Abstract
Industrialization is inducing water pollution by pharmaceuticals, fertilizers and cosmetics. Many emerging pollutants are non-biodegradable, toxic and recalcitrant to conventional wastewater treatments, thus calling for improved remediation techniques such as advanced oxidation processes which allow complete mineralization of pollutants. Here we review advanced oxidation processes with focus on ozonation and photocatalysis for the degradation of organic and microbial contaminants in wastewaters. Ozonation efficiency is limited by ozone-resistant pollutants, whereas photocatalysis is slow due to charge recombination, yet photocatalytic ozonation overcomes these limitations. Photocatalytic ozonation indeed shows synergy indices of up to 5.8 for treating wastewaters. This resulted in faster reaction kinetics, enhanced pollutant degradation with mineralization achieved in most cases, and reduction of toxicity up to 100%. We also discuss energy requirements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The protection of natural water resources and development of new technologies for water and wastewater treatment for reuse are key priorities of the twenty-first century. The environmental degradation caused by emerging biorecalcitrant organic compounds such as pharmaceuticals, cosmetics, fertilizers and resistant microbial pollutants is a global concern resulting in scarcity of fresh water in various parts of the world (Valério et al. 2020). However, the current conventional wastewater treatment technologies are often not effective in meeting the stringent effluent standards targeting the removal of emerging contaminants (Dewil et al. 2017). There is need to develop more effective treatment technologies that satisfy a range of requirements such as complete removal of biorecalcitrant organic pollutants, inactivation of resistant pathogens, less costly, energy efficient and environmentally friendly (Singh 2012; Mecha et al. 2017a, b). In this regard, advanced oxidation processes, especially those driven by solar light, have great potential in wastewater remediation targeting emerging contaminants (Rizzo et al. 2019; Rodríguez et al. 2019).
The advanced oxidation processes are destructive technologies which degrade contaminants. However, despite their overall high degradation efficiency, large-scale practical implementation has not been realized (Matafonova and Batoev 2018). This is partly due to high process costs since they are energy intensive. Also information regarding their performance is not standardized, for instance, a direct comparison of different advanced oxidation processes is difficult. In fact even the use of the electrical energy per order (EEO) concept for comparison is hampered by variation in influencing factors (Miklos et al. 2018). The advanced oxidation processes break down complex organics into simpler, less harmful ones such as carbon dioxide and water, a process known as mineralization (Bethi et al. 2016). However, in cases where mineralization is not achieved, the intermediate products produced may be toxic, thus re-contaminating the treated water and thereby endangering humans, ecological systems and the environment (Wang et al. 2018). This makes it necessary therefore to study the toxicity of treated water before discharge or reuse.
Among the advanced oxidation processes, ozonation and photocatalysis have received wide attention and recently photocatalytic ozonation has come to the limelight. Thus, here in, we review the principles of operation of advanced oxidation processes; performance of ozonation and its limitations; performance of heterogeneous photocatalysis and its limitations; combination of photocatalysis and ozonation to overcome the challenges of the individual processes; application of photocatalytic ozonation in wastewater treatment. We also address pertinent aspects required for real application of photocatalytic ozonation such as synergy of the combined processes, toxicity of treated water, energy requirements and photocatalyst recovery and reuse. Figure 1 shows a laboratory scale photocatalytic ozonation system.
Advanced oxidation processes
Principles of advanced oxidation processes
The advanced oxidation processes are near-ambient temperature and pressure processes that involve the generation of highly reactive radicals (Glaze et al. 1987; Miklos et al. 2018). Although advanced oxidation processes make use of different reaction systems, they are all characterized by the production of highly reactive hydroxyl (·OH) radicals. The standard redox potential of ·OH radicals (2.8 V) is much higher compared to that of common oxidants such as ozone (2.07 V), hydrogen peroxide (1.77 V) or even chlorine (1.36 V) (Pelaez et al. 2012). The ·OH radicals are non-selective and therefore can virtually degrade any organic contaminant present in wastewater including those that are biorecalcitrant (Andreozzi et al. 1999; Valério et al. 2020). This is a useful attribute for an oxidant to be used in the treatment of wastewaters which normally contains a variety of pollutants. The ·OH radicals can react in aqueous solution through three possible mechanisms: (1) hydrogen abstraction (Eq. 1), (2) electron transfer (Eq. 2) and (3) radical addition (Eq. 3):
The advanced oxidation processes when properly developed can provide a complete solution to the problem of pollutant abatement (through mineralization) in contrast to the phase separation processes (such as membrane separation and adsorption), which produce sludge that requires final disposal and introduces secondary pollution.
