Abstract
Global change has affected the Antarctic Peninsula influencing the abundance of krill, one of the main preys of penguins. In areas where breeding penguin populations overlap, species with a more diverse diet have generally been less affected than krill-specialist species, which have shown population declines. Human activities can add to these changes, as penguins are sensitive to anthropic impacts such as contamination. Our objective was to assess whether selected physiological parameters of Adélie and Gentoo penguins reflect their contrasting population trends in a colony located at Punta Stranger (25 de Mayo Island/King George, South Shetland Islands) where they breed sympatrically. During 2012, we assessed the leukocyte profile, heterophil to lymphocyte ratio (H/L), erythrocytic nuclear abnormalities (ENAs), hematocrit, biochemical profile, and a measure of immune function (bacterial agglutination) in adults and chicks of both species. Higher values of ENAs, indicative of genotoxic damage caused by contaminants, are in accordance with a greater sensitivity to ongoing global changes by Adélie penguins. Levels of cholesterol and triglycerides strengthen this idea since individuals could be investing more resources in energy reserves to successfully cope with challenging environmental conditions during the breeding season. The remaining physiological parameters did not provide a clear picture. Furthermore, some results could be related to differences in diet. Gentoos show greater prey diversity than Adélie penguins, incorporating a richer parasite fauna, which could explain their higher heterophils and H/L. The physiological parameters measured here serve as baseline for a sustained monitoring of these rapidly changing populations. Further physiological variables, including stress hormone and indices of oxidative stress, remain to be assessed as potential indicators of population susceptibility to global change in this system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last three decades, all Antarctic ecosystems, and specially the Antarctic Peninsula, have been significantly affected by climatic changes illustrated by warming sea surface (SCAR 2010). Consequently, the extent of sea ice has been significantly reduced (Stammerjohn et al. 2008), resulting in the alteration of critical habitats for the fauna. In particular, sea ice reduction has resulted in habitat conditions more suitable for ice-intolerant penguin species such as Gentoo penguins (Pygoscelis papua), whose populations have increased in numbers, whereas populations of ice-dependent (or ice-obligate) penguin species such as Adélie penguins (P. adeliae) have declined on the Antarctic Peninsula (Trathan et al. 1996; Forcada and Trathan 2009; Lynch et al. 2012a; Ducklow et al. 2013). Another major effect of sea ice reduction has been the modification of food webs. Reduced abundance of main prey items such as the Antarctic krill (Euphausia superba) is expected to have important repercussions on its predator populations, which include squids, fishes, seals, whales, and seabirds (Ainley et al. 2010). Among the species that depend most on krill are the Antarctic Pygoscelid penguins (Williams 1995). Nevertheless, not all species and populations are being equally affected (Beaulieu et al. 2013). For instance, it has been proposed that in areas where breeding populations overlap, species with a wider prey spectrum and fewer habitat requirements for breeding should be less affected than krill-specialist species that depend on sea ice for breeding (Forcada and Trathan 2009; Ainley et al. 2010). Supporting this hypothesis, populations of Adélie penguins in the South Shetland Islands, which have a diet based mainly on krill (Libertelli et al. 2003; Miller et al. 2009) and have the highest dependence on sea ice for breeding, have been particularly impacted. Their breeding populations have decreased by 65 % over the last 25 years (Carlini et al. 2009) in association with the reduction in krill biomass (Atkinson et al. 2004; Emslie and Patterson 2007). This has also been the case for Chinstrap penguin (P. antarctica) populations in the South Shetlands, which have decreased by 60 % on average (Barbosa et al. 2012). On the other hand, populations of Gentoo penguins, an ice-intolerant species with a more diverse diet composed by krill, fish, and squid (Libertelli et al. 2003; Miller et al. 2009), have mainly remained stable or even increased in size (Carlini et al. 2009; Juáres et al. 2009). Ongoing global changes also include other factors that can add to these climate-driven effects. For instance, penguins seem to be sensitive to increased human activities (e.g., tourism, scientific bases) and their consequences (e.g., disturbance, pollution) (Barbosa et al. 2013; Bertellotti et al. 2013; Ducklow et al. 2013).
