body composition (Battley et al. 2000; Dietz et al. 1999; Karasov and Pinshow 1998; Piersma and Lindström 1997). Relatively few studies have directly compared BMR across different stages of long-distance migration cycles, but those that have done so have revealed substantial phenotypic flexibility. In yellow-rumped warblers migrating northwards during spring, total BMR was 31% higher compared to during the southward autumn migration (Swanson and Dean 1999). Great knots migrating from northwestern Australia to southern China exhibited reductions of 42–46% in total BMR and 33–40% in mass-specific BMR during their migratory flight (Battley et al. 2001, 2000).
A more common approach to investigating metabolic adjustments in long-distance migrants is to compare metabolic rates among studies conducted in different regions (Kersten et al. 1998; Kvist and Lindström 2001; Lindström 1997; Lindström and Klaassen 2003). Although this approach suffers from the limitation of methodological variation across studies (Kvist and Lindström 2001), these studies have been instrumental in revealing metabolic adjustments in long-distance migrants. The pattern that has emerged for a number of species that migrate between Arctic breeding grounds and winter quarters in tropical Africa is that BMR is highest in the Arctic and lowest on the African winter range (Kvist and Lindström 2001; Lindström and Klaassen 2003). In a study of 19 wader species, Kvist and Lindström (2001) found an average reduction in BMR of 16% between the Arctic and southern Sweden, and a further but non-significant average reduction of 8% between southern Sweden and Africa.
The most rapid changes in avian BMR yet documented have been reported in migrants. In a single thrush nightingale (Luscinia luscinia) that underwent a simulated 6,300 km migration in a wind tunnel, total BMR increased on average by 22.7% during each 60 h refueling period between 12 h flights, concomitant with an average increase in M b of 24.3% (Lindström et al. 1999). Garden warblers (Sylvia borin) that were starved in order to simulate a migratory flight exhibited a decrease in mass-specific BMR of approximately 20% over a 2–6 day period (Klaassen and Biebach 1994).
Short-term thermal acclimation
In a number of species, BMR has been found to vary over time scales of days to weeks within captive individuals in response to changes in thermal environments (Table 1). Gelineo’s (1964) observation that birds typically up-regulate BMR when acclimated to cold conditions has been supported by several more recent studies (Klaassen et al. 2004; McKechnie et al. 2007; Tieleman et al. 2003; Vezina et al. 2006; Williams and Tieleman 2000). The magnitude of BMR adjustments associated with short-term thermal acclimation varies among species and may involve increases in BMR of up to 42% (Table 1). Even among closely related species, metabolic responses to acclimation differ. In four species of larks, the BMR of individuals acclimated to T a = 15°C ranged from 5 to 29% higher than that of individuals acclimated to T a = 35°C (Tieleman et al. 2003). When variation in acclimation T a among recent studies is taken into account, the magnitude of BMR adjustments ranges from 0.2 to 1.5% per 1°C change in acclimation T a (McKechnie et al. 2007). The latter values were calculated using the full range of acclimation T as used in each study, although metabolic upregulation may a priori be expected only at acclimation Tas below the thermoneutral zone (TNZ). However, in laughing doves with a lower critical limit of thermoneutrality (Tlc) of ca. 30°C, BMR upregulation between acclimation T a = 35°C and 22°C was quantitively similar to upregulation between T a = 22°C and 10°C, with the result that BMR was linearly related to acclimation T a (McKechnie et al. 2007). If BMR upregulation had occurred only in response to acclimation T a < T lc, then the relationship between BMR and acclimation T a would presumably have been non-linear over the three acclimation T as used (10, 22 and 35°C).
The majority of studies of BMR adjustments during short-term thermal acclimation have involved comparisons of BMR among groups of experimental birds, each acclimated to a single T a. In a study that examined variation within individuals, rather than among experimental groups, laughing doves (Streptopelia senegalensis) exhibited BMR adjustments that were reversible in direction, with individuals that initially upregulated BMR during acclimation to cold temperatures subsequently downregulating BMR when moved into a warm room and vice versa (McKechnie et al. 2007).
