Abstract
The overuse of antibiotics has led to an increase in bacterial resistance and, in turn, to a decreasing efficiency of the rare available antibiotics. Alternatively, gold nanoparticles are promising antibacterials due to their high specific surface area, easy modification by functional groups and broad-spectrum antibacterial activity. Their antibacterial properties are closely related to particle size, dispersibility and surface modification, which can be tuned by adjusting reaction conditions. Here, we review the synthesis and antibacterial performance of gold nanoparticles in the raw form or modified with metal, organic compounds and carbon. We present the effect of reaction conditions on particle dispersibility and size. We compare the various synthesis methods. Antibacterial activities and their mechanisms are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abuse of antibiotics has become a growing problem in recent years. When antibiotics enter the environment, they damage the ecosystem’s bacteria, aquatic organisms, soil organisms and plants. Of particular concern, bacteria eventually develop resistance or even multi-drug-resistant (MDR) “super bacteria.” They can produce inactivating enzymes and block drugs by altering cytomembrane permeability (Hu et al. 2019). Today, more than 70% of bacteria have become resistant to one or more antibiotics (Allahverdiyev et al. 2011). That is forcing doctors to increase antibiotic doses, which can cause adverse reactions. And for some severe bacterial infections, there may be no medicine available.
It is urgent to find alternative materials instead of antibiotics to play an antibacterial role. One lead in this direction is that biomolecules immobilized on the surface of nanoscale particles have demonstrated significant antibacterial activity (Brown et al. 2012). The high specific surface area allows nanoscale particles to bind relatively high concentrations of functional ligands or act as carriers for other active substances, enhancing their interaction with target bacteria (Li et al. 2014a).
The nanoscale materials that have demonstrated antibacterial activity include Ag, Cu and Zn ions and zinc and copper oxides (Zhao et al. 2018). Nanoscale gold particles (AuNPs) have shown uniquely advantageous antimicrobial activity. As the least active metal, gold has very stable chemical properties, is non-toxic and has good biocompatibility (Hammer and Norskov 1995). And gold is multivalent; it can bind many types of ligands (Gupta et al. 2016), and AuNPs’ very large specific surface area provides abundant sites for binding with target bacteria (Dreaden et al. 2012; Nirmala Grace and Pandian 2007). The antibacterial spectrum is broad. AuNPs have shown antibacterial ability against both Gram-positive and Gram-negative bacteria (Slavin et al. 2017). Compared with normal antibiotics, AuNPs do not easily produce drug resistance (Zhao et al. 2018) because they target a variety of molecules (DNA and protein) in bacteria, making it difficult for bacteria to establish a system that can defend against all damage.
The preparation and antibacterial properties of AuNPs have been widely studied, but to date no comprehensive summary has been published. This article reviews the progress in recent years in the preparation and antibacterial action of nanoscale gold and its composites, as indicated in Fig. 1. The antibacterial effects of gold nanocomposites are reviewed along with possible mechanisms. Those antibacterial mechanisms mainly involve damaging the cytoderm and biofilms, producing reactive oxygen species and releasing metal ions which induce damage in bacterial cells. The review’s aim is to provide a better basis for preparing AuNPs and to disseminate some up-to-date ideas for obtaining more effective antibacterial agents.
Preparation methods
Nanoscale gold particles
Gold has oxidation states between − 1 and + 5, but mainly + 1 and + 3. Adding a reducing agent to reduce Au3+ or Au+ to Au0 is the usual first step in the synthesis of nanoscale gold particles (AuNPs). In addition, the addition of polymers, surfactants or sulfates can enhance the stable dispersion of the particles formed. A large number of incompletely coordinated atoms on the surface of the AuNPs can then bind with numerous ligands to form functionalized gold nanoparticles. Increasing the incidence of defects by grinding allows the nanoparticles to absorb and bind more effectively. And today the use of microwaves, electromagnetism or radiation to assist the functionalization of nanoscale gold particles is becoming more widespread. The size, morphology and dispersibility of the gold nanoparticles required vary according to the application. Reaction temperature, reaction time, and the type and amounts of additives all affect the final state of gold nanoparticle synthesis.
An efficient preparation method is the key to obtaining high-quality AuNPs. Chloroauric acid is the raw material most commonly used, but it is very corrosive, requiring the use of plastic or glass equipment. Glassware should be soaked in aqua regia and washed with ultrapure water because foreign impurities may replace the stabilizer and cause aggregation of the nanoparticles. In addition, AuNPs should be stored in a brown bottle protected from light.
The seed growth method
In the seed growth method, chloroauric acid is reduced using sodium borohydride with a protective agent. AuNPs about 1–2 nm in diameter are used as seeds. Growth liquid is added to allow the seed crystals to continue to grow, and AuNPs 10–20 nm in diameter are obtained. The other components of the growth solution are silver nitrate, ascorbic acid and cetyltrimethylammonium bromide. The detailed steps are shown in Fig. 2a. The cetyltrimethylammonium bromide is a stabilizer and a regulator which adjusts the growth rate of the gold crystals along different crystal planes. HAuCI4 solution and sodium borohydride are successively added to the brown-yellow seed solution stabilized by cetyltrimethylammonium bromide with vigorous stirring over about 2 h. Sodium borohydride is a strong reducing agent which reduces Au3+ directly to Au0 to obtain the seed solution. The cetyltrimethylammonium bromide is stirred into a certain volume of AgNO3 solution at room temperature, and then, HAuCI4 solution is added. At this time, the solution appears bright yellow. Finally, a certain volume of ascorbic acid solution, a weak reducing agent, is added to prepare a colorless growth solution. Au3+ is reduced to Au+. Finally, the seed solution is poured into the growth solution and mixed well and left to grow steadily for about 16 h, during which time the solution turns soil red. At this point, Au0 acts as a catalyst to reduce Au+ to Au0 in the presence of ascorbic acid, so that the auric nuclei continue to grow slowly (Li et al. 2017).
Li and his colleagues describe preparing gold nanorods using the seed growth method (Li et al. 2017). Studies have shown that when the concentration of ascorbic acid is 0.60 mM, the concentration of silver nitrate is 0.08 mM, a 16 h reaction time generates the best gold nanorods with a large aspect ratio. A group led by Gonzalez-Rubio controlled the size and morphology of gold nanorods by optimizing the seed growth stage and improved the reproducibility of the preparation (Gonzalez-Rubio et al. 2019). One advantage of the seed growth method is that AuNPs of various sizes and morphologies can be obtained through appropriate regulation of the proportions of seed crystals and silver nitrate, the ascorbic acid concentration, the reaction time and temperature. The seed growth method is particularly suitable for systems that have strict particle size and morphology requirements.
Biological extract synthesis
Biological extract synthesis is a relatively green methodology which has emerged in response to the toxicity of some of the compounds added in other syntheses. It uses biological extracts as stabilizers, reducing agents and capping agents to synthesize highly dispersible and stable bioactive AuNPs. It has the advantages of safety, mild reaction conditions, simple operation and low cost and can be operated on a large scale without additional reducing agents and stabilizers. The detailed preparation steps of a biological extract synthesis are shown in Fig. 2b.
The biological extract is obtained through a series of treatments such as washing, water bath heating and paper filtration. Reducing substances such as vitamins, sugars and hydroxyl groups are extracted in this way from grapefruit skins and used as reducing agents and stabilizers (Yuan et al. 2017). The biological extract is added dropwise to a solution of HAuCI4 and mixed well. A change in color from light brown to dark brown indicates the formation of AuNPs. Yuan’s group successfully synthesized gold with particle size of 10 nm in this way. Their AuNPs showed bacteriostatic effectiveness with both E. coli and S. aureus, and the bacteriostatic effect increased with the AuNP concentration. AuNPs have also been prepared using extracts of Sueda fruciotosa (Khan et al. 2017), longan juice (Khan et al. 2016) and mushroom (Lee et al. 2015).
Chemical reduction
The principle of the chemical reduction method is that a reducing agent gives electrons to Au3+ so that it is reduced to Au0. Common reducing agents are sodium borohydride, ascorbic acid, sodium citrate, H2O2 and others. Some reducing agents can also act as stabilizers. Stabilizers and surfactants such as sulfates, phosphorus ligands and cetyltrimethylammonium bromide can effectively forestall agglomeration of the nanoparticles. Surfactants can also control their growth.
The most typical chemical reduction methods are sodium citrate reduction and the Brust–Schiffrin method. Sodium citrate reduction is the most convenient and the most commonly used. It can generate spherical gold particles between 10 and 50 nm in diameter. However, it is difficult to prepare smaller particles. As shown in Fig. 2c, sodium citrate is added dropwise to a boiling aqueous solution of chloroauric acid. After about 25 s, the solution turns pale blue, indicating that it has been nucleated. It becomes bright red after 70 s, and monodisperse spherical particles are formed. Frens’ experiments showing that the reduction of chloroauric acid is basically complete after boiling for 5 min. Longer heating or additional citrate produces no substantial change to the suspension (Frens 1973).