Classification of advanced oxidation processes
Advanced oxidation processes fall under two general categories. The first utilizes light energy such as ultraviolet (UV) light in conjunction with other chemical additives. Under this category are processes such as UV/H2O2, UV/ozone (O3), UV/titanium dioxide (TiO2) and UV/Fenton. When no light source is used, the technology can be termed as a dark oxidative process. Processes in this category include ozonation, Fenton’s reagent, ultrasound and microwaves among others (Gilmour 2012). Thus, advanced oxidation processes include chemical oxidants (H2O2, ozone, etc.), Fenton and photo-Fenton processes (Fe2+/H2O2/UV), photocatalytic processes (semiconductor with UV/visible light), supercritical water oxidation, electron beams and ultrasounds (Rizzo 2011; Khataee and Fathinia 2013). These processes are based on the in situ generation of highly reactive transitory species (H2O2, ·OH, O2 −, O3) for mineralization of refractory organic compounds and inactivation of waterborne pathogens (Hoigne 1998; Esplugas et al. 2002; Tsydenova et al. 2015) simultaneously. Due to rapid oxidation reactions, advanced oxidation processes are characterized by high reaction rates and short treatment times, which make them promising in wastewater treatment (Hoigne 1998; Esplugas et al. 2002). A general classification of advanced oxidation processes is given in Table 1.
Advantages of advanced oxidation processes
The advanced oxidation processes have unique advantages over conventional treatment processes such as (1) operation under ambient conditions of temperature and pressure, (2) effectiveness in destroying biorecalcitrant organic compounds, (3) mineralization of organic contaminants into carbon dioxide if desired, without any waste disposal problem, and (4) production of minimal harmful by-products (Zhou and Smith 2002; Parsons 2004). In most instances, advanced oxidation processes are used to supplement rather than to replace conventional systems and to enhance the treatment of organic micropollutants and pathogens. They are therefore used as pretreatment to convert recalcitrant pollutants into biodegradable compounds that can then be treated by conventional biological methods. They are also used for the degradation of recalcitrant pollutants as a post-treatment after the biological process to polish the effluent before discharge or reuse (Wang and Xu 2012). The main idea of the combination is the use of a more expensive technology only in the first or final step of the treatment to reduce costs (Černigoj 2007).
Disadvantages of advanced oxidation processes
The major disadvantage of these processes is their high cost resulting from the costly reagents and light energy sources like UV light (Esplugas et al. 2002). However, this can be addressed for instance by the development of visible light active catalysts (hence enable the use of natural sunlight) and improved reactor design to ensure optimal utilization of the oxidants. Recent research efforts have focused more on those photocatalytic processes, which can be driven by solar irradiation to reduce dependency on electrical energy and hence reduce costs (Mecha et al. 2016a, b). The use of renewable and free solar energy in such processes could substantially decrease treatment costs and be more environmental friendly for wastewater decontamination. Among the many advanced oxidation processes that have been studied, ozonation and photocatalysis are prominent for wastewater treatment (Esplugas et al. 2002) and are explored further in this review.
Ozonation
Introduction to ozone
Ozone is a gas with a pungent smell that is generated on-site from dry air or pure oxygen. The formation of ozone is endothermic, and ozone is thermodynamically unstable and thus readily reverts to oxygen (\(3{\text{O}}_{2} \leftrightarrow 2{\text{O}}_{3}\)) (Zhou and Smith 2002; Gardoni et al. 2012). Ozone is a strong oxidizing agent and is used as a chemical reagent in synthesis, water and wastewater treatment and bleaching agent. Its use in water and wastewater treatment is based on its effectiveness in (2) disinfection, (2) oxidation of biorecalcitrant pollutants, (3) removal of taste and odour and colour and (4) reduction of turbidity (Gray 2014; Mecha et al. 2018). Advantages of ozone include: (1) it can be easily produced from air or oxygen by electric discharge; (2) it reacts readily with organic and inorganic compounds; (3) multiple applications such as disinfection, reduction of chemical oxygen demand, colour, odour and turbidity of the water treated; and (4) any excesses of ozone in water decompose readily to oxygen, without leaving any residue.