Mechanisms of within-individual BMR adjustments
In a review of the mechanisms driving seasonal metabolic adjustments in birds, Swanson (2007) has identified three major physiological and morphological pathways whereby metabolic rates are up- or downregulated, namely adjustments in transport capacities for oxygen and metabolic substrates, adjustments in the mass-independent metabolic intensities of specific organs, and/or adjustments in organ masses. Whereas adjustments in transport capacities are important for changes in summit metabolism (M sum) and maximal metabolic rate (MMR), BMR represents minimum maintenance energy turnover and predominantly reflects the mass and metabolic intensities of the major organs (Swanson 2007). Intra-specific variation in BMR has been correlated with the mass of organs such as the heart, intestine, liver, spleen and kidneys (Chappell et al. 1999; Daan et al. 1990; Hammond et al. 2000; for review see Table 1 of Piersma 2002). However, Vézina and Williams (2005) have pointed out that relationships between organ masses and BMR are often inconsistent among studies, probably reflecting changes in mass-specific metabolic intensity within and among organs. In European starlings (Sturnus vulgaris), the mass-specific metabolic intensity of organs (measured as citrate synthase activity) varied through time, but not in a consistent fashion among organs (Vézina and Williams 2005). Further evidence for the importance of adjustments in mass-specific metabolic intensity comes from the observation that captive red knots exhibited increases in lean mass, yet a decrease in BMR when switched to a less digestible diet (Piersma et al. 2004).
Short-term adjustments of BMR during thermal acclimation appear to be driven primarily by changes in organ mass. The energy demand hypothesis proposes that the masses of organs involved in supplying energy for thermogenesis are matched to changing food requirements (Williams and Tieleman 2000). According to this idea, increased food intake at low T a leads to increases in the masses of organs, which in turn results in an elevation in BMR (Tieleman et al. 2003; Williams and Tieleman 2000). Several studies have reported increases in food intake and the masses of organs such as the liver, kidney and intestines in birds acclimated to cold conditions (Tieleman et al. 2003; Williams and Tieleman 2000). However, increased BMR in birds acclimated/acclimatized to cold conditions may also reflect the upregulation of tissues responsible for heat generation, such as the pectoral muscles, with a concomitant increase in maintenance costs (Piersma 2002). Recently, Vezina et al. (2006) used ultrasonography to examine body composition correlates of metabolic adjustments during short-term thermal acclimation in red knots (Calidris canutus islandica), and found that, in general, within-individual changes in BMR were related to neither gizzard size nor pectoral muscle thickness. Instead, these authors argued that the elevated BMR of cold-acclimated birds reflected increases in the masses of organs such as the liver, as has been observed during acclimation in a number of species of larks (Tieleman et al. 2003; Williams and Tieleman 2000).
Implications of phenotypic flexibility in avian BMR for comparative analyses
Metabolic scaling
Plastic allometry has been documented in morphological traits (Schlichting and Pigliucci 1998), and recent work has revealed similar plasticity in avian metabolic scaling. The allometric exponent for avian BMR depends on the origin of populations used for metabolic measurements, with captive-raised birds exhibiting a significantly shallower slope (0.670) than wild-caught birds (0.744; McKechnie et al. 2006). Whereas the environmental factors and physiological mechanisms that contribute to these differences remain unclear, it is likely that both phenotypic flexibility and developmental plasticity (sensu Piersma and Drent 2003) are involved (McKechnie et al. 2006). Plasticity in metabolic scaling is not limited to birds; there is increasing evidence that scaling coefficients among arthropods are significantly affected by metabolic responses to acclimation (Chown and Terblanche 2007; Terblanche et al. 2007). Collectively, these findings significantly complicate the process of controlling for the effects of M b in comparative analyses. In the case of avian BMR, the origins of study populations need to be taken into account, and pooling data for wild-caught and captive-raised individuals will potentially confound analyses of metabolic variation correlated with environmental factors and/or organismal traits.
Identifying metabolic adaptation
The realization that avian BMR exhibits considerable phenotypic flexibility greatly complicates the process of identifying metabolic adaptation. Traditional approaches to comparing BMR among groups of species, or comparing the BMR of a species of interest to allometrically expected values, implicitly assume that BMR is a fixed, taxon-specific trait. The latter limitation also applies to analyses that involve methods for detecting phylogenetic signals and controlling for phylogenetic non-independence of data (Blomberg et al. 2003; Freckleton et al. 2002; Garland et al. 1993, 1992; Garland and Ives 2000; Martins and Hansen 1997; Pagel 1994, 1999), although potential bias associated with within-species variation and/or traits that exhibit low repeatabilities is increasingly being recognized (Adolph and Hardin 2007; Ives et al. 2007). In the case of avian BMR, the trait values observed in experimental populations are dependent on the acclimation/acclimatization states of the individuals involved, and the conclusions reached in comparisons of observed vs. predicted BMR values similarly depend on acclimation/acclimatization history (Fig. 2).