The advantage of the sodium citrate reduction method is that highly dispersed AuNPs of different particle sizes can be obtained simply by changing the amount of sodium citrate added. The disadvantage is that the nanogold interface obtained by this method is not clean due to the decomposition of sodium citrate during the synthesis (Panahi et al. 2017).
The Brust–Schiffrin method (Brust et al. 1955) is also known as the two-phase method. Chloroauric acid solution and p-mercaptophenol are dissolved in methanol. The addition of acetic acid prevents protonation of the p-mercaptophenol. Tetraoctylammonium bromide is used as a phase transfer agent to transfer HAuCl4 from the aqueous to the organic phase. Sodium borohydride, a strong reducing agent, is added in the presence of dodecyl mercaptan, and rapid browning of the solution marks the formation of gold clusters. The particle size varies with the ratio of gold to dodecyl mercaptan. Dodecyl mercaptan is a surfactant. It is amphiphilic and so can be adsorbed on the particles’ surface. Nanoparticles coated with surfactant can remain stable in a solid or liquid state for a long time. This method is diagrammed in Fig. 2d.
AuNPs obtained by the Brust–Schiffrin method are small and very stable. The isolated gold nanoparticles can be re-dispersed in organic solvents. The disadvantage is that the preparation method is complicated.
Metal-modified gold nanoparticles
In recent years, nanoscale particles of metals such as gold, silver, copper, palladium and platinum have been widely used in medical treatment (Annamalai et al. 2013; Ayaz Ahmed et al. 2016; Boomi et al. 2013; Li et al. 2014b; Valodkar et al. 2011; Wang et al. 2016a). However, along with their advantages, metal nanoparticles have shortcomings which limit their practical application. For example, silver nanoparticles have diverse antibacterial mechanisms, but their cytotoxicity limits their application (Wang et al. 2016b). Both platinum and gold nanoparticles are bacteriostatic (Ayaz Ahmed et al. 2016; Li et al. 2014b), but they are costly and material scarcity is a major problem. Combinations of metal nanoparticles can sometimes alleviate these shortcomings. For example, due to the action of strong electron ligands, combining silver and gold can increase the effective concentration range of silver ions (Wang et al. 2016b), enhancing their antibacterial activity. Adding a third metal can sometimes offer further improvement.
The seed growth method
Nanoparticles grown from seed crystals tend to be small and controllable with good dispersibility. The particle size and its dispersion can be controlled by controlling reaction conditions such as the concentration, pH and the temperature of the solution. Emam used Arabic gum as a biosynthetic agent to produce Ag–Au bimetallic nanoscale composite particles using seed-mediated growth techniques, as Fig. 3a shows (Emam 2019). Acacia gum can be used as both a reducing agent for metal ions in producing nanoscale structures and as a crystal growth modifier for nanocomposites. Silver nitrate, gold chloride and sodium hydroxide are added to the prepared Arabic gum with stirring at room temperature or 70 °C. The order of addition of the alkali and metal salts, the concentration of the glue and the reaction temperature all affect the particle size generated. Increasing the reaction temperature reduces the average size of the bimetallic particles from 6.5 to 3.1 nm. Adding alkali after the metal salt increases the size of Ag–Au composite particles from 3.1 to 12.7 nm.
Typical methods for synthesizing metal-modified nanoscale gold particles. a The seed growth method (reprinted with permission from (Emam 2019), Copyright (2019) Springer); b the precipitation–deposition method; c microwave-assisted continuous chemical reduction
The precipitation–deposition method
A group led by Mishra reports (Mishra et al. 2016) synthesizing Au/Fe2O3 composite particles 19–24 nm in diameter by co-precipitation using Fe(NO3)3·9H2O, Na2CO3 and HAuCl4 as raw materials. They mixed aqueous solutions of Fe(NO3)3·9H2O and HAuCl4 with stirring at 80 °C. An aqueous Na2CO3 solution was added dropwise until the precipitation was complete. After the formation of the precipitate, the solution was cooled to room temperature and aged overnight. After washing, drying and calcining the precipitate at 400 °C for 4 h, the preparation was complete.
Han’s laboratory synthesized mesoporous porous TiO2 by a solvothermal method (Ye et al. 2011), and then deposited Au on the TiO2 by the precipitation–deposition method (Elmoula et al. 2009; Zanella et al. 2002). The mesogenic porous TiO2 anatase was prepared from tetrabutyl titanate. 0.2 mL of tetrabutyl titanate was added dropwise to 10 mL of acetic acid under continuous stirring, which was placed in an autoclave and heated at 200 °C for 24 h. Chloroauric acid was heated in an oil bath to 80 °C and 1 g of the mesoporous TiO2 was added with continuous stirring. After stirring for 5 min, the pH of the suspension was adjusted to 8 with 1 M NaOH solution. After 4 h of vigorous stirring, the suspension was centrifuged, and the precipitate was washed with water and dried. It was then calcined at 300 °C for 4 h and ground for later use. That synthesis is diagrammed in Fig. 3b. Precipitation–deposition is the ideal method for loading gold. The target product can be adjusted by adjusting the concentration of chloroauric acid, the pH and the ratio of gold to the substrate (Moreau et al. 2005).
Microwave-assisted continuous chemical reduction
Adding a third metal can add new dimensions to the properties of bimetallic materials and offer additional scope for their optimization. Yadav and his colleagues successfully synthesized Au/Pt/Ag trimetallic nanoparticles by the microwave-assisted continuous chemical reduction shown in Fig. 3c (Yadav et al. 2018). The gold solution was prepared by sodium citrate reduction. H2PtCl6·xH2O solution and sodium citrate solution were added at room temperature; then, the mixture was heated in a microwave oven to yield a Au/Pt bimetallic nanocomposite. An aqueous solution of AgNO3 was then added and the mixture was microwaved in a loop mode, yielding a black Au/Pt/Ag tri-metal nanocomposite.
Gold nanoparticles modified with organic compounds
Macromolecules can stabilize nanoscale gold particles (AuNPs) and also give them additional functionality. The physicochemical method for obtaining polymer-modified gold nanoparticles has been extensively studied (Dzhardimalieva et al. 2011; Feuz et al. 2012; Raula et al. 2003). There are three generic methods for preparing organic-coated gold nanoparticles.
The interface polymerization method
The interfacial polymerization method is a two-phase system in which an aqueous solution of toluene and chloroauric acid is the oil phase and there is an aqueous phase. The size and morphology of the composite material can be adjusted by controlling the amount of the reactants and the reaction time. The specific preparation process is shown in Fig. 4a. The toluene organic phase is added to the aqueous chloroauric acid solution to form a two-phase interface. Aniline solution is added, and the reaction immediately occurs at the interface, producing a purple-red color which darkens subsequently. Eventually, a black substance appears at the bottom, an Au–polyaniline nanocomposite.
Experiments conducted by Jin’s group (Jin et al. 2016) show that when using interfacial polymerization to synthesize such materials, controlling the amount of chloroauric acid can change the morphology of the Au–polyaniline composite. With 5 mL of 10 mM chloroauric acid nanospheres formed. With 1 mL of 10 mM chloroauric acid lollipop-shaped particles formed. AuNPs modified with organic monomers can be used for monitoring plasma-driven reactions using surface-enhanced Raman imaging. The interface polymerization method does not require adding any reducing agent. Organic monomers acting as ligands can also serve as reducing agents to directly reduce metal ions to metal nanoparticles because the reduction potential of gold-containing compounds is higher than that of organic monomers.
One-step polymerization
The one-step method is another common method for preparing AuNPs modified with organics. Unlike the interfacial polymerization method, in the one-step method the organic compound and the chloroauric acid solution are mixed directly, and the reaction conditions are adjusted to obtain the target product. Li’s group ultrasonically mixed an ethanol solution of aniline with HAuCl4 solution to obtain a wine-red suspension (Li et al. 2016a). Oxidative polymerization of the aniline formed polyaniline, while the chloroauric acid was reduced to metallic gold, as indicated in Fig. 4b. After centrifugation and purification, an Au–polyaniline composite was obtained.
Zhao’s group used indole derivatives as reducing agents and ligands to synthesize indole derivative-modified gold nanoparticles in one step (Zhao et al. 2018). Tween was added to the chloroauric acid solution, stirred to uniformity, and the reaction was continued by adding a indole solution to obtain indole derivative-modified gold nanoparticles. The synthesis of AuNPs modified with other indole derivatives such as tryptophan, 5-aminoindole was consistent with the basic indole derivative-modified gold nanoparticles method. The one-step method involves no complicated preparation steps, avoids material loss and damage and maximizes the original properties of the material. However, the Au–polyaniline composite prepared by this method has a low molecular weight insufficient for some purposes and low conductivity.