Several parameters have been measured on Pygoscelis penguins breeding sympatrically in relation to the effects of environmental changes, including trophic ecology, colony size, chick growth, breeding success, and breeding phenology (Forcada et al. 2006; Carlini et al. 2009; Chapman et al. 2011; Lynch et al. 2012b). Few studies to date, however, have compared physiological parameters among populations of Pygoscelid penguins with disparate demographic trends (e.g., Barbosa et al. 2007a, b; Beaulieu et al. 2013). Physiological traits at the individual level are promising indicators of population health and can signal a problem even before demographic consequences can be observed (Carey 2005; Wikelski and Cooke 2006). Thus, the use of physiological variables in the context of conservation biology has grown considerably in the last decade (Cooke et al. 2013). Nevertheless, physiological traits need to be evaluated before concluding they reflect population trends and can thus serve as reliable indicators of conservation concerns (Beaulieu et al. 2013).
The objective of this work was to test whether selected physiological parameters reflect the observed higher sensitivity to global changes of Adélie penguins compared with Gentoo penguins at Punta Stranger (25 de Mayo Island/King George Island, South Shetland Islands), an area where these species breed sympatrically. In the last 10 years, the population of ice-dependent Adélie has shrunk by ~60 %, whereas the Gentoo one has increased by ~70 % at this site (Carlini et al. 2009), providing an excellent system for testing which physiological parameters are better indicators of these contrasting demographic trends. For this, we considered 14 blood and biochemical markers of general body condition that are related to health, nutrition, immune function, and genotoxicity. We predicted that if these physiological parameters were good indicators of population trends, then Adélie penguins (i.e., the species with a declining population) would show lower general body condition and health status indices (including evidence of immunodepression) and/or higher levels of stress and genotoxicity than Gentoo penguins (i.e., the species with a growing population).
Materials and methods
The study was conducted during the penguin breeding season in January 2012 at Punta Stranger, Potter Peninsula, 62° 15′S, 58° 37′W (Fig. 1). Adults and chicks of Adélie and Gentoo penguins were sampled. Adults were chosen at random, captured, and immediately sampled in the colony. All adults sampled were reproductive individuals. Chicks were sampled during the guard phase (15–30 days after birth) to avoid potential variation related to different stages of the breeding period. We sampled a total of 84 individuals (20 Adélie and 20 Gentoo adults, and 20 Adélie and 24 Gentoo chicks). Sampled birds showed no external signs of illness or injuries.
Blood samples (1.0 ml) were obtained from the metatarsal vein. All birds were sampled within 5 min of capture to minimize the potential effect of handling stress on the parameters measured (Davis 2005). Blood was placed into microcapillary tubes for hematocrit measurement and into heparinized eppendorf tubes for separation of the plasma portion. Thin blood smears were prepared with a drop of fresh blood, air-dried, fixed with ethanol for 3 min, and stained with Tinción 15 (Biopur).
Microcapillary tubes were spun in a centrifuge for 12 min at 12,000g, and hematocrit was determined with a microhematocrit ruler. Hematocrit is considered a physiological index of condition in birds when evaluated with other hematological parameters (Fair et al. 2007), providing an estimate of aerobic capacity (Beldomenico et al. 2008). Blood smears were used for analysis of the complete leukocyte profile, the heterophil to lymphocyte ratio (H/L), and records of erythrocytic nuclear abnormalities (ENAs). The leukocyte profile provides valuable information on the cellular components of the immune system and was assessed by estimation of the total leukocyte count (or total white blood cell count, total WBC) and of its five leukocyte types (heterophils, eosinophils, basophils, lymphocytes, and monocytes) (Roitt et al. 2001). The H/L is considered a reliable measure of stress in vertebrates, including birds (Davis et al. 2008), and ENAs are commonly used in ecotoxicology studies as indices of genotoxicity caused by pollution (Van Ngan et al. 2007). Blood smears were examined with a light microscope to estimate the total WBC by counting all leukocytes in ten consecutive 400× monolayer fields (D’Amico et al. 2010). Leukocyte proportions were obtained as the part of each leukocyte type in a sample of 100 leukocytes under 1000× microscope magnification (Campbell 1995). The number of ENAs was scored for each blood smear in relation to 2000 mature erythrocytes (Carabajal et al. 2012). Nuclear abnormalities were classified following Kursa and Bezrukov (2008) as: (1) micronucleus, (2) segmented nucleus or two-lobe nucleus, and (3) tailed and buddings nucleus. The sum of all ENAs per individual was used for statistical analysis.