Mechanism of ozone oxidation
In aqueous solution, ozone reacts with various constituents in two ways: (1) direct oxidation by molecular ozone which involves selective reactions, such as electrophilic, nucleophilic or dipolar addition reactions with low reaction rates (Hoigne 1998), and (2) indirect mechanism through the decomposition of ozone to produce ·OH radicals, which are non-selective and highly reactive (Miklos et al. 2018). Ozone and ·OH radicals are strong chemical oxidants and are involved in disinfection and oxidation of contaminants (Gray 2014). The overall reaction for the production of ·OH from ozone is given as:
Limitations of ozonation
A major limitation of ozone is that it oxidizes refractory organic compounds, but only minimal mineralization is achieved (Bashiri and Rafiee 2014) due to the formation of ozone recalcitrant intermediate compounds (Chin and Berube 2005; Mecha et al. 2016c). When used for disinfection, regrowth of the microorganisms cannot be prevented because of the difficulty in maintaining residual ozone. This requires the use of a secondary disinfectant such as chlorine to maintain a residual especially if the treated water is intended for consumption (Demir and Atguden 2016). Other disadvantages include: (1) the yield of ozone generator is low (6–12% from oxygen and 4–6% from air); (2) ozone is unstable and has to be generated on-site due to challenges with storage and transportation; and (3) limitations of mass transfer of ozone into water.
Heterogeneous photocatalysis
Overview of heterogeneous photocatalysis
Heterogeneous photocatalysis has become an increasingly viable technology in environmental remediation. A photocatalytic process usually requires the following elements: a semiconductor or photocatalyst, a light source, a reactor system, the pollutant and oxygen (Vamathevan et al. 2001). The oxidizing species either free holes or ·OH radicals are generated under ambient conditions. Heterogeneous photocatalytic technologies have advantages over other advanced oxidation processes such as operation under ambient conditions of temperature and pressure, the use of oxygen from the air as oxidant, the possibility of using solar light to drive the process and the complete destruction of most contaminants without generating secondary waste (mineralization). These attributes are very important from the energy consumption and environmental impact perspectives (van Grieken et al. 2009a; Zangeneh et al. 2015).
Properties of photocatalysts
Photocatalysts are materials that are activated by adsorbing photons and are capable of accelerating reactions without being consumed (Umar and Aziz 2013). Some basic requirements of a good photocatalyst include high photoactivity, biological and chemical inertness, photostability and non-selectivity in most cases (Pirkanniemi and Sillanpää 2002; Kumar and Bansal 2013). To reduce the electrical energy requirements, it is desirable for the photocatalyst to be able to utilize not only UV light, but also visible light from solar energy. The photocatalyst also needs to be inexpensive. Based on these properties, the most popular photocatalyst for use in water treatment is titanium dioxide because it encompasses most of the above-mentioned properties (Andreozzi et al. 1999; Rizzo et al. 2019).
Titanium dioxide as a potential photocatalyst
Titanium dioxide exists in three crystalline forms, namely, brookite, rutile and anatase. Among these, rutile and anatase are the commonly used forms; however, anatase is mostly used in photocatalytic treatment of wastewater. The composition of titanium dioxide is temperature dependent; for instance, rutile is more stable than anatase thermodynamically, but at temperatures below 600 °C the formation of anatase is kinetically favoured (Carp et al. 2004). In most of the photocatalytic studies, anatase has been shown to be more photoactive as compared to rutile. This is attributed to the fact that anatase has a slightly higher Fermi level, higher capacity to adsorb oxygen and a higher degree of hydroxylation of the surface. In terms of light absorption, rutile is able to absorb light with a wavelength of 415 nm, whereas anatase only absorbs at 385 nm (Fujishima et al. 2008). A commercially widely used titanium dioxide, Degussa P25, has been used in many studies due to its high photoactivity under UV light irradiation. It is non-porous, is composed of 70–90% anatase and 10–30% rutile, has a surface area of 55 ± 15 m2/g and crystallite sizes of 30 nm (Hoffmann et al. 1995; Valério et al. 2020). In most cases, mixed phase titanium dioxide photocatalysts are found to perform better (Carp et al. 2004).