Two-step polymerization
There is also a two-step process. The first step is to prepare a polyaniline solution with reducing properties. The second is to reduce the chloroauric acid to obtain Au–polyaniline nanocomposite. A group led by Ma first polymerized aniline; then, HAuCl4 was reduced on polyaniline nanotubes to generate modified AuNPs on the nanotubes (Ma et al. 2016). This is sketched in Fig. 4c. The two-step method can also be used to prepare AuNPs in the first step. The second step is then to mix AuNPs and aniline to obtain Au–polyaniline nanocomposites by in situ polymerization. Compared with the one-step method, the two-step method can better regulate the product’s morphology and particle size. However, the force binding the gold nanoparticles and the polyaniline is weak, and the gold nanoparticles easily fall off.
Carbon-modified gold nanoparticles
Little has been published about the idea of modifying gold nanoscale particles with inorganic carbon. There is, however, a sol–gel method and a one-step in situ growth method. Combining inorganic carbon with nanoscale gold particles gives it more applications by improving the dispersibility, fluorescence and biocompatibility of gold nanoparticles.
The sol–gel method
A group led by Wang has successfully synthesized the composite Au@CdS/g-C3N4 (Wang et al. 2018). Au colloid was first prepared by the citrate reduction method. A suspension of cysteine-capped Au nanoparticles was prepared by mixing cystine into a suspension of the nanoparticles. Cysteine and Cd(NO3)2 were dissolved in deionized water to obtain a cysteine/Cd2+ solution. A certain amount of Au colloid was then added to the cysteine/Cd2+ solution with vigorous stirring. The protonated graphitic carbon nitride (g-C3N4) expanded body was added, stirred, and the mixture was heated to 130 °C for 6 h for hydrothermal reaction. After washing and drying, Au@CdS/g-C3N4 composite was obtained.
The structure of the composite is regulated by controlling the mass ratio of the precursors. This method is suitable for the synthesis of various M@XS/N multi-component complexes. The synthesis steps are shown in Fig. 5. In the figure, M stands for any noble metal nanoparticle, XS stands for sulfide, and N can be a compound with a lamellar structure (such as g-C3N4 or graphene). Note, however, that the pure M@XS product is obtained in only low yield, so the method may not be suitable for large-scale use.
A one-step in situ growth method
Combination with inorganic carbon affects the catalytic activity of nanoscale gold particles (AuNPs). The g-C3N4 is a polymeric semiconductor with good thermal and chemical stability. It has a wide absorption spectrum and can effectively activate oxygen molecules to produce superoxide radicals. Therefore, g-C3N4 has been widely used as visible light-activated catalyst. Fu and his colleagues report uniformly loading 2.8 nm AuNPs on the surface of g-C3N4 nanosheets using a one-step in situ growth method (Fu et al. 2017). The g-C3N4 nanosheet is first prepared using an ultrasonic stripping technique. The chloroauric acid solution is then added to a dispersion of g-C3N4, and sodium borohydride solution is added with stirring to continue the reaction. After centrifugal drying, the Au/g-C3N4 product is obtained. The method is simple and can yield small AuNPs. Studies have shown that the photocatalytic activity of Au/g-C3N4 driven by visible light is significantly improved compared with that of ordinary gold. This may be attributed to the interaction between gold and g-C3N4 causing charge transfer.
Green synthesis using biological extracts
Carbon quantum dots are highly dispersed carbon nanoparticles. They have useful optical properties and good biocompatibility. Very small gold clusters (< 2 nm) have enhanced fluorescence attributed to the oscillation of free electrons within them. Venkateswarlu’s group reports that the synthesis of Au/Carbon quantum dots nanocomposites is promoted by using onion leaf extract as a reducing agent (Venkateswarlu et al. 2019). Onions contain flavonoids and phenolic compounds that act as reducing agents, stabilizers and carbon precursors. The specific preparation steps are as follows. The onion leaves are water-washed and filtered to obtain an extract. The extract is poured into a freshly prepared mixture of chloroauric acid and glutathione and heated in a microwave oven. Freeze-drying then yields the target product. The addition of carbon precursors stabilizes the product for at least 300 days with excellent detection limits for Hg2+.
Antibacterial properties of functionalized gold nanoparticles and the mechanisms involved
The different preparation methods lead to different nanoparticle sizes and size distributions. That in turn affects their antibacterial properties. Differences in surface modification can affect the antibacterial properties of nanoparticles in two ways. One is to change the gold nanoparticles’ morphology, structure and dispersion. The second is to increase their antibacterial effectiveness. For example, the molecules with special optical, electrochemical or biological activity can be connected to the surface of gold nanoparticles by some synthetic methods (Liu and Lammerhofer 2019).
Size, dispersibility and antibacterial activity
Gold’s stability and low toxicity and the high specific surface area and easy functionalization of nanoparticles have meant that nanoscale gold particles (AuNPs) have been widely studied and applied as an effective antibacterial agent (Connor et al. 2005; Dizaj et al. 2014; Gupta et al. 2016). The antibacterial properties of AuNPs are shown in Table 1. The size and dispersibility of nanoparticles are important for their antibacterial effectiveness. Generally speaking, smaller AuNPs 2–15 nm in diameter is more used in tissue immunology, biochemistry and high-powered microscopy. In environmental testing, DNA testing and drug delivery, medium-sized AuNPs with a diameter of 20–60 nm are mostly used. Larger AuNPs with a diameter of 80–250 nm are used in medical, electrical and X-ray optics (Shah et al. 2015). With the morphology of gold nanoparticles changes from dispersed small particles to large aggregates, the color of a suspension of them changes from red to blue.
Silver nanoparticles follow the rule that the smaller the size, the better the antibacterial effect, and gold nanoparticles also follow this pattern. Vanaraj’s group used a methanol extract of C. ternatea leaves to prepare 100 nm AuNPs (Vanaraj et al. 2017). In an anti-biofilm activity experiment against Pseudomonas aeruginosa, the rate of biofilm formation was inhibited 94.4%, but only after the concentration of AuNPs reached 100 μg/mL. Lanh and his colleagues used a seeding method to produce gold nanorods 10 nm in diameter (Lanh et al. 2015), and studies have shown that the minimum inhibitory concentrations of gold nanorods against E. coli, S. Typhimuriumm, S. aureus and L. monocytogenes are 0.05 μg/mL, 0.2 μg/mL, 0.008 μg/mL and 0.0002 μg/mL, respectively.
Emam prepared Au–Ag bimetallic nanocomposites with different particle sizes. Studies have shown that smaller bimetallic nanocomposites exhibit better antibacterial activity. Bimetallic particles 3.1 nm in diameter were observed to eliminate 83.2–87% of bacteria after 6 h. Under the same conditions, however, the bacteriostatic rate of bimetallic nanocomposites with an average particle size of 22.1 nm was only 60.9% (Emam 2019). In addition, small gold nanoclusters 1–2 nm in equivalent diameter can be formed by controlling the reaction conditions. Bacteria can take in such small clusters with nutrients and they then interfere with the bacteria’s normal metabolism. Active oxygen species accumulate in the bacteria and the stability of their cytomembranes is reduced. Cell death is the likely result.
The effect of dispersibility on the antibacterial properties of AuNPs cannot be ignored. The antibacterial advantages of small nanoparticles are limited by their dispersibility. Generally, the proportion of surface atoms increases rapidly when the size of nanoparticles is below 10 nm. The atoms concentrated on the particle’s surface are highly active and tend to stabilize by binding with other atoms. Any aggregation of nanoparticles reduces their contact area with bacteria, which weakens their antibacterial properties. Other studies have shown that small gold nanoparticles can enter cells by binding to the pore proteins and changing the pathway of porin (Wigginton et al. 2010). Reducing the particle size and increasing the dispersibility therefore help to improve nanoparticles’ antibacterial properties.
Various surfactants, stabilizers and reducing agents are usually used in the preparation of AuNPs. The resulting particle size is related to the type and amount of reducing agent. Common reducing agents include sodium citrate, sodium borohydride, ascorbic acid, hydroxylamine hydrochloride, amino acids, H2O2 and others (Liu and Lammerhofer 2019). White phosphorus or ascorbic acid is usually used to prepare AuNPs 5–12 nm in diameter. It is difficult for ascorbic acid to reduce trivalent gold particles to zero, but it can reduce the valence to one. Therefore, ascorbic acid is mainly used in the growth solutions of the seeded growth method. For the preparation of AuNPs with particle size larger than 12 nm, sodium citrate is used. Sodium citrate has weak reducing properties, so larger AuNPs can be prepared, and it acts as a stabilizer at the same time. Sodium borohydride is a strong reducing agent, which dissociates hydrogen ion during the reaction process. Sodium borohydride will show obvious nucleation during the reduction process. It cannot be used together with a strong stabilizer, or no large nanoparticles will be obtained. The size of AuNPs is inversely proportional to the amount of reductant used (Panahi et al. 2017).