Blood stored in eppendorf tubes was centrifuged to separate the plasma portion for biochemical and immunological determinations. Plasma was processed on a spectrophotometer (METROLAB 1600 Plus, UV–Vis Metrolab) to determine the concentration of total proteins (g/dl), cholesterol (mg/dl), triglycerides (mg/dl), and glucose (mg/dl). Increases in total proteins are commonly associated with the intake of high protein food. Total protein levels and also lipids are important during the breeding season because they represent the nutrient reserve available for reproduction and to cope with severe environmental conditions (Morrison et al. 2005). Energy stored as triglycerides in adipose tissues is primarily used to support aerobic activities (Brown 1996). Increases in cholesterol are related to the quality of food eaten, while decreases may be related to anabolic processes (Alonso-Alvarez et al. 2002). Glucose is the main carbohydrate source of cellular energy, and its values depend on the type and quality of food eaten (Brown 1996). As glucose is consumed very fast, its blood concentration tends to be low when birds are active, whereas it increases at rest (Jenni-Eiermann and Jenni 1994). The ability of humoral components in plasma to agglutinate foreign particles constitutes an index of constitutive innate immunity, an important first line of defense against invading pathogens (Roitt et al. 2001; Matson et al. 2005). Agglutination of Escherichia coli (ATCC 8739) by plasma components was measured following protocols that have been used in fish (Sahoo et al. 2008 and references therein) and that we adapted for use in penguins. Briefly, bacteria were grown in tryptic soy (TS) broth and then fixed in 1 % formalin overnight at 4 °C. Fixed bacteria were washed three times with phosphate buffered saline (PBS) and adjusted to a concentration of approx. 1 × 109 bacteria/ml. Plasma samples (15 µl) were added to the first column of a 96-well plate and serially twofold diluted along the rows with PBS. A negative control (PBS instead of plasma) was included in each plate. Then, 15 µl of fixed bacteria were added to all wells. Plates were vortexed and incubated at 40 °C overnight. Agglutination titers were determined as −log2 of the highest dilution showing bacterial agglutination. Inter-plate variation, calculated as the coefficient of variation (CV), was 6.1 %.
The 14 physiological parameters were statistically analyzed using general linear models (GLM) with the software JMP Pro 10.0.0 (SAS Institute Inc 2012). Models included the fixed effects of penguin species (Adélie and Gentoo) and age group (adults and chicks). The interaction term between these main effects was also tested in the models to determine whether species differences depended on whether adults or chicks were considered. Body mass, structural size (bill length and height), and body condition (residuals of the regression of body mass on structural size) were evaluated as potential covariates but did not explain significant variation in any of the response variables (all P > 0.05) and were therefore excluded from the final models. Residual plots were visually inspected for signs of non-normality, and variables were log10 transformed for analysis when necessary. Untransformed raw data are depicted in tables and graphs for visual clarity. To maintain an experiment-wise error rate of 0.05, we used a Bonferroni adjustment (Rice 1989) of α = 0.003 for n = 14 parameters compared.