The maximum and minimum BMR observed during acclimation in laughing doves (Streptopelia senegalensis; pair of circles; data from McKechnie et al. 2007) and hoopoe larks (Alaemon alaudipes; pair of squares; data from Williams and Tieleman 2000), and during seasonal acclimatization in house sparrows (Passer domesticus; pair of diamonds; data from Arens and Cooper 2005). These species were chosen as they provide instructive examples over a threefold range of body mass. Data for wild-caught populations of 137 species (McKechnie et al. 2006) are shown as triangles. The solid line is a conventional least-squares linear regression fitted to the latter data, and the dashed lines are phylogenetically independent 95% prediction intervals for the BMR of P. domesticus, calculated following Garland and Ives (2000). Note that the lower (summer) value for P. domesticus falls within the prediction intervals, whereas the upper (winter) value lies above the upper prediction interval
There is considerable overlap in magnitude between adjustments of BMR within species/individuals (Table 1) and variation reported among groups of species that differ in habitat or other traits. For instance, the BMR of desert birds is on average 17% lower than that of non-desert birds (Tieleman and Williams 2000), and BMR in tropical species is 10–18% lower than in temperate species (Wiersma et al. 2007). However, BMR may vary within individuals by >30% in response to acclimation or acclimatization (Table 1). The latter observation reiterates the importance of taking phenotypic flexibility into account as a source of variation in comparative analyses of BMR among groups of taxa that differ in habitat, diet or other traits. Currently, metabolic adjustments in response to acclimation or acclimatization have been examined in too few species to directly assess the relative contributions of phenotypic flexibility and genotypic adaptation to observed relationships between avian BMR and variables such as aridity (Tieleman and Williams 2000), diet (McNab 1988) and temperature (White et al. 2007).
The changing view of avian metabolic diversity
Birds, like other organisms, show considerable variation in physiological traits, and analyses of BMR have been instrumental in elucidating patterns of avian metabolic diversity. Traditionally, comparative analyses of avian BMR have focused on several key sources of variation, namely allometric scaling (Brody and Proctor 1932; Lasiewski and Dawson 1967; McKechnie et al. 2006), metabolic variation among higher-order taxa (Bennett and Harvey 1987; Lasiewski and Dawson 1967; Reynolds and Lee 1996), and/or Mb-independent variation correlated with climate (Ellis 1984; Tieleman and Williams 2000; Weathers 1979; White et al. 2007) and factors such as foraging mode and diet (Bennett and Harvey 1987; McNab 1988, 2001, 2003). In the vast majority of cases, these analyses have involved a single BMR value per species, which has usually been implicitly assumed to represent a fixed, species-specific value. In contrast, the emerging view of avian BMR is of a trait continually adjusted in response to fluctuating environments (Broggi et al. 2007; Swanson and Olmstead 1999). The realization that avian BMR exhibits considerable phenotypic flexibility and varies within individual birds through time calls for new approaches to comparing metabolic rates among taxa. Below, I outline several key questions that need to be addressed.
What are the shapes of avian BMR reaction norms?
Several properties of a reaction norm [i.e., the range of phenotypic trait values that a single genotype can produce (Schlichting and Pigliucci 1998)] apply to patterns of phenotypic flexibility in avian BMR, including the magnitude, shape and rapidity of responses. Whereas numerous studies have reported the magnitude of BMR adjustments, information on the shape of BMR reaction norms is virtually non-existent, because most studies involved acclimation to one of only two T as (Gelineo 1969; Klaassen et al. 2004; Tieleman et al. 2003; Williams and Tieleman 2000). In a study where laughing doves (Streptopelia senegalensis) were acclimated to one of three T as (10, 22, or 35°C), BMR was linearly related to acclimation T a (McKechnie et al. 2007), despite the fact that one of the acclimation T as used was ca. 5°C above the T lc for this species (see discussion above). These data for S. senegalensis suggest that avian BMR reaction norms may be approximately linear in shape. However, up- or downregulation of BMR is presumably limited by physiological and morphological constraints, and we might thus expect that avian BMR reaction norms represent linear relationships between upper and lower bounds (Fig. 3a).
A hypothetical reaction norm (a) for avian BMR relative to air temperature (T a), where BMR is linearly related to the T a between upper and lower bounds. b–f are graphical representations of five non-mutually exclusive hypotheses concerning differences that might exist between desert (dashed line) and non-desert (solid line) species. The dashed lines in (b)–(f) represent two arbitrary acclimation T as, and illustrate how the predicted differences in BMR between desert and non-desert species vary between scenarios. In scenario (b), BMR is more flexible in desert species in the sense that it can be adjusted over a wider range of T a. In scenario (c), the slope of the reaction norm is steeper in desert species, and a larger difference in BMR is predicted for a given combination of acclimation T a values. In (d)–(f), the slopes of the reaction norms are the same, but the reaction norm of desert species is shifted downwards compared to non-desert species (d), and shifted downwards as well as towards higher T a values (e). In (f), the shape of the reaction norm is identical for desert and non-desert species, but desert species adjust BMR more rapidly
The rapidity of avian BMR adjustments (i.e., the rate at which BMR is up- or downregulated) also remains largely unknown. In the majority of studies involving short-term thermal acclimation, birds were acclimated for approximately 3 weeks. In two species of larks, BMR measured 2 weeks after the start of thermal acclimation did not differ from BMR measured after 3 weeks, revealing that BMR adjustments took place in less than 2 weeks (Tieleman et al. 2003). In laughing doves in which BMR was measured every few days following a change in thermal environment, BMR was adjusted by 0.5–0.7% per day (Chetty 2006).