In order to maintain stable nanoparticle dispersion, physical and chemical dispersion methods are employed. Chemical dispersion requires selection of an appropriate dispersant, normally a surfactants or polymer, to control particle interactions. Surfactants are mainly used as stabilizers, but they can also control the size and morphology of nanoparticles. Physical dispersion mainly depends on mechanical and ultrasonic dispersion. In addition, the electrostatic repulsion effect of the charged ligand can also achieve the purpose of dispersion (Shah et al. 2015).
Surface functionalization and antibacterial activity
Single nanomaterials cannot meet the growing functional needs of practical applications. Compounding multiple materials can realize better regulation of the material’s structure and function. Nanoscale gold itself is expensive and relatively scarce. Combining it with metals, organic compounds or inorganic carbon can both reduce the cost and increase its functionality. In recent years, bimetallic and trimetallic nanoparticles have been widely used for their unique properties (Karthikeyan and Loganathan 2012; Li et al. 2016b; Luo et al. 2017; Pandey and Pandey 2016; Rao and Paria 2015; Venkatesan and Santhanalakshmi 2010; Wang and Yamauchi 2011; Wang et al. 2016b; Yang et al. 2017). The antibacterial properties of metal-modified nanoscale gold particles (AuNPs) are shown in Table 2. Some organic compounds also exhibit good antibacterial activity. And using inorganic carbon as a supporting material in synthesizing AuNPs can effectively prevent the particles’ agglomeration. The antibacterial properties of AuNPs modified with organic compounds and inorganic carbon are shown in Table 3.
Metal-modified gold nanoparticles
The antibacterial properties of AuNPs can be improved by combining them with other metals, metal oxides or metal hydroxides. Au and Ag at low concentrations are less toxic to normal cells, but more lethal to bacteria. Therefore, silver and gold composites have been widely studied as antimicrobial agents. The diameter of the inhibition zone of the 10 nm Au–Ag nanocomposite prepared by Bankura was 24 nm against B. subtilis, 21 nm against B. cereus, 17 nm against E. coli and 20 nm against P. aeruginosa. And the antibacterial action was superior to that of gold nanoparticles alone (Bankura et al. 2014). Another study showed that Au–Pt nanoaggregates have enhanced antibacterial potential compared to Au and Pt single metal nanoparticles (Britto Hurtado et al. 2020).
Ding’s experiments showed that Au@AgNPs had strong antibacterial activity against S. aureus, with a minimum inhibitory concentration (MIC) of 7.5 pM (Ding et al. 2017). This is because positively charged Au@Ag NPs can aggregate with the surface of negatively charged bacteria, producing strong two-photon photoluminescence. That has a strong photothermal effect, so Au@AgNPs can effectively remove 85% of a bacterial biofilm in 4 min under near-infrared radiation (Ding et al. 2017). Nanostructures with a photothermal effect convert light energy into heat and kill bacteria by local heating. Common photocatalytic nanomaterials include ZnO, TiO2, Fe2O3 and others.
Gholap’s group used a hydrothermal method to prepare Au@ZnO composite at a concentration of 20 μg/mL which reduced the growth of Bacillus subtilis by 99.9% (Gholap et al. 2016). The experiment also found that the antibacterial mechanism of Au@ZnO is the generation of superoxide anion radicals under irradiation.
In addition to bimetallic nanocomposites, trimetallics have also been widely studied and applied. The addition of a third metal can add new dimensions and improve the properties of the materials. A group led by Yadava prepared a Au–Pt–Ag nanoscale fluid by green, microwave-assisted, continuous chemical reduction (Yadav et al. 2018). Trimetallic nanofluids show good antimicrobial activity. When the concentration of the composite was 15.625 mg/mL, the MIC for Gram-positive S. aureus was 31.25 mg/mL and that for E. faecalis was 15.625 mg/mL. For Gram-negative E. coli, Salmonella typhi and Klebsiella the MIC was 15.625 mg/mL. That was better antibacterial activity than bimetal and single metal nanofluids at lower concentration.
Gold nanoparticles modified with organic compounds
Given the antibacterial activity of some organic compounds, researchers have combined them with AuNPs seeking better antibacterial effects than those of gold nanoparticles alone. Indole derivatives, for example, can produce antibacterial compounds after certain chemical reaction, which confirms that they have antibacterial potential.
Zhao’s experiments have shown that when AuNPs are modified with tryptophan and 5-aminopurine, the resulting Au–tryptophan and Au-5-aminopurine composites have excellent antibacterial activity against both bacteria and multi-drug-resistant (MDR) bacteria (Zhao et al. 2018). After 0.5 h of contact with either composite nanoparticle the elimination of viable MDR E. coli and polymyxin-resistant K. pneumoniae reached 99.9%. Even at the high bacterial concentration of 5 × 108 CFU/mL, more than 99.99% of polymyxin-resistant K. pneumoniae was inactivated after 4 h. Hareesh and his colleagues prepared a hexagonal gold–polycarbonate matrix of 110 nm particles by a radiation-assisted method (Hareesh et al. 2015). Their experiments showed good antibacterial action against Gram-positive and Gram-negative bacteria and showed the inhibitory effect of a dose of gamma radiation on the overall growth of bacteria. Two of the nanocomposites synthesized by Boomi’s group through chemical methods were polyaniline/Ag–Au and polyaniline/Au–Pd (Boomi and Prabu. 2013; Boomi et al. 2014). Gold colloids did not show any inhibition zone for the four pathogens they studied, but when modified by polyaniline they showed inhibitory effects on all of the pathogens. They were also stable for several months.
Gold nanoparticles modified with carbon
One way to improve the antibacterial properties of nanomaterials is to synthesize gold nanoparticles on a supporting material. This can effectively solve the problem of nanoparticle agglomeration. A group led by Gao synthesized a stable and dispersed Au/C shell-nucleus composite by a redox method (Gao et al. 2010). Au deposited on the surface of carbon spheres formed a gold shell 10 nm thick. Gold nanoparticles can inhibit microorganisms’ growth better when dispersed on the surface of carbon spheres because they have more opportunities to contact bacteria.
Antibacterial mechanisms
As the problem of clinical bacteria resistance becomes more and more serious, single antibiotics have begun to lose bacteriostatic effectiveness. The hydrophilicity of some antibiotics causes them to cross through the water-filled channels of bacteria’s membrane pore proteins. That leads to short residence time in the cells and weakens the bactericidal effect. The combination of nanogold and antibiotics has significantly improved antibacterial ability.
Gold nanoparticles sometimes break down cell walls in entering cells. They can thus help some antibiotics that cannot penetrate the cell wall barrier. The Au/AgNPs@Vancomycin synthesized by Lu’s group in one step showed better antibacterial activity and weaker bacterial resistance than free vancomycin (Lu et al. 2017). The effect of vancomycin is to increase the adsorption of bacteria on Au/AgNPs@Vancomycin particles. Au/AgNPs@Vancomycin showed high antibacterial activity against Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria, with minimum inhibitory concentration (MIC) as low as 30 nmol mL−1. 99% of the bacteria were killed within 5 h. Ag/AuNPs@Van2’s cytotoxicity was lower. A group led by Khandelwal has synthesized nanoscale gold particles (AuNPs) labeled with cefradine (an antibiotic) (Khandelwal et al. 2015). A small amount of gentamycin sulfate combined with AuNPs can obtain more significant antibacterial properties than a single antibiotic (Fiori-Duarte et al. 2020; Zhou et al. 2020; Zou et al. 2020).
Experiments have shown that inhibition by AuNPs results from direct contact which induces cell wall rupture rather than by generating reactive oxygen species (ROS). Wang’s laboratory synthesized Au@Ag-loaded Fe3O4 magnetic nanoparticles using a polyethyleneimine-assisted ligation method (Wang et al. 2016c). They showed good antibacterial activity against Gram-negative E. coli and Gram-positive bacteria S. aureus. In addition, streptomycin modification can significantly improve the antibacterial activity of Fe3O4–Au@Ag nanoparticles. Streptomycin modification decreased the MIC by 25% for E. coli and 40% for S. aureus, indicating synergy between Fe3O4–Au@Ag and antibiotics.
A group led by Coradeghini has tested the idea that AuNPs’ uptake into cells increases with smaller particle size (Coradeghini et al. 2013). They found some effect of particle size on antibacterial mechanisms. Smaller nanoparticles can enter cells. Larger ones cause cell lysis and death by acting on the surface. However, there is as yet no complete description of these mechanisms. The contact area of highly dispersed nanoparticles is larger, and their antibacterial activity is obvious.