Results
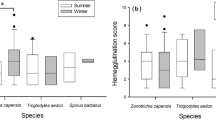
Descriptive statistics for the 14 physiological markers of adults and chicks of the two penguin species are shown in Table 1. Eleven of the 14 measured parameters showed statistically significant differences by species, by age, and/or their interaction (Fig. 2). Total WBC differed between species only for adults (species × age: F 1, 1 = 23.76, P < 0.0001), with Adélies showing lower levels than Gentoos (Fig. 2a). The percentage of lymphocytes (Fig. 2b) was higher in Adélies than Gentoos (species: F 1, 1 = 29.86, P < 0.001), and higher in adults than chicks (age: F 1, 1 = 23.76, P < 0.0001), showing a nonsignificant trend toward an interaction (P > 0.003). The percentage of heterophils was higher in Gentoos irrespective of age (species: F 1, 1 = 32.15, P < 0.0001, Fig. 2c), whereas the percentage of basophils was overall higher in Adélies than in Gentoos (Fig. 2d), showing a difference between age groups only in the former species (species × age: F 1, 1 = 9.78, P = 0.0025). The remaining components of the leukocyte profile (i.e., eosinophils and monocytes) did not show any significant effects (all P > 0.003). The lower percentage of lymphocytes displayed by Gentoos coupled with their higher percentage of heterophils resulted in a higher H/L ratio in this species (species: F 1, 1 = 34.53, P < 0.0001, Fig. 2e). There was also a nonsignificant trend (P > 0.003) toward higher H/L ratios in adults than chicks of both species. Bacterial agglutination only showed an effect of age, with titers being higher in adults than chicks (age: F 1, 1 = 47.86, P < 0.0001, Fig. 2f). Of the variables involving red blood cells, hematocrit showed a complex species by age interaction (F 1, 1 = 41.37, P < 0.0001, Fig. 2g), whereas ENAs were significantly higher in Adélies irrespective of age (species: F 1, 1 = 17.52, P < 0.0001, Fig. 2h). Finally, biochemical parameters, with the exception of glucose levels, showed significant patterns. Whereas total protein only showed an age effect with higher levels in adults than chicks (age: F 1, 1 = 127.84, P < 0.0001, Fig. 2i), both triglyceride and cholesterol levels had significant interaction effects between species and age (F 1, 1 = 9.91, P = 0.0023, Fig. 2j; F 1, 1 = 30.92, P < 0.0001, Fig. 2k, respectively). For both lipid components, differences between species were observed only in adults, with higher levels displayed by Adélies.
Boxplots of physiological parameters of chicks and adults of Adélie and Gentoo penguins showing significant effects of species, age group, and/or their interaction. Boxplots depict medians (horizontal lines inside boxes), 25 and 75 percentiles (edges of boxes), 10 and 90 percentiles (whiskers), and outlying points included in the analyses (dots). Letters at the top of each panel correspond to levels of Tukeys HSD (honestly significant difference) post hoc test. Levels not connected by the same letter are significantly different. WBC white blood cell counts, H/L heterophil to lymphocyte ratio, ENAs erythrocyte nuclear abnormalities, RBCs red blood cells
Discussion
We compared physiological parameters related to general body condition, immune function, and genotoxicity of adults and chicks of two penguin species breeding sympatrically at Punta Stranger but showing strongly contrasting population trends. We predicted that if these parameters constitute good indicators of population trends and sensitivity to ongoing global changes, then the declining species (Adélie) would show signs of deteriorated physiology compared to the species undergoing population growth (Gentoo). Of the 14 physiological parameters we evaluated, only a few could potentially be considered indicators of the greater sensitivity to global changes displayed by Adélie penguins. Other parameters provide no evidence of this and/or alternative interpretations for species differences can be proposed. In addition, the species by age interaction observed for some parameters defy a straightforward interpretation of the patterns.
Higher number of erythrocytic nuclear abnormalities (ENAs) in Adélie adults and chicks suggests that they are more sensitive to contaminants than Gentoo penguins. ENAs count is one of the main methods for detecting genomic damage related to environmental deterioration and pollution (Van Ngan et al. 2007). Previous studies have shown that penguins at the Punta Stranger colony present high levels of contamination by heavy metals (Jerez et al. 2011). At this site, Adélie penguins showed the highest levels of chrome (Cr) and selenium (Se) in feathers, while Gentoo penguins showed the highest levels of zinc (Zn) (Jerez et al. 2011). Both penguin species showed similar percentages of lead (Pb) in feathers, these being the highest levels reported in comparison with other populations of Adélie and Gentoo penguins along the Antarctic Peninsula (Jerez et al. 2011). Pb and Cr are heavy metals directly related to human contaminant activities, and analysis of penguin feathers showed that these elements are most abundant in areas with major human presence, such as 25 de Mayo Island/King George (Jerez et al. 2011). Moreover, ice melt due to global warming may be releasing these pollutants that had been accumulated around the base over decades, exacerbating their effect on the nearby penguin populations (Cabrerizo et al. 2013). Interestingly, genomic damage and/or instability has also been linked to diseases (e.g., cancer) (Kursa and Bezrukov 2008). Few studies deal with this issue in wild animals, even fewer in Antarctic fauna, and thus more research on this topic is warranted.