How do the properties of BMR reaction norms differ among avian taxa?
The first attempt to frame a hypothesis of avian metabolic adaptation in terms of the properties of BMR reaction norms was recently made by Tieleman et al. (2003), who used four species of larks to test the idea that species inhabiting environments of high temporal heterogeneity (deserts) have evolved greater phenotypic flexibility than species in more predictable (non-desert) habitats. In this study, larks were acclimated to one of two acclimation T as (15 or 35°C) and the magnitude of BMR adjustments compared across species, but no correlation was found with habitat aridity. The “greater flexibility in desert species” hypothesis put forward by Tieleman et al. (2003) provides a useful example with which to explore the approach of generating metabolic adaptation hypotheses in a reaction norm framework. The shapes of reaction norms for avian BMR could potentially differ between desert and non-desert species in a number of ways (Fig. 3b–e) similar to the various patterns of physiological acclimation documented in ectotherms (Cossins and Bowler 1987; Precht 1973; Prosser 1973). Alternately, the shapes of BMR reaction norms might be similar, but the rapidity of responses may differ (Fig. 3f). Tieleman et al. (2003) implicitly tested the prediction that, for a given change in acclimation Ta, desert species exhibit a larger fractional adjustment in BMR, i.e., the slope of the BMR vs.. T a reaction norm is steeper in desert birds (Fig. 3c). However, patterns of phenotypic flexibility could potentially differ in a number of other ways that were not considered in the latter study (Fig. 3).
Does the magnitude of BMR adjustments scale with body mass?
Studies of BMR adjustments during acclimatization and/or acclimation have been conducted under a wide range of conditions, often precluding direct comparisons of the magnitude of BMR adjustments among taxa. Before hypotheses concerning the adaptive value of inter- and/or intraspecific variation in the properties of BMR reaction norms can be tested, particularly in the context of mechanistic links between BMR and more ecologically relevant measures of energy expenditure, other sources of variation need to be accounted for. A simple model for the effect of BMR upregulation on the lower critical limit of thermoneutrality (T lc) reveals that the fractional BMR increment required for a given shift in T lc decreases with increasing M b (Fig. 4). Thus, I predict that for a given set of T as in experiments involving short-term thermal acclimation, fractional increments in BMR will be larger in smaller species. A quantitatively similar prediction can be made for shifts in the temperature at cold limit [T CL, i.e., the lowest T a at which a normothermic T b can be defended (Saarela et al. 1988)] through adjustments in M sum.
Downward shifts in the lower critical limit of thermoneutrality (T lc) require larger fractional increments in basal metabolic rate (BMR) in smaller species. The increments in BMR (%) required to decrease the T lc by 2, 5 or 10°C were calculated using the equation: BMR = C min (T b−T lc), where BMR is in J g−1 h−1, C min is minimum wet thermal conductance [J (g h°C)−1] and T b is body temperature (°C). Initial T lc was calculated using BMR values predicted by McKechnie and Wolf (2004a); values of C min were predicted by Schleucher and Withers (2001), and T b = 38.5°C
Does the rapidity of BMR adjustments scale with body mass?
Processes related to rates of change in organ mass and/or the properties of tissues that determine metabolic intensity may a priori be expected to be M b-dependent. Carleton and Martinez del Rio (2005) tested the hypothesis that isotopic incorporation rates are related to metabolic rate, and found that the fractional rates of 13C incorporation into avian blood scale to approximately M b −0.25. Since the rate at which carbon turns over in an organism’s blood is proportional to mass-specific metabolic rate, it is reasonable to expect that rates of change in organ mass and/or cellular properties also vary with M b. Hence, I predict that the rate of change in BMR during acclimation scales allometrically with a similar exponent to mass-specific metabolic rate.
Conclusions
Comparative analyses of the metabolic rates of birds date back to at least the 1920s (Benedict and Fox 1927; Giaja and Males 1928; Terroine and Trautmann 1927), and nearly a century later comparisons of BMR across taxa remain a fundamental aspect of avian ecological and evolutionary physiology (McKechnie et al. 2006; White et al. 2007; Wiersma et al. 2007; Wikelski et al. 2003). There is increasing evidence, however, that phenotypic flexibility in BMR is a general feature of avian physiological phenotypes. The realization that avian BMR is a flexible trait necessitates a critical re-examination of comparative methods. Most importantly, the hypotheses tested using comparative data need to explicitly take phenotypic flexibility into account (e.g., Tieleman et al. 2003).