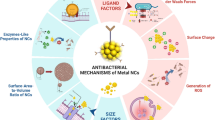
Differences in surface modification also make a difference. The action modes of nanoparticles mainly include damaging the cytoderm, damaging cytomembranes and modifying the interior of bacterial cells. Alternatively, some nanoparticles modified with a photocatalytic metal rely on light excitation to generate free radicals for antibacterial purposes. The main antibacterial mechanisms of AuNPs are shown in Fig. 6.
Possible antibacterial mechanisms of gold nanoparticles (AuNPs). a Positively-charged nanoparticles electrostatically adsorbed to the negatively-charged cytoderm; b the accumulation of nanoparticles disrupts the intra-cellular balance, resulting in reactive oxygen species (ROS) oxidation of biofilms and oxidative stress; c the combination of gold ions and proteins destroys the microbes’ respiratory systems and electron transport chains; d the ROS damage cellular DNA and proteins; e metal ions react with sulfhydryl groups to destroy cellular synthetase activity
Cytoderm damage
Cytoderm damage is results from the electrostatic attraction between positively charged nanoparticles and the negatively charged cytoderm, as indicated in Fig. 6a. Nanoparticles attached to the cytoderm can penetrate the cell by releasing copious ions to produce toxicity. Scanning electron microscopy has shown that AuNPs labeled with cefradine cause cell wall damage through direct physical contact. Moreover, the measurement of ROS through fluorescent probes ruled out the possibility of ROS leading to the observed loss of cell viability (Khandelwal et al. 2015). Experiments have shown that gold nanorods causes membrane damage through electrostatic attraction between the gold and the cytoderm, which eventually leading to cell death (Arockia Jency et al. 2020; Lanh et al. 2015).
Interestingly, many studies have found that gold nanoparticles have different effects on Gram-negative and Gram-positive bacteria. Gao’s group dispersed gold nanoparticles uniformly on the surface of carbon spheres. Such Au/C core–shell nanomaterials proved more effective in killing Gram-negative than Gram-positive bacteria. The MIC for E. coli was 3200 μg/mL. Only when the concentration of the composite reached 5000 μg/mL did it show good antibacterial ability against Gram-positive bacteria (Gao et al. 2010). This may be due to differences in the cytoderms. Gram-positive bacteria contain a large amount of peptidoglycan and have a thick cytoderm (20–80 nm). Gram-negative bacteria only have a thin layer of peptidoglycan, and the cytoderm is thin (10 nm). The metal ions released by the nanoparticles can more easily invade the thin-walled Gram-negative bacteria. But not all the research results agree. Therefore, in addition to the influence of the cell wall structure, there are other factors.
Cytomembrane damage
Biofilms are extracellular molecules wrapped around the surface of bacteria. Their high resistance is one thing that makes bacterial diseases difficult to cure. Bacteria produce ROS when exposed to gold nanoparticles. Because the cell membrane is negatively charged, it is not easily penetrated by negatively charged reactive oxygen species. However, H2O2 can penetrate the cell membrane and induce cell membrane damage (Padmavathy and Vijayaraghavan 2008).
Another way for nanoparticles to play an antibacterial role is to break up the cytomembrane and let the cell contents flow out. The adsorption of gold nanoparticles on the membrane can cause the necessary membrane damage (Liu et al. 2006) as indicated in Fig. 6b. The TEM results published by Zhao show that the cytomembranes of bacteria treated with Au–tryptophan and Au-5-aminopurine nanocomposites were broken, while the cytomembranes of untreated bacteria were intact (Zhao et al. 2018). This suggests that the antibacterial mechanism of gold nanocomposites is the destruction of cell membrane.
Photothermal therapy uses near-infrared radiation to induce AuNPs to produce surface plasmon resonance, which destroys the cytomembrane structure and kills bacteria by local heating (Aksoy et al. 2020; Hsiao et al. 2015). Zhang’s group synthesized a series of gold composites with modified titanium oxide using a hot sol method (Zhang et al. 2016). Experiments show that surface plasmon resonance produced by AuNPs can significantly improve the visible light absorption characteristics of TiO2. The 1% Au–TiO2 composite was able to kill E. coli in the dark. After 5 h of treatment, the survival rate of E. coli was 73.3%. The cell death may have been due to electrons generated by the Au nanoparticles on the cytomembrane (Li et al. 2014c).
Gold ion release
The antibacterial activity of gold nanoparticles is partly due to the release of gold ions (Wang et al. 2014). The higher the added concentration of gold nanoparticles, the more gold ions will be released, and the better the antibacterial effect will be (Tamayo et al. 2014). Smaller nanoparticles release Au+ faster due to their greater specific surface area. When bacteria are exposed to the nanoparticles, the Au+ released is evenly distributed around the bacteria. They penetrate cell walls and enter the cells where they can react with thiol groups to form Au-thiol groups. Thiol groups on cysteine can cause protein coagulation. Disulfide bridging between cysteine residues is involved in protein folding (Slavin et al. 2017). The combination of gold ions and cysteine also disrupts the microbes’ respiration and electron transport systems, as indicated in Fig. 6c. There are also mercaptan groups in the cell wall synthetase pathway. Binding sulfhydryl groups destroys synthase activity, halting cell division and proliferation and causing cell death. The process is represented in Fig. 6e.
Internal effects on bacterial cells
Active oxygen cane be oxygen-containing free radicals or forms of peroxide without free radicals. It is formed in basic metabolism. Reactive oxygen maintained at an appropriate level has a positive effect on cells. However, the level of reactive oxygen species rises significantly when cells face external stimuli. Excess reactive oxygen species can then have negative effects. AuNPs in contact with bacteria generate O2−, H2O2, HO2· and ·OH. These ROS cause oxidative stress which leads to lipid peroxidation in the cytomembrane. That is, ROS react with macromolecular substances such as phospholipids, enzymes and nucleic acids of the cytomembrane to form lipid peroxidation products. That increases the cytomembrane’s permeability, which eventually leads to changes in cell structure and function.
In addition, AuNPs can cause bacterial death by causing ROS production within cells, resulting in protein aggregation and DNA destruction as Fig. 6d illustrates (Wang et al. 2016b). The mechanisms of action of ROS are shown in Fig. 7 (Slavin et al. 2017). He and his colleagues synthesized ZnO/Au hybrid nanostructures using a photoreduction method and used electrons photoinduced by ZnO to reduce Au3+ on the ZnO surface (He et al. 2014). Gold was deposited on the ZnO surface, which caused ·OH, O2− and H2O2 to be generated by light excitation. This is the main mechanism of its antibacterial ability. The antibacterial efficiency of ZnO/Au composite nanomaterials against E. coli is three times that of ZnO alone. In addition to lipid peroxidation, oxidative stress can also cause damage in the form of DNA strand breaks and base modifications, as indicated in Fig. 6d. Gold nanoparticles that enter the cell affect protein synthesis by inhibiting the binding of ribosomal subunits to tRNA.
In practical applications, gold nanoparticles must not only have excellent antibacterial ability, but also maintain low toxicity to normal cells. AuNPs have good biocompatibility (Norouzi 2020). Small AuNPs may enter normal cells with nutrients. It is worth noting that the decomposition of foreign substances in the lysosomes of normal cells can partially antagonize the adverse effects of AuNPs. Lysosomes are not found in bacteria. In other words, AuNPs can exert their antibacterial effect without damaging normal cells.
Conclusion
There are two main approaches useful for preparing functionalized nanoscale gold composites. One is to optimize the material’s particle size, morphology and dispersibility by controlling the reaction conditions for the best performance. The other is to bolster the nanomaterial’s properties with ligands. The preparation of nanoscale gold mainly relies on the seeded growth method, green synthesis with plant extracts or chemical reduction. The resulting material can then be functionalized. The antibacterial properties of nanoscale gold particles (AuNPs) are closely related to the particle size, dispersibility and surface modification of the nanoparticles. AuNPs mainly cause cell lysis and death by electrostatic adsorption on the bacterial surface, membrane damage and reactive oxygen species (ROS) damage to proteins and DNA. AuNPs have good biocompatibility and no cytotoxicity.
At present, most of the antibacterial studies with AuNPs have been with conventional bacteria. There have been relatively few studies with multi-drug-resistant bacteria. That is an essential direction for future research. Antibacterial activity is expressed in terms of minimum inhibitory concentration (MIC), zone of inhibition, bacteria reduction, survival percentage and the rate of bacteria inactivation. Because of the different units, it is difficult to make objective comparisons among different studies. This is one of many aspects of nanoscience that need to be standardized. In addition, combining theoretical research findings with clinical application is a difficult point. There are still no firm conclusions for many issues. Among them are the site and specificity of nanoparticles’ action on the cytomembrane and their transmembrane action. Whether AuNPs still have good long-term biocompatibility in vivo also requires further verification.