Hematological and biochemical parameters can provide valuable information on the physiological state and adaptation of individuals to changes in their habitat, nutritional status, and general body condition (Quillfeldt et al. 2004). For instance, some birds like migratory shorebirds show lower values of lipids and total proteins after they arrive to their non-breeding sites in comparison with when they are in migration (D’Amico et al. 2010). In the present study, contrary to our prediction, adult Adélie showed higher hematocrit, triglycerides, and cholesterol levels than adult Gentoo penguins (while there were no differences in total protein and glucose levels), suggesting that they were actually storing more fat. Fat stores have been linked to higher performance (i.e., production, activity or response to environmental condition) and fitness in birds (Brown 1996). Thus, our result suggests that the population decline of Adélie penguins at this site is not mainly related to food availability/nutritional deficiency of adults or that, alternatively, adults of these species need to make a large investment in food storage to ensure enough energetic resources for reproduction and survival. Even though the latter is applicable to both species, ice-dependent Adélie penguins may have to invest disproportionally more resources than Gentoos to attain similar fitness benefits given their higher dependence on decreasing stocks of Antarctic krill (Libertelli et al. 2003; Atkinson et al. 2004, 2008; but see Chapman et al. 2011; Sailley et al. 2013) and on decreasing sea ice (Ainley et al. 2010).
Leukocyte profiles, which are indices of health, immunological status, and stress levels of the birds, did not provide a clear picture. Some of the differences observed between the two species could be related to differences in their diet rather than to differential stress and/or sensitivity to environmental conditions. For instance, Gentoo penguins show greater prey diversity and thus incorporate a richer parasite fauna through their diet than Adélie penguins (Diaz et al. 2013). Higher total WBC can be indicative of higher exposure to parasites and/or pathogens (Roitt et al. 2001). Heterophils, one of the most abundant phagocytic cells in birds, are the first line of defense against gastrointestinal parasites incorporated through the diet (Shutler and Marcogliese 2011). Consequently, the greater gastrointestinal parasite diversity and infestation that has been shown to occur in Gentoo penguins (Diaz et al. 2013) could explain their higher total WBC (only in adults), percentage of heterophils, and H/L ratios documented in our study. A similar result has been found when comparing Antarctic and non-Antarctic penguins (D’Amico et al. 2014). Bacterial agglutination by plasma components, an index of constitutive humoral innate immunity, did not differ between Adélie and Gentoo penguins but was higher in adults than chicks of both species, probably reflecting the ontogeny of this immune component (Palacios et al. 2009). The only other study that measured an immune-related parameter in these two species found that Gentoo penguins showed higher circulating immunoglobulin levels than Adélie penguins in 25 de Mayo Island (Barbosa et al. 2007a), which could also result from their higher parasite exposure through the diet mentioned earlier. Overall, these data do not support the idea that individuals in the declining population are immunocompromised compared to individuals in the growing population. Nevertheless, given the complexity of the immune system, a more complete test of this hypothesis warrants incorporation of a broader set of immunological assays covering diverse aspects of both innate and acquired immunity (e.g., Palacios et al. 2012).