Limitations of titanium dioxide photocatalysis
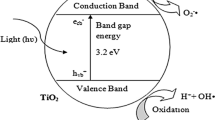
The efficiency of photocatalytic reactions is dependent on the degree of electron–hole recombination inherent in processes and the light absorption capability of photocatalysts (Vamathevan et al. 2002). Consequently, the conspicuous drawback of titanium dioxide is that after excitation, the photogenerated charge carriers depict a high rate of recombination. The electron–hole recombination declines the overall efficiency of the semiconductor by decreasing the quantum yield of the desired oxidation/reduction processes. This results in slow reaction kinetics resulting from charge recombination. Furthermore, titanium dioxide has a wide band gap (3.2 eV for anatase), which requires UV light to produce ·OH radicals during the photocatalytic process (Nahar et al. 2006; Ambrus et al. 2008). This constitutes a significant energy consumption problem, thereby increasing the electricity requirements. Moreover, it limits the application of solar radiation, which contains 4–6% UV irradiation. On the other hand, visible light constitutes a large portion of solar light spectrum (approximately 45%) (Castillo et al. 2013), and to apply it in photocatalytic treatment of wastewater, there is need to develop visible light-responsive photocatalysts. This can be achieved by catalyst modification processes to cause a red shift in the semiconductor’s light response to the visible spectrum.
The development of a new titanium dioxide photocatalysts with increased activities under visible light can be attained through various modification techniques such as bulk modification, surface modification and sensitization of titanium dioxide (Zaleska 2008; Mital and Manoj 2011; Lazar et al. 2012; Zangeneh et al. 2015). The major practices involve catalyst modification by doping using metal and non-metal ions, metal coating, surface sensitization and increase in surface area by design and development of secondary titania photocatalyst. Among these modifications, metal-ion doping is reported to be effective in improving the visible light activity of titanium dioxide (Silva 2008; Mecha et al. 2016b).
Modification of titanium dioxide using metal-ion doping
Doping titanium dioxide with metal ions is an important approach in band gap engineering to change the optical response of titanium dioxide. The principle entails the introduction of localized bands of orbitals within the titanium dioxide band gap (bathochromic shift). Consequently, this reduces the recombination of photogenerated electrons and holes and extends the light absorption of the photocatalyst into the visible region resulting from charge-transfer transition between the d electrons of the metals and the conduction band or valance band of titanium dioxide (Zangeneh et al. 2015).
Transition metal doping species improve the trapping of electrons to inhibit electron–hole recombination during illumination (Mital and Manoj 2011). However, not all transition metals can achieve this; only transition metals such as Fe3+ and Cu2+ inhibit electron–hole recombination (Vamathevan et al. 2001). They also increase the electron–hole pair separation efficiency, thus inhibiting their recombination and hence improving the photocatalytic activity under visible light irradiation (Pham and Lee 2014). Noble metals such as platinum, gold and silver have high Schottky barriers and thus act as electron traps and facilitate electron–hole separation. There are different mechanisms for noble metal doping on titanium dioxide depending on the photoreaction conditions. They may (1) enhance the electron–hole separation by acting as electron traps, (2) enable visible light absorption and enhance plasmon resonance surface electron excitation under visible light and (3) modify the surface properties of photocatalysts (Sobana et al. 2006).
Although visible light activity of metal-ion-doped titanium dioxide leads to the reduction of the energy requirements, it still does not solve the challenge of slow reaction kinetics encountered in photocatalysis. Therefore, despite the great potential of heterogeneous photocatalysis for the removal of persistent non-biodegradable organic pollutants from wastewater, it suffers from the significant challenge of low oxidation rate (Augugliaro et al. 2006). This limitation may be addressed through the combination of photocatalysis with other oxidation processes such as ozonation, which is a better electron scavenger than oxygen.
Coupling photocatalysis and ozonation
Motivation for combining photocatalysis and ozonation
Ozone oxidation of organic pollutants is generally a fast process; however, a significant mineralization of the pollutant rarely occurs because of the formation of ozone-resistant and stable degradation by-products such as carboxylic acids (Hsu et al. 2007). These carboxylic acids are formed by opening the aromatic ring and are very resistant to oxidation by ozone, and hence, they limit the mineralization potential (Kasprzyk-Hordern et al. 2003; Zou and Zhu 2008). On the other hand, photocatalysis presents the advantage of achieving complete contaminant mineralization. However, long degradation time is required because of the low oxidation rates (Agustina et al. 2005). To improve the overall performance, heterogeneous photocatalysis has in recent times been combined with other processes, which affect the chemical kinetics and/or the overall efficiency. For instance, the efficiency of titanium dioxide photocatalytic process can be improved by adding oxidant species such as ozone (Rajeswari and Kanmani 2009a). When photocatalysis is coupled with ozonation, the combination influences the photocatalytic mechanisms by increasing the efficiency and decreasing the reaction time in respect of the individual processes (Augugliaro et al. 2006). It reduces the ozone dosage required (Silva et al. 2019), which further leads to low costs of water treatment and reduced formation of ozone disinfection by-products (Meunier et al. 2006). Photocatalytic ozonation has a superior performance than the individual processes (Shinpon et al. 2002; Rajeswari and Kanmani 2009a). For instance, Müller et al. (1998) showed that the advantages of photocatalysis achieving a constant decline in dissolved organic carbon, and of ozonation preventing the accumulation of high intermediate concentrations, were beneficial during photocatalytic ozonation of 2,4-dichlorophenoxyacetic acid. Therefore, integrating ozonation and photocatalysis enables the exploration of the advantages of both processes and synergies, while also overcoming their individual limitations. Moreover, the similarities between the mechanism of photocatalysis and ozonation and operation under some common process conditions point towards the synergy between these methods leading to better results as compared to individual techniques (Gogate and Pandit 2004). Consequently, there is a growing shift from the use of individual processes to combined oxidation processes, which result in increased overall degradation of several pollutants. This has been attributed to enhanced generation of ·OH radicals, eventually increasing the oxidation rates, or improving the contacting of the generated free radicals with the pollutants and effective utilization of oxidants (Wang and Xu 2012).