Abbreviations
- AuNPs:
-
Nanoscale gold particles
- g-C3N4 :
-
Graphitic carbon nitride
- MDR:
-
Multi-drug resistant
- MIC:
-
Minimum inhibitory concentration
- MRSA:
-
Methicillin-resistant S. aureus
- ROS:
-
Reactive oxygen species
References
Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM (2013) Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem 10:S3029–S3039. https://doi.org/10.1016/j.arabjc.2013.11.044
Aksoy I, Kucukkececi H, Sevgi F, Metin O, Hatay Patir I (2020) Photothermal antibacterial and antibiofilm activity of black phosphorus/gold nanocomposites against pathogenic bacteria. ACS Appl Mater Interfaces 12(24):26822–26831. https://doi.org/10.1021/acsami.0c02524
Allahverdiyev AM, Abamor ES, Bagirova M, Rafailovich M (2011) Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol 6(8):933–940. https://doi.org/10.2217/fmb.11.78
Annamalai A, Christina VL, Sudha D, Kalpana M, Lakshmi PT (2013) Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloid Surface B 108:60–65. https://doi.org/10.1016/j.colsurfb.2013.02.012
Arockia Jency D, Sathyavathi K, Umadevi M, Parimaladevi R (2020) Enhanced bioactivity of Fe3O4–Au nanocomposites—a comparative antibacterial study. Mater Lett. https://doi.org/10.1016/j.matlet.2019.126795
Ayaz Ahmed KB, Raman T, Anbazhagan V (2016) Platinum nanoparticles inhibit bacteria proliferation and rescue zebrafish from bacterial infection. RSC Adv 6(50):44415–44424. https://doi.org/10.1039/c6ra03732a
Bankura K, Maity D, Mollick MM, Mondal D, Bhowmick B, Roy I, Midya T, Sarkar J, Rana D, Acharya K, Chattopadhyay D (2014) Antibacterial activity of Ag-Au alloy NPs and chemical sensor property of Au NPs synthesized by dextran. Carbohydr Polym 107:151–157. https://doi.org/10.1016/j.carbpol.2014.02.047
Boomi P, Prabu HG (2013) Synthesis, characterization and antibacterial analysis of polyaniline/Au–Pd nanocomposite. Colloid Surface A Physicochem Eng Asp 429:51–59. https://doi.org/10.1016/j.colsurfa.2013.03.053
Boomi P, Prabu HG, Mathiyarasu J (2013) Synthesis and characterization of polyaniline/Ag-Pt nanocomposite for improved antibacterial activity. Colloid Surface B 103:9–14. https://doi.org/10.1016/j.colsurfb.2012.10.044
Boomi P, Prabu HG, Manisankar P, Ravikumar S (2014) Study on antibacterial activity of chemically synthesized PANI-Ag–Au nanocomposite. Appl Surf Sci 300:66–72. https://doi.org/10.1016/j.apsusc.2014.02.003
Boomi P, Poorani GP, Selvam S, Palanisamy S, Jegatheeswaran S, Anand K, Balakumar C, Premkumar K, Prabu HG (2020) Green biosynthesis of gold nanoparticles using Croton sparsiflorus leaves extract and evaluation of UV protection, antibacterial and anticancer applications. Appl Organomet Chem. https://doi.org/10.1002/aoc.5574
Britto Hurtado R, Cortez-Valadez M, Flores-Lopez NS, Flores-Acosta M (2020) Agglomerates of Au–Pt bimetallic nanoparticles: synthesis and antibacterial activity. Gold Bull 53(2):93–100. https://doi.org/10.1007/s13404-020-00277-y
Brown AN, Smith K, Samuels TA, Lu J, Obare SO, Scott ME (2012) Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol 78(8):2768–2774. https://doi.org/10.1128/AEM.06513-11
Brust M, Fink J, Bethell D, Schiffrin DJ, Kiely C (1955) Synthesis and reactions of functionalised gold nanoparticles. J Chem Soc Chem Commun 16(16):1655–1656. https://doi.org/10.1039/c39950001655
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1(3):325–327. https://doi.org/10.1002/smll.200400093
Coradeghini R, Gioria S, García CP, Nativo P, Franchini F, Gilliland D, Ponti J, Rossi F (2013) Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol Lett 217(3):205–216. https://doi.org/10.1016/j.toxlet.2012.11.022
Ding X, Yuan P, Gao N, Zhu H, Yang YY, Xu QH (2017) Au–Ag core-shell nanoparticles for simultaneous bacterial imaging and synergistic antibacterial activity. Nanomedicine 13(1):297–305. https://doi.org/10.1016/j.nano.2016.09.003
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K (2014) Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl 44:278–284. https://doi.org/10.1016/j.msec.2014.08.031
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779. https://doi.org/10.1039/c1cs15237h
Dzhardimalieva GI, Pomogailo AD, Golubeva ND, Pomogailo SI, Roshchupkina OS, Novikov GF, Rozenberg AS, Leonowicz M (2011) Metal-containing nanoparticles with core-polymer shell structure. Colloid J 73(4):458–466. https://doi.org/10.1134/s1061933x11040041
Elmoula MA, Panaitescu E, Phan M, Yin D, Richter C, Lewis LH, Menon L (2009) Controlled attachment of gold nanoparticles on ordered titania nanotube arrays. J Mater Chem 19(26):4483–4487. https://doi.org/10.1039/b903197a
Emam HE (2019) Arabic gum as bio-synthesizer for Ag–Au bimetallic nanocomposite using seed-mediated growth technique and its biological efficacy. J Polym Environ 27(1):210–223. https://doi.org/10.1007/s10924-018-1331-3
Feuz L, Hook F, Reimhult E (2012) Design of intelligent surface modifications and optimal liquid handling for nanoscale bioanalytical sensors. Surfce Modif Liq Handl Biosens. https://doi.org/10.1002/9781118181249.ch3
Fiori-Duarte AT, de Paiva REF, Manzano CM, Lustri WR, Corbi PP (2020) Silver(I) and gold(I) complexes with sulfasalazine: spectroscopic characterization, theoretical studies and antiproliferative activities over Gram-positive and Gram-negative bacterial strains. J Mol Struct 1214(2020):128158. https://doi.org/10.1016/j.molstruc.2020.128158
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 241(105):20–22. https://doi.org/10.1038/physci241020a0
Fu Y, Huang T, Jia B, Zhu J, Wang X (2017) Reduction of nitrophenols to aminophenols under concerted catalysis by Au/g-C3N4 contact system. Appl Catal B Environ 202:430–437. https://doi.org/10.1016/j.apcatb.2016.09.051
Gao Y-H, Zhang N-C, Zhong Y-W, Cai H-H, Liu Y-l (2010) Preparation and characterization of antibacterial Au/C core-shell composite. Appl Surf Sci 256(22):6580–6585. https://doi.org/10.1016/j.apsusc.2010.04.051
Gholap H, Warule S, Sangshetti J, Kulkarni G, Banpurkar A, Satpute S, Patil R (2016) Hierarchical nanostructures of Au@ZnO: antibacterial and antibiofilm agent. Appl Microbiol Biotechnol 100(13):5849–5858. https://doi.org/10.1007/s00253-016-7391-1
Gonzalez-Rubio G, Kumar V, Llombart P, Diaz-Nunez P, Bladt E, Altantzis T, Bals S, Pena-Rodriguez O, Noya EG, MacDowell LG (2019) Disconnecting symmetry breaking from seeded growth for the reproducible synthesis of high quality gold nanorods. ACS Nano 13(4):4424–4435. https://doi.org/10.1021/acsnano.8b09658
Gupta A, Moyano DF, Parnsubsakul A, Papadopoulos A, Wang LS, Landis RF, Das R, Rotello VM (2016) Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl Mater Interfaces 8(22):14096–14101. https://doi.org/10.1021/acsami.6b02548
Hammer B, Norskov JK (1995) Why gold is the noblest of all the metals. Nature 376(6537):238–240. https://doi.org/10.1038/376238a0
Hareesh K, Deore AV, Dahiwale SS, Sanjeev G, Kanjilal D, Ojha S, Dhole NA, Kodam KM, Bhoraskar VN, Dhole SD (2015) Antibacterial properties of Au doped polycarbonate synthesized by gamma radiation assisted diffusion method. Radiat Phys Chem 112:97–103. https://doi.org/10.1016/j.radphyschem.2015.03.023
He W, Kim HK, Wamer WG, Melka D, Callahan JH, Yin JJ (2014) Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J Am Chem Soc 136(2):750–757. https://doi.org/10.1021/ja410800y