In summary, the higher values of ENAs of Adélie compared with Gentoo penguins support the use of this measure as an indicator of the greater sensitivity of the former species to environmental contaminants at Punta Stranger, which could be magnified by the effects of climate change (i.e., ice melting, Cabrerizo et al. 2013) in Antarctica. Some biochemical parameters of adult Adélie penguins could also be considered indicators of their greater sensitivity to global changes if individuals needed larger fat reserves to successfully cope with the greater environmental challenges they face during the breeding season. However, most other physiological parameters measured in this study did not show clear patterns between the two species, especially for chicks. Before concluding that these parameters are generally poor indicators of demographic trends, it is important to consider whether the sampled individuals and/or the specific moment when they are sampled could influence the results. In our case, despite the strongly contrasting population trends of Adélie and Gentoo penguins at Punta Stranger, both populations show similar breeding success (number of chicks in crèche/number of nests with eggs) (Carlini et al. 2009). Thus, our finding of few inter-species differences in several physiological parameters measured during the breeding season might not be as surprising, although similar breeding success does not necessarily imply that adults of both species are paying the same physiological costs of reproduction and/or that chicks fledged by both species are of the same quality. Indeed, Carlini et al. (2009) suggest that changes in population sizes of Adélie and Gentoo penguin populations at Punta Stranger are likely driven by factors that operate mainly over winter. Furthermore, the authors suggest that although those factors could affect adults, they most likely affect juvenile survival of the two species differently, leading thus to marked differences in recruitment of new individuals to the breeding populations (Carlini et al. 2009). Thus, we can hypothesize that the physiological parameters included in this study could better reflect the population trends if evaluated in juvenile penguins during the winter season. Nevertheless, the physiological data collected in this study serve as baseline for sustained monitoring of these rapidly changing populations. Future research will assess additional indices of stress, such as levels of corticosterone and oxidative damage, as potential physiological indicators of population trends in penguins. The ecophysiological approach illustrated by the present study could be extended to other sentinel species that can also express physiological responses to environmental changes, such as seals, whales, and species dependent directly or indirectly on krill.
References
Ainley D, Russell J, Jenouvrier S, Woehler E, O’B Lyver F, Fraser WR, Kooyman GL (2010) Antarctic penguin response to habitat change as Earth’s troposphere reaches 28C above preindustrial levels. Ecol Monogr 80:49–66
Alonso-Alvarez C, Ferrer M, Velando A (2002) The plasmatic index of body condition in Yellow-legged Gulls Larus cachinnans: a food-controlled experiment. Ibis 144:147–149
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Atkinson A, Siegel V, Pakhomov E, Rothery P, Loeb V, Ross RM, Quentin LB, Schimidt K, Fretwell P, Murphy EJ, Tarling GA, Fleming AH (2008) Oceanic circumpolar habitats of Antarctic krill. Mar Ecol Prog Ser 362:1–23
Barbosa A, Merino S, Benzal J, Martinez J, Garcia-Fraile S (2007a) Geographic variation in immunoglobulin levels in pygocelid penguins. Polar Biol 30:219–225
Barbosa A, Merino S, Benzal J, Martinez J, Garcia-Fraile S (2007b) Population variability in heat shock proteins among three Antarctic penguin species. Polar Biol 30:1239–1244
Barbosa A, Benzal J, De León A, Moreno J (2012) Population decline of chinstrap penguins (Pygoscelis antarctica) on Deception Island, South Shetlands, Antarctica. Polar Biol 35:1453–1457
Barbosa A, Eva De Mas E, Benzal J, Diaz JI, Motas M, Jerez S, Pertierra L, Benayas J, Justel A, Lauzurica P, Garcia-Peña FJ, Serrano T (2013) Pollution and physiological variability in gentoo penguins at two rookeries with different levels of human visitation. Antarct Sci. doi:10.1017/S0954102012000739