Finally, ecological and evolutionary physiologists need to move away from the approach of measuring BMR just once per species (e.g., McKechnie and Lovegrove 2001, 2003; McNab 2001, 2005), since the BMR observed during once-off measurements depends on a number of factors related to acclimation/acclimatization and is thus usually not directly comparable across taxa. However, multiple measurements across seasons or in response to different acclimation T as will often be difficult or even impossible in the case of species that are rare and/or occur in inaccessible areas, and there are a number of ways in which workers can improve the comparability of once-off BMR measurements. In addition to restricting analyses to data from either wild-caught or captive-raised populations (McKechnie et al. 2006; White et al. 2007), one approach is to standardize the time of year during which measurements are made. The latter approach was recently used by Wiersma et al. (2007) who included only BMR data measured in summer for their comparisons of tropical and temperate species.
Abbreviations
- BMR:
-
Basal metabolic rate
- T a :
-
Air temperature
- M b :
-
Body mass
- M sum :
-
Summit metabolism
- FMR:
-
Field metabolic rate
- MMR:
-
Maximal metabolic rate
- T CL :
-
Temperature at cold limit
References
Adolph SC, Hardin JS (2007) Estimating phenotypic correlations: correcting for bias due to intraindividual variability. Funct Ecol 21:178–184
Afik D, Caviedes-Vidal E, Martinez del Rio C, Karasov WH (1995) Dietary modulation of intestinal hydrolytic enzymes in yellow-rumped warblers. Am J Physiol 269:R413–R420
Arens JR, Cooper SJ (2005) Metabolic and ventilatory acclimatization to cold stress in house sparrows (Passer domesticus). Physiol Biochem Zool 78:579–589
Battley PF, Dekinga A, Dietz MW, Piersma T, Tang S, Hulsman K (2001) Basal metabolic rate declines during long-distance migratory flight in great knots. Condor 103:838–845
Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K (2000) Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc R Soc Lond B 267:191–195
Bech C, Langseth I, Gabrielsen GW (1999) Repeatability of basal metabolism in breeding female kittiwakes Rissa tridactyla. Proc R Soc Lond B 266:2161–2167
Benedict FG, Fox EL (1927) The gaseous metabolism of large birds under aviary life. Proc Am Philos Soc 66:511–534
Bennett PM, Harvey PH (1987) Active and resting metabolism in birds: allometry, phylogeny and ecology. J Zool Lond 213:327–363
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Brody S, Proctor RC (1932) Growth and development, with special reference to domestic animals. XXIII. Relation between basal metabolism and mature body weight in different species of mammals and birds. Mo Univ Agric Exp Stn Res Bull 166:89–101
Broggi J, Hohtola E, Koivula K, Orell M, Thomson RL, Nilsson J-A (2007) Sources of variation in winter basal metabolic rate in the great tit. Funct Ecol 21:528–533
Carleton SA, Martinez del Rio C (2005) The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia 144:226–232
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Chetty K (2006) Phenotypic flexibility in the basal metabolic rate of laughing doves (Streptopelia senegalensis) in response to short-term thermal acclimation. MSc. thesis, University of the Witwatersrand, Johannesburg
Chown SL, Terblanche JS (2007) Physiological diversity in insects: ecological and evolutionary contexts. Adv Insect Physiol 33:50–152
Cooper SJ (2000) Seasonal energetics of mountain chickadees and juniper titmice. Condor 102:635–644
Cooper SJ, Swanson DL (1994) Seasonal acclimatization of thermoregulation in the black-capped chickadee. Condor 96:638–646
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, London
Daan S, Masman D, Groenewold A (1990) Avian basal metabolic rates: their association with body compostition and energy expenditure in nature. Am J Physiol 259:R333–R340
Davydov AF (1972) Seasonal variations in the energy metabolism and thermoregulation at rest in the black-headed gull. Sov J Ecol 2:436–439
Dawson WR (2003) Plasticity in avian responses to thermal challenges— an essay in honor of Jacob Marder. Isr J Zool 49:95–109
Dawson WR, Buttemer WA, Carey C (1985) A reexamination of the metabolic response of house finches to temperature. Condor 87:424–427
Dawson WR, Carey C (1976) Seasonal acclimation to temperature in Cardueline finches. J Comp Physiol 112:317–333
Dawson WR, Whittow GC (2000) Regulation of body temperature. In: Sturkie PD (ed) Avian physiology. Academic, New York, pp 343–390
Dietz MW, Piersma T, Dekinga A (1999) Body-building without power training: endogenously regulated pectoral muscle hypertrophy in confined shorebirds. J Exp Biol 202:2831–2837