Hsiao C-W, Chen H-L, Liao Z-X, Sureshbabu R, Hsiao H-C, Lin S-J, Chang Y, Sung H-W (2015) Effective photothermal killing of pathogenic bacteria by using spatially tunable colloidal gels with nano-localized heating sources. Adv Funct Mater 25(5):721–728. https://doi.org/10.1002/adfm.201403478
Hu Y, Zhang T, Jiang L, Yao S, Ye H, Lin K, Cui C (2019) Removal of sulfonamide antibiotic resistant bacterial and intracellular antibiotic resistance genes by UVC-activated peroxymonosulfate. Chem Eng J 368:888–895. https://doi.org/10.1016/j.cej.2019.02.207
Huo D, He J, Li H, Yu H, Shi T, Feng Y, Zhou Z, Hu Y (2014) Fabrication of Au@Ag core-shell NPs as enhanced CT contrast agents with broad antibacterial properties. Colloid Surface B 117:29–35. https://doi.org/10.1016/j.colsurfb.2014.02.008
Jin W, Han L, Han X, Zhang B, Xu P (2016) Interfacial synthesis of lollipop-like Au-polyaniline nanocomposites for catalytic applications. RSC Adv 6(85):81983–81988. https://doi.org/10.1039/c6ra15446h
Karthikeyan B, Loganathan B (2012) Strategic green synthesis and characterization of Au/Pt/Ag trimetallic nanocomposites. Mater Lett 85:53–56. https://doi.org/10.1016/j.matlet.2012.06.070
Khan AU, Yuan Q, Wei Y, Khan SU, Tahir K, Khan ZUH, Ahmad A, Ali F, Ali S, Nazir S (2016) Longan fruit juice mediated synthesis of uniformly dispersed spherical AuNPs: cytotoxicity against human breast cancer cell line MCF-7, antioxidant and fluorescent properties. RSC Adv 6(28):23775–23782. https://doi.org/10.1039/c5ra27100b
Khan ZUH, Khan A, Chen Y, Ullah Khan A, Shah NS, Muhammad N, Murtaza B, Tahir K, Khan FU, Wan P (2017) Photo catalytic applications of gold nanoparticles synthesized by green route and electrochemical degradation of phenolic Azo dyes using AuNPs/GC as modified paste electrode. J Alloy Compd 725:869–876. https://doi.org/10.1016/j.jallcom.2017.07.222
Khan SA, Shahid S, Lee CS (2020) Green synthesis of gold and silver nanoparticles using leaf extract of clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. https://doi.org/10.3390/biom10060835
Khandelwal P, Singh DK, Sadhu S, Poddar P (2015) Study of the nucleation and growth of antibiotic labeled Au NPs and blue luminescent Au8 quantum clusters for Hg2+ ion sensing, cellular imaging and antibacterial applications. Nanoscale 7(47):19985–20002. https://doi.org/10.1039/c5nr05619e
Lagashetty A, Ganiger SK, Shashidhar (2019) Synthesis, characterization and antibacterial study of Ag–Au Bi-metallic nanocomposite by bioreduction using piper betle leaf extract. Heliyon 5(12):e02794. https://doi.org/10.1016/j.heliyon.2019.e02794
Lanh LT, Hoa TT, Cuong ND, Khieu DQ, Quang DT, Van Duy N, Hoa ND, Van Hieu N (2015) Shape and size controlled synthesis of Au nanorods: H2S gas-sensing characterizations and antibacterial application. J Alloy Compd 635:265–271. https://doi.org/10.1016/j.jallcom.2015.02.146
Lee KD, Nagajyothi PC, Sreekanth TVM, Park S (2015) Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J Ind Eng Chem 26:67–72. https://doi.org/10.1016/j.jiec.2014.11.016
Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF, Sahar A, Riley MA, Rotello VM (2014a) Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8(10):10682–10686. https://doi.org/10.1021/nn5042625
Li J, Zhou H, Qian S, Liu Z, Feng J, Jin P, Liu X (2014b) Plasmonic gold nanoparticles modified titania nanotubes for antibacterial application. Appl Phys Lett 104(26):261110(261111–261115). https://doi.org/10.1063/1.4885401
Li R, Li Z, Wu Q, Li D, Shi J, Chen Y, Yu S, Ding T, Qiao C (2016a) One-step synthesis of monodisperse AuNPs@PANI composite nanospheres as recyclable catalysts for 4-nitrophenol reduction. J Nanopart Res 18(6):142. https://doi.org/10.1007/s11051-016-3452-8
Li J, Lv L, Zhang G, Zhou X, Shen A, Hu J (2016b) Core–shell fructus Broussonetia-like Au@Ag@Pt nanoparticles as highly efficient peroxidase mimetics for supersensitive resonance-enhanced Raman sensing. Anal Methods 8(9):2097–2105. https://doi.org/10.1039/c5ay03124a
Li D, Liu N, Gao Y, Lin W, Li C (2017) Thermosensitive polymer stabilized core-shell AuNR@Ag nanostructures as “smart” recyclable catalyst. J Nanopart Res 19(11):377. https://doi.org/10.1007/s11051-017-4070-9
Liu S, Lammerhofer M (2019) Functionalized gold nanoparticles for sample preparation: a review. Electrophoresis. https://doi.org/10.1002/elps.201900111
Liu N, Chen X-G, Park H-J, Liu C-G, Liu C-S, Meng X-H, Yu L-J (2006) Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr Polym 64:60–65. https://doi.org/10.1016/j.carbpol.2005.10.028
Lu Z, Zhang J, Yu Z, Liu X, Zhang Z, Wang W, Wang X, Wang Y, Wang D (2017) Vancomycin-hybrid bimetallic Au/Ag composite nanoparticles: preparation of the nanoparticles and characterization of the antibacterial activity. New J Chem 41(13):5276–5279. https://doi.org/10.1039/c7nj01660c
Luo L, Duan Z, Li H, Kim J, Henkelman G, Crooks RM (2017) Tunability of the adsorbate binding on bimetallic alloy nanoparticles for the optimization of catalytic hydrogenation. J Am Chem Soc 139(15):5538–5546. https://doi.org/10.1021/jacs.7b01653
Ma X, Yang J, Cai W, Zhu G, Liu J (2016) Preparation of Au nanoparticles decorated polyaniline nanotube and its catalytic oxidation to ascorbic acid. Chem Res Chin U 32(4):702–708. https://doi.org/10.1007/s40242-016-5460-8
Mishra M, Park H, Chun D-M (2016) Photocatalytic properties of Au/Fe2O3 nano-composites prepared by co-precipitation. Adv Powder Technol 27(1):130–138. https://doi.org/10.1016/j.apt.2015.11.009
Moreau F, Bond G, Taylor A (2005) Gold on titania catalysts for the oxidation of carbon monoxide: control of pH during preparation with various gold contents. J Catal 231(1):105–114. https://doi.org/10.1016/j.jcat.2005.01.030
Nirmala Grace A, Pandian K (2007) Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloid Surface A 297(1–3):63–70. https://doi.org/10.1016/j.colsurfa.2006.10.024
Nithya P, Sundrarajan M (2020) Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: antibacterial and anti-cancer activities. J Photochchem Photobiol B Biol 202:111706. https://doi.org/10.1016/j.jphotobiol.2019.111706
Norouzi M (2020) Gold nanoparticles in glioma theranostics. Pharmacol Res 156(2020):104753. https://doi.org/10.1016/j.phrs.2020.104753
Padmavathy N, Vijayaraghavan R (2008) Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater 9(3):035004. https://doi.org/10.1088/1468-6996/9/3/035004
Panahi Y, Mohammadhosseini M, Nejati-Koshki K, Abadi AJ, Moafi HF, Akbarzadeh A, Farshbaf M (2017) Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug Res (Stuttg) 67(2):77–87. https://doi.org/10.1055/s-0042-115171
Pandey PC, Pandey G (2016) One-pot two-step rapid synthesis of 3-aminopropyltrimethoxysilane-mediated highly catalytic Ag@(PdAu) trimetallic nanoparticles. Catal Sci Technol 6(11):3911–3917. https://doi.org/10.1039/c5cy02040a
Rao KJ, Paria S (2015) Mixed phytochemicals mediated synthesis of multifunctional Ag–Au–Pd nanoparticles for glucose oxidation and antimicrobial applications. ACS Appl Mater Inter 7(25):14018–14025. https://doi.org/10.1021/acsami.5b03089
Raula J, Shan J, Nuopponen M, Niskanen A, Jiang H, Kauppinen EI, Tenhu H (2003) Synthesis of gold nanoparticles grafted with a thermoresponsive polymer by surface-induced reversible-addition-fragmentation chain-transfer polymerization. Langmuir 19(8):3499–3504. https://doi.org/10.1021/la026872r