Beaulieu M, Thierry AM, González-Acuña D, Polito MJ (2013) Integrating oxidative ecology into conservation physiology. Conserv Physiol 1:cot001. doi:10.1093/conphys/cot001
Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M (2008) The dynamics of health in wild field vole populations: a haematological perspective. J Anim Ecol 77:984–997
Bertellotti M, D’Amico VL, Cejuela E (2013) Tourist activities focusing on Antarctic Penguins. Ann Tourism Res 42:428–431
Brown M (1996) Assessing body condition in birds. In: Nolan V, Ketterson ED (eds) Current ornithology. volume 13, chapter 3. Plenum Press, New York
Cabrerizo A, Dachs J, Barceló D, Jones KC (2013) Climatic and biogeochemical controls on the remobilization and reservoirs of persistent organic pollutants in Antarctica. Environ Sci Technol 47:4299–4306
Campbell TW (1995) Avian hematology and cytology. Iowa State University Press, Ames
Carabajal E, Massari N, Croci M, Lamas DM, Prestifilippo JP, Ciraolo P, Bergoc RM, Rivera ES, Medina VA (2012) Radioprotective potential of histamine on rat small intestine and uterus. Eur J Histochem 56:302–310
Carey C (2005) How physiological methods and concepts can be useful in conservation biology. Int Comp Biol 45:4–11
Carlini R, Coria NR, Santos MM, Negrete J, Juares MA, Daneri GA (2009) Responses of Pygoscelis adeliae and P. papua populations to environmental changes at Isla 25 de Mayo (King George Island). Polar Biol 32:1427–1433
Chapman EW, Hofmann EE, Patterson DL, Ribic CA, Fraser WR (2011) Marine and terrestrial factors affecting Adélie penguin Pygoscelis adeliae chick growth and recruitment off the western Antarctic Peninsula. Mar Ecol Prog Ser 436:273–289
Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Cons Physiol 1:cot004. doi:10.1093/conphys/cot004
D’Amico VL, Bertellotti M, Baker AJ, González PM (2010) Hematological and plasma biochemistry values for endangered red knots (Calidris canutus rufa) at wintering and migratory sites in Argentina. J Wildl Dis 46:644–648
D’Amico VL, Bertellotti M, Díaz JI, Coria N, Vidal V, Barbosa A (2014) Leucocyte levels in some Antarctic and non-Antarctic penguins. Ardeola 61:145–152
Davis AK (2005) Effects of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76:334–338
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–777
Diaz JI, Fusaro B, Longarzo L, Coria NR, Vidal V, Jerez S, Ortiz J, Barbosa A (2013) Gastrointestinal helminths of Gentoo penguins (Pygoscelis papua) from Stranger point, 25 de Mayo/King George Island, Antarctica. Parasitol Res 112:1877–1881
Ducklow HW, Fraser WR, Meredith MP, Stammerjohn SE, Doney SC, Martinson DG, Sailley SF, Schofield OM, Steinberg DK, Venables HJ, Amsler CD (2013) West Antarctic Peninsula: an ice-dependent coastal marine ecosystem in transition. Oceanography 26:190–203
Emslie SD, Patterson WP (2007) Abrupt recent shift in 13C and 15N values in Adélie penguin eggshell in Antarctica. PNAS 104:11666–11669
Fair J, Whitaker S, Pearson B (2007) Sources of variation in haematocrit in birds. Ibis 149:535–552
Forcada J, Trathan PN (2009) Penguin responses to climate change in the Southern Ocean. Glob Change Biol 15:1618–1630
Forcada J, Trathan PN, Reid K, Murphy EJ, Croxall JP (2006) Contrasting population changes in sympatric penguin species in association with climate warming. Glob Change Biol 12:411–423
Jenni-Eiermann S, Jenni L (1994) Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the Garden Warbler. Auk 111:888–899
Jerez S, Motas M, Palacios MJ, Valera F, Cuervo JJ, Barbosa A (2011) Concentration of trace elements in feathers of three Antarctic penguins: geographical and interspecific differences. Environ Pollut 159:2412–2419
Juáres MA, Santos MM, Negrete J, Libertelli MM, Gray M, Moreira ME, Carlini A, Coria NR (2009) Tendencias poblacionales de pingüinos Pygoscelidos en tres localidades de las Islas Shetland del Sur, Antártida. V Simposio Latinoamericano sobre Investigaciones Antárticas y Simposio Ecuatoriano de Ciencia Polar. Resumen. p 28
Kursa M, Bezrukov V (2008) Health status in an Antarctic top predator: micronuclei frequency and white blood cells differentials in the south polar skua (Catharacta maccormicki). Polarforschung 77:1–5
Libertelli MM, Coria N, Marateo G (2003) Diet of the Adélie penguin during three consecutive chick rearing periods at Laurie Island. Polish Polar Res 24:133–142