Ellis HI (1984) Energetics of free-ranging seabirds. In: Whittow GC, Rahn H (eds) Seabird energetics. Plenum, New York
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726
Garland T, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer simulation. System Biol 42:265–292
Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. System Biol 41:18–32
Garland T, Ives AR (2000) Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat 155:346–364
Gelineo S (1964) Organ systems in adaptation: the temperature regulating system. In: Dill DB (ed) Handbook of physiology, Section 4, Adaptation to the environment. American Physiological Society, Washington DC
Gelineo S (1969) Heat production in birds in summer and winter. Srpska Akad. Nauka I Umetnosti Belgrad, Bull Classe Sci Math Sci Nat 12:99–105
Giaja J, Males B (1928) Sur la valeur du métabolisme de base de quelques animaux en fonction de leur surface. Annales de Physiologie et de Physicochimie Biologique 4:875–904
Hammond KA, Chappell MA, Cardullo RA, Lin R-I, Johnsen TS (2000) The mechanistic basis of aerobic performance variation in red junglefowl. J Exp Biol 203:2053–2064
Hart JS (1962) Seasonal acclimatization in four species of small wild birds. Physiol Zool 35:224–236
Haugen MJ, Tieleman BI, Williams JB (2003) Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J Exp Biol 206:3581–3588
Hayworth AM, Weathers WW (1984) Temperature regulation and climatic adaptation in black-billed and yellow-billed magpies. Condor 86:19–26
Hissa R, Palonkangas R (1970) Thermoregulation in the titmouse (Parus major L.). Comp Biochem Physiol 33:941–953
Hoffman TCM, Walsberg GE (1999) Inhibiting ventilatory evaporation produces an adaptive increase in cutaneous evaporation in mourning doves Zenaida macroura. J Exp Biol 202:3021–3028
Irving L, Krog H, Monson M (1955) The metabolism of some Alaskan animals in winter and summer. Physiol Zool 28:173–185
Ives AR, Midford PE, Garland T (2007) Within-species variation and measurement error in phylogenetic comparative methods. System Biol 56:252–270
Karasov WH (1996) Digestive plasticity in avian energetics and feeding ecology. In: Carey C (ed) Avian energetics and nutritional ecology. Chapman and Hall, New York, pp 61–84
Karasov WH, Pinshow B (1998) Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a spring stopover site. Physiol Biochem Zool 71:435–448
Kersten M, Bruinzeel LW, Wiersma P, Piersma T (1998) Reduced basal metabolic rate of migratory waders wintering in coastal Africa. Ardea 86:71–80
Klaassen M, Biebach H (1994) Energetics of fattening and starvation in the long-distance migratory garden warbler, Sylvia borin, during the migratory phase. J Comp Physiol B 164:362–371
Klaassen M, Oltrogge M, Trost L (2004) Basal metabolic rate, food intake, and body mass in cold- and warm-acclimated garden warblers. Comp Biochem Physiol A 137:639–647
Kvist A, Lindström Å (2001) Basal metabolic rate in migratory waders: intra-individual, intraspecific, interspecific and seasonal variation. Funct Ecol 15:465–473
Lasiewski RC, Dawson WR (1967) A re-examination of the relation between standard metabolic rate and body weight in birds. Condor 69:13–23
Levey DJ, Place AR, Rey PJ, Martinez del Rio C (1999) An experimental test of dietary enzyme modulation in pine warblers Dendroica pinus . Physiol Biochem Zool 72:576–587
Liknes ET, Scott SM, Swanson DL (2002) Seasonal acclimatization in the American goldfinch revisited: to what extent do metabolic rates vary seasonally? Condor 104:548–557
Liknes ET, Swanson DL (1996) Seasonal variation in cold tolerance, basal metabolic rate, and maximal capacity for thermogenesis in white-breasted nuthatches Sitta carolinensis and downy woodpeckers Picoides pubescens, two unrelated arboreal temperate residents. J Avian Biol 27:279–288
Lindström Å (1997) Basal metabolic rates of migrating waders in the Eurasian Arctic. J Avian Biol 28:87–92
Lindström Å, Klaassen M (2003) High basal metabolic rates of shorebirds while in the Arctic: a circumpolar view. Condor 105:420–427
Lindström Å, Klaassen M, Kvist A (1999) Variation in energy intake and basal metabolic rate of a bird migrating in a wind tunnel. Funct Ecol 13:352–359
Maddocks TA, Geiser F (2000) Seasonal variations in thermal energetics of Australian silvereyes (Zosterops lateralis). J Zool Lond 252:327–333
Marder J, Arieli U (1988) Heat balance of acclimated pigeons Columba livia exposed to temperatures of up to 60°C T a. Comp Biochem Physiol 91A:165–170
Martinez del Rio C, Brugger KW, Rios JL, Vergara ME, Witmer MC (1995) An experimental and comparative study of dietary modulation of intestinal enzymes in the European starling. Physiol Zool 68:490–511
Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149:646–667
McKechnie AE, Chetty K, Lovegrove BG (2007) Phenotypic flexibility in basal metabolic rate in laughing doves: responses to short-term thermal acclimation. J Exp Biol 210:97–106
McKechnie AE, Freckleton RP, Jetz W (2006) Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc R Soc Lond B 273:931–937
McKechnie AE, Lovegrove BG (2001) Thermoregulation and the energetic significance of clustering behavior in the white-backed mousebird (Colius colius). Physiol Biochem Zool 74:238–249
McKechnie AE, Lovegrove BG (2003) Facultative hypothermic responses in an Afrotropical arid-zone passerine, the red-headed finch (Amadina erythrocephala). J Comp Physiol B 173:339–346
McKechnie AE, Wolf BO (2004a) The allometry of avian basal metabolic rate: good predictions need good data. Physiol Biochem Zool 77:502–521
McKechnie AE, Wolf BO (2004b) Partitioning of evaporative water loss in white-winged doves: plasticity in response to short-term thermal acclimation. J Exp Biol 207:203–210
McNab BK (1988) Food habits and the basal rate of metabolism in birds. Oecologia 77:343–349
McNab BK (1997) On the utility of uniformity in the definition of basal rates of metabolism. Physiol Zool 70:718–720
McNab BK (2001) Energetics of toucans, barbets and a hornbill: implications for avian frugivory. Auk 118:916–933
McNab BK (2003) Ecology shapes bird bioenergetics. Nature 426:620–621
McNab BK (2005) Food habits and the evolution of energetics in birds of paradise (Paradisaeidae). J Compar Physiol B 175:117–132
Merola-Zwartjes M, Ligon JD (2000) Ecological energetics of the Puerto Rican tody: heterothermy, torpor and intra-island variation. Ecology 81:990–1002
Nagy KA (1987) Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr 57:111–128
O’Conner TP (1995) Metabolic characteristics and body composition in house finches: effects of seasonal acclimatization. J Compar Physiol B 165:298–305
Pagel M (1994) Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc R Soc Lond B 255:37–45
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Piersma T (2002) Energetic bottlenecks and other design constraints in avian annual cycles. Integr Compar Biol 42:51–67
Piersma T, Cadée N, Daan S (1995) Seasonality in basal metabolic rate and thermal conductance in a long distance migrant shorebird, the knot (Calidris canutus). J Compar Physiol 165:37–45
Piersma T, Drent J (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18:228–233
Piersma T, Gessaman JA, Dekinga A, Visser GH (2004) Gizzard and other lean mass components increase, yet basal metabolic rates decrease, when red knots Calidris canutus are shifted from soft to hard-shelled food. J Avian Biol 35:99–104
Piersma T, Lindström A (1997) Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol Evol 12:134–138
Pohl H (1971) Seasonal variation in metabolic functions of bramblings. Ibis 113:185–193
Pohl H, West GC (1973) Daily and seasonal variation in metabolic response to cold during rest and exercise in the common redpoll. Comp Biochem Physiol 45A:851–867
Precht H (1973) Limiting temperatures of life functions. In: Precht H, Christophersen J, Hensel H, Larcher W (eds) Temperature and life. Spinger, Berlin, pp 400–440
Prosser CL (1973) Comparative animal physiology. Saunders, Philadelphia
Reynolds PS, Lee RM (1996) Phylogenetic analysis of avian energetics: passerines and non-passerines do not differ. Am Nat 147:735–759
Rezende EL, Swanson DL, Novoa FF, Bozinovic F (2002) Passerines versus nonpasserines: so far, no statistical differences in the scaling of avian energetics. J Exp Biol 205:101–107
Ricklefs RE, Konarzewski M, Daan S (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1071
Riddle O, Smith GC, Benedict FG (1934) Seasonal and temperature factors and their determination in pigeons of percentage metabolism change per degree of temperature change. Am J Physiol 107
Rising JD, Hudson JW (1974) Seasonal variation in the metabolism and thyroid activity of the black-capped chickadee (Parus atricapillus). Condor 76:198–203
Rønning B, Moe B, Bech C (2005) Long-term repeatability makes basal metabolic rate a likely heritable trait in the zebra finch Taeniopygia guttata. J Exp Biol 208:4663–4669
Saarela S, Hohtola E (2003) Seasonal thermal acclimatization in sedentary and active pigeons. Isr J Zool 49:185–193
Saarela S, Klapper B, Heldmaier G (1988) Thermogenic capacity of greenfinches and siskins in winter and summer. In: Bech C, Reinertsen RE (eds) Physiology of cold adaptation in birds. Plenum, New York, pp 115–122
Schleucher E, Withers PC (2001) Re-evaluation of the allometry of wet thermal conductance for birds. Comp Biochem Physiol A 129:821–827