Shah M, Badwaik V, Kherde Y, Waghwani HK, Modi T, Aguilar ZP, Rodgers H, Hamilton W, Marutharaj T, Webb C, Lawrenz MB, Dakshinamurthy R (2015) Gold nanoparticles: various methods of synthesis and antibacterial applications. Front Biosci 19:1320–1344. https://doi.org/10.2741/4284
Slavin YN, Asnis J, Hafeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol 15(1):65. https://doi.org/10.1186/s12951-017-0308-z
Sun Z, Zheng W, Zhu G, Lian J, Wang J, Hui P, He S, Chen W, Jiang X (2019) Albumin broadens the antibacterial capabilities of nonantibiotic small molecule-capped gold nanoparticles. ACS Appl Mater Interfaces 11(49):45381–45389. https://doi.org/10.1021/acsami.9b15107
Tamayo LA, Zapata PA, Vejar ND, Azócar MI, Gulppi MA, Zhou X, Thompson GE, Rabagliati FM, Páez MA (2014) Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater Sci Eng C 40:24–31. https://doi.org/10.1016/j.msec.2014.03.037
Valodkar M, Modi S, Pal A, Thakore S (2011) Synthesis and anti-bacterial activity of Cu, Ag and Cu–Ag alloy nanoparticles: a green approach. Mater Res Bull 46(3):384–389. https://doi.org/10.1016/j.materresbull.2010.12.001
Vanaraj S, Jabastin J, Sathiskumar S, Preethi K (2017) Production and characterization of bio-AuNPs to induce synergistic effect against multidrug resistant bacterial biofilm. J Clust Sci 28(1):227–244. https://doi.org/10.1007/s10876-016-1081-0
Venkatesan P, Santhanalakshmi J (2010) Designed synthesis of Au/Ag/Pd trimetallic nanoparticle-based catalysts for Sonogashira coupling reactions. Langmuir 26(14):12225–12229. https://doi.org/10.1021/la101088d
Venkateswarlu S, Govindaraju S, Sangubotla R, Kim J, Lee MH, Yun K (2019) Biosynthesized highly stable Au/C nanodots: ideal probes for the selective and sensitive detection of Hg(2+) ions. Nanomaterials (Basel) 9(2):245. https://doi.org/10.3390/nano9020245
Vilas V, Philip D, Mathew J (2016) Biosynthesis of Au and Au/Ag alloy nanoparticles using Coleus aromaticus essential oil and evaluation of their catalytic, antibacterial and antiradical activities. J Mol Liq 221:179–189. https://doi.org/10.1016/j.molliq.2016.05.066
Wang L, Yamauchi Y (2011) Strategic synthesis of trimetallic Au@Pd@Pt Core-Shell nanoparticles from poly(vinylpyrrolidone)-based aqueous solution toward highly active electrocatalysts. Chem Mater 23(9):2457–2465. https://doi.org/10.1021/cm200382s
Wang L, He H, Yu Y, Sun L, Liu S, Zhang C, He L (2014) Morphology-dependent bactericidal activities of Ag/CeO2 catalysts against Escherichia coli. J Inorg Biochem 135:45–53. https://doi.org/10.1016/j.jinorgbio.2014.02.016
Wang J, Li J, Guo G, Wang Q, Tang J, Zhao Y, Qin H, Wahafu T, Shen H, Liu X, Zhang X (2016a) Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: new insight into the antimicrobial action of silver. Sci Rep 6:32699. https://doi.org/10.1038/srep32699
Wang Y, Wan J, Miron RJ, Zhao Y, Zhang Y (2016b) Antibacterial properties and mechanisms of gold–silver nanocages. Nanoscale 8(21):11143–11152. https://doi.org/10.1039/c6nr01114d
Wang C, Xu S, Zhang K, Li M, Li Q, Xiao R, Wang S (2016c) Streptomycin-modified Fe3O4–Au@Ag core-satellite magnetic nanoparticles as an effective antibacterial agent. J Mater Sci 52(3):1357–1368. https://doi.org/10.1007/s10853-016-0430-6
Wang G, Feng H, Jin W, Gao A, Peng X, Li W, Wu H, Li Z, Chu PK (2017a) Long-term antibacterial characteristics and cytocompatibility of titania nanotubes loaded with Au nanoparticles without photocatalytic effects. Appl Surf Sci 414:230–237. https://doi.org/10.1016/j.apsusc.2017.04.053
Wang Z, Dong K, Liu Z, Zhang Y, Chen Z, Sun H, Ren J, Qu X (2017b) Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 113:145–157. https://doi.org/10.1016/j.biomaterials.2016.10.041
Wang H, Peng D, Hu X, Ma X, Ye W, Zhang W, Chang Y, Dong S (2018) Target preparation of multicomponent composites Au@CdS/g-C3N4 as efficient visible light photocatalysts with the assistance of biomolecules. Mater Res Bull 108:176–186. https://doi.org/10.1016/j.materresbull.2018.09.009
Wang X, Guo J, Zhang Q, Zhu S, Liu L, Jiang X, Wei DH, Liu RS, Li L (2020) Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnol 31(13):134004. https://doi.org/10.1088/1361-6528/ab59eb
Wigginton NS, Titta AD, Piccapietra F, Dobias J, Nesatyy VJ, Suter MJF, Bernier-Latmani R (2010) Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity. Environ Sci Technol 44(6):2163–2168. https://doi.org/10.1021/es903187s
Yadav N, Jaiswal AK, Dey KK, Yadav VB, Nath G, Srivastava AK, Yadav RR (2018) Trimetallic Au/Pt/Ag based nanofluid for enhanced antibacterial response. Mater Chem Phys 218:10–17. https://doi.org/10.1016/j.matchemphys.2018.07.016
Yang S, Rui KH, Tang XY, Xu Q, Shi M (2017) Rhodium/silver synergistic catalysis in highly enantioselective cycloisomerization/cross coupling of keto-vinylidenecyclopropanes with terminal alkynes. J Am Chem Soc 139(16):5957–5964. https://doi.org/10.1021/jacs.7b02027
Ye J, Liu W, Cai J, Chen S, Zhao X, Zhou H, Qi L (2011) Nanoporous anatase TiO2 mesocrystals: additive-free synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior. J Am Chem Soc 133(4):933–940. https://doi.org/10.1021/ja108205q
Yuan CG, Huo C, Gui B, Cao WP (2017) Green synthesis of gold nanoparticles using Citrus maxima peel extract and their catalytic/antibacterial activities. IET Nanobiotechnol 11(5):523–530. https://doi.org/10.1049/iet-nbt.2016.0183
Zanella R, Giorgio S, Henry CR, Louis C (2002) Alternative methods for the preparation of gold nanoparticles supported on TiO2. J Phys Chem B 106(31):7634–7642. https://doi.org/10.1021/jp0144810
Zhang J, Suo X, Zhang J, Han B, Li P, Xue Y, Shi H (2016) One-pot synthesis of Au/TiO2 heteronanostructure composites with SPR effect and its antibacterial activity. Mater Lett 162:235–237. https://doi.org/10.1016/j.matlet.2015.09.136
Zhao X, Jia Y, Li J, Dong R, Zhang J, Ma C, Wang H, Rui Y, Jiang X (2018) Indole derivative-capped gold nanoparticles as an effective bactericide in vivo. ACS Appl Mater Interfaces 10(35):29398–29406. https://doi.org/10.1021/acsami.8b11980
Zhou L, Yu K, Lu F, Lan G, Dai F, Shang S, Hu E (2020) Minimizing antibiotic dosage through in situ formation of gold nanoparticles across antibacterial wound dressings: a facile approach using silk fabric as the base substrate. J Clean Prod 243(2020):118604. https://doi.org/10.1016/j.jclepro.2019.118604
Zou Y, Xie R, Hu E, Qian P, Lu B, Lan G, Lu F (2020) Protein-reduced gold nanoparticles mixed with gentamicin sulfate and loaded into konjac/gelatin sponge heal wounds and kill drug-resistant bacteria. Int J Biol Macromol 148:921–931. https://doi.org/10.1016/j.ijbiomac.2020.01.190
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos: 51878321 and 51968032) and the Applied Basic Research Foundation of Yunnan Province (No: 2019FB015).
Author information
Authors and Affiliations
Contributions
Zhixiang Xu put forward the conception for the review article. The literature search and data analysis were performed by Xiao Gu and Lipeng Gu. The first draft of the manuscript was written by Xiao Gu and Fengxia Han, and other authors (Huayu Xu, Bo Chen, Xuejun Pan) critically revised the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, X., Xu, Z., Gu, L. et al. Preparation and antibacterial properties of gold nanoparticles: a review. Environ Chem Lett 19, 167–187 (2021). https://doi.org/10.1007/s10311-020-01071-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01071-0