Lynch HJ, Naveen R, Trathan PN, Fagan WF (2012a) Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93:1367–1377
Lynch HJ, Fagan WF, Naveen R, Trivelpiece SG, Trivelpiece WZ (2012b) Differential advancement of breeding phenology in response to climate may alter staggered breeding among sympatric pygoscelid penguin. Mar Ecol Prog Ser 454:135–145
Matson KD, Ricklefs RE, Klasing KC (2005) A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev Comp Immunol 29:275–286
Miller AK, Karnovsky NJ, Trivelpiece WZ (2009) Flexible foraging strategies of gentoo penguins Pygoscelis papua over 5 years in the South Shetland Islands, Antarctica. Mar Biol 156:2527–2537
Morrison RIG, Davidson NC, Piersma T (2005) Transformations at high latitudes: why do red knots bring body stores to the breeding grounds? The Condor 107:449–457
Palacios MG, Cunnick JE, Vleck D, Vleck CM (2009) Ontogeny of innate and adaptive immune defense components in free-living tree swallows, Tachycineta bicolor. Dev Comp Immunol 33:456–463
Palacios MG, Cunnick J, Winkler D, Vleck CM (2012) Interrelations among immune defense indices reflect major components of the immune system in a free living vertebrate. Physiol Biochem Zool 85:1–10
Quillfeldt P, Masello JF, Möstl E (2004) Blood chemistry in relation to nutrition and ectoparasite load in Wilson’s storm-petrels Oceanites oceanicus. Polar Biol 27:168–176
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Roitt I, Brostoff J, Male D (2001) Immunology. Mosby, London
Sahoo PK, Das Mahapatra K, Saha JN, Barat A, Sahoo M, Mohanty BR, Gjerde B, Ødegard J, Rye M, Salte R (2008) Family association between immune parameters and resistance to Aeromonas hydrophila infection in the Indian major carp, Labeo rohita. Fish Shellfish Immun 25:163–169
Sailley SE, Ducklow HW, Moeller HV, Fraser WR, Schofield OM, Steinberg DK, Garzio LM, Doney SC (2013) Carbon fluxes and pelagic ecosystem dynamics near two western Antarctic Peninsula Adélie penguin colonies: an inverse model approach. Mar Ecol Prog Ser 492:253–272
SAS Institute Inc (2012) SAS Campus Drive, Cary, North Carolina
SCAR (2010) Antarctic climate change and the environment. Published by the Scientific Committee on Antarctic Research, Cambridge. ISBN 978-0-948277-22-1
Shutler D, Marcogliese DJ (2011) Leukocyte profiles of Northern Leopard Frogs, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands. Copeia 2:301–307
Stammerjohn SE, Martinson DG, Smith RC, Yuan X, Rind D (2008) Trends in Antarctic annual sea ice retreat and advance and their relation to ENSO and southern annular mode variability. J Geophys Res 113:C03S90. doi:10.1029/2007JC004269
Trathan PN, Croxall JP, Murphy EJ (1996) Dynamics of Antarctic penguin populations in relation to inter-annual variability in sea-ice distribution. Polar Biol 16:321–330
Van Ngan PV, Gomes V, Passos MJACR, Ussami KA, Campos DYF, Da Silva AJ, Pereira BA (2007) Biomonitoring of the genotoxic potential (micronucleus and erythrocyte nuclear abnormalities assay) of the Admiralty Bay water surroundings the Brazilian Antarctic Research Station “Comandante Ferraz”, King George Island. Polar Biol 30:209–217
Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21:38–46
Williams TD (1995) The penguins. Oxford University Press, Oxford
Acknowledgments
We very much appreciated the hospitality and logistic support of the Argentinean Antarctic Base “Carlini (ex Jubany).” We thank J. Menucci for providing the maps and two anonymous reviewers for their suggestions that helped improve our manuscript. We are grateful for the logistic support and permits provided by the Instituto Antártico Argentino (IAA–DNA). AB was supported by the project CTM2011-24425. Permits to work at the site and handle penguins were given by Dirección Nacional del Antártico.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is an invited contribution on Life in Antarctica: Boundaries and Gradients in a Changing Environment as the main theme of the XIth SCAR Biology Symposium. J.-M. Gili and R. Zapata Guardiola (Guest Editors).
Rights and permissions
About this article
Cite this article
D’Amico, V.L., Coria, N., Palacios, M.G. et al. Physiological differences between two overlapped breeding Antarctic penguins in a global change perspective. Polar Biol 39, 57–64 (2016). https://doi.org/10.1007/s00300-014-1604-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1604-9