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer Associates, Sunderland
Sharbaugh SM (2001) Seasonal acclimatization to extreme climatic conditions by black-capped chickadees (Poecile atricapilla) in interior Alaska (64°N). Physiol Biochem Zool 74:568–575
Southwick EE (1980) Seasonal thermoregulatory adjustments in white-crowned sparrows. Auk 97:76–85
Swanson DL (1990) Seasonal variation in cold hardiness and peak rates of cold-induced thermogenesis in the dark-eyed junco (Junco hyemalis). Auk 107:561–566
Swanson DL (1991) Seasonal adjustments in metabolism and insulation in the dark-eyed junco. Condor 93:538–545
Swanson DL (2007) Seasonal metabolic variation in birds: functional and mechanistic correlates. In: Curr Ornithol, vol 17 (in press)
Swanson DL, Dean KL (1999) Migration-induced variation in thermogenic capacity in migratory passerines. J Avian Biol 30:245–254
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperatures on metabolism in passerine birds. Physiol Biochem Zool 72:566–575
Swanson DL, Weinacht DP (1997) Seasonal effects on metabolism and thermoregulation in northern bobwhite. Condor 99:478–489
Terblanche JS, Janion C, Chown SL (2007) Variation in scorpion metabolic rate and rate–temperature relationships: implications for the fundamental equation of the metabolic theory of ecology. J Evol Biol 20:1602–1612
Terroine EF, Trautmann S (1927) Influence de la température extérieure sur la production calorique des Homéothermes et loi des surfaces. Annales de Physiologie et de Physicochimie Biologique 3:422–457
Tieleman BI, Williams JB (2000) The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol Biochem Zool 73:461–479
Tieleman BI, Williams JB, Buschur ME, Brown CR (2003) Phenotypic variation of larks along an aridity gradient: are desert birds more flexible? Ecology 84:1800–1815
Tieleman BI, Williams JB, Visser GH (2004) Energy and water budgets of larks in a life history perspective: parental effort varies with aridity. Ecology 85:1399–1410
Veghte JH (1964) Thermal and metabolic responses of the gray jay to cold stress. Physiol Zool 37:316–328
Veghte JH (1975) Thermal exchange between the raven (Corvus corax) and its environment. PhD. Thesis, University of Michigan, Ann Arbor
Vezina F, Jalvingh K, Dekinga A, Piersma T (2006) Acclimation to different thermal conditions in a northerly wintering shorebird is driven by body mass-related changes in organ size. J Exp Biol 209:3141–3154
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Wallgren H (1954) Energy metabolism of two species of Emberiza. Acta Zoologica Fennica 84:5–110
Weathers WW (1979) Climatic adaptation in avian standard metabolic rate. Oecologia 42:81–89
Weathers WW, Caccamise DF (1978) Seasonal acclimatization to temperature in monk parakeets. Oecologia 35:173–183
Weathers WW, Sullivan KA (1993) Seasonal patterns of time and energy allocation by birds. Physiol Zool 66:511–536
West GC (1972a) The effect of acclimation and acclimatization on the resting metabolic rate of the common redpoll. Comp Biochem Physiol 43A:293–310
West GC (1972b) Seasonal differences in resting metabolic rate of Alaskan ptarmigan. Comp Biochem Physiol A 41:867–876
White CR, Blackburn TM, Martin GR, Butler PJ (2007) The basal metabolic rate of birds is associated with habitat temperature and precipitation, not productivity. Proc R Soc B 274:287–293
White CR, Seymour RS (2004) Does basal metabolic rate contain a useful signal? Mammalian BMR allometry and correlations with a selection of physiological, ecological, and life-history variables. Physiol Biochem Zool 77:929–941
Wiersma P, Muñoz-Garcia A, Walker A, Williams JB (2007) Tropical birds have a slow pace of life. Proc Natl Acad Sci USA 104:9340–9345
Wijnandts H (1984) Ecological energetics of the long-eared owl (Asio otus). Ardea 72:1–92
Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E (2003) Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc R Soc Lond B 270:2383–2388
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Acknowledgments
I thank Ian Hume for inviting me to write this review. The manuscript benefitted greatly from a discussion with Steven Chown, and from the constructive comments of Craig Willis and an anonymous reviewer. The work was facilitated by funding from the DST/NRF Centre of Excellence at the Percy FitzPatrick Institute and the University of the Witwatersrand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
McKechnie, A.E. Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J Comp Physiol B 178, 235–247 (2008). https://doi.org/10.1007/s00360-007-0218-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0218-8