Factors influencing photocatalysis and ozonation
To explore the combination of photocatalysis and ozone in wastewater treatment, it is necessary to understand the main factors affecting the performance of these processes. In addition, understanding the impact of various process parameters that govern photocatalytic and ozone degradation efficiency is paramount from the design and the operational points of view when choosing a sustainable technique for the treatment of wastewater. Photocatalysis and ozonation reaction rates are affected by operating conditions such as reactor type, oxygen concentration, ozone concentration, solution pH, catalyst loading, substrate concentration and water matrix. The physical and chemical intrinsic properties of the photocatalyst such as the crystal composition, surface area and crystallite size are also important factors. These are described in detail in a previous study (Mecha 2017) and briefly summarized below.
Reactor design/type
Photocatalytic reactors for wastewater treatment can be categorized based on the following aspects: (1) the state of the catalyst in the reactor (slurry or immobilized catalyst photoreactors), (2) the source of irradiation (natural, e.g. sunlight, or artificial, e.g. UV lamp) and (3) the position of the light source (immersed or external) (Silva 2008). In slurry reactors, fine particles of the solid semiconductor material are dispersed in the liquid phase using either mechanical or magnetic stirrers. An air supply is usually provided to scavenge the electrons and prevent electron–hole charge recombination; aeration also helps in catalyst dispersion. Slurry reactors are often used to study degradation kinetics since they are characterized by large catalytic surface area and low mass transfer limitations compared to immobilized catalyst systems (Choi et al. 2009). Regarding the source of light, artificial radiation sources include arc lamps, incandescent lamps, fluorescent lamps and lasers. Instead of artificial light sources, solar radiation can also be used and it is a more convenient and economical source of light, especially in places with high insolation levels. The source of light can be immersed (common in commercial UV reactors) in the reactor or be external (common in solar radiation reactors) to the reactor. Nevertheless, irrespective of the reactor design selected, the primary focus should be that uniform irradiation of the entire catalyst surface is achieved at the incident light intensity. Photocatalytic ozonation reactors are essentially similar to photocatalytic reactors except that instead of oxygen being the electron scavenger, ozone is used.
Irradiation intensity
The extent of light absorption by the photocatalyst and the rate of electron–hole formation depend on the light intensity (Cassano and Alfano 2000). Although the form of the light does not affect the reaction pathway (Gaya and Abdullah 2008), with increase in light intensity, the catalyst absorbs more photons, thus enhancing the production of electron–hole pairs, ·OH radicals and contaminant degradation (Zangeneh et al. 2015).
Oxygen and ozone concentration
Oxygen acts as an electron scavenger/acceptor in photocatalytic reactions to produce super oxide radical ions \(( {\text{O}}_{2}^{ \cdot - } )\), and an optimal oxygen supply should be used (Kabra et al. 2004). It has been reported that oxygen does not affect the adsorption on the titanium dioxide catalyst surface as the reduction reaction takes place at a different location from where oxidation occurs (Gaya and Abdullah 2008). Nevertheless, the dissolved oxygen improves the separation of photogenerated electrons, thus preventing electron–hole recombination (Yamazaki et al. 2001). The absence of oxygen suppresses photocatalytic activity because of the back-electron transfer from charged species present on photocatalyst surface (Chatterjee and Dasgupta 2005). An increase in the ozone concentration increases the pollutants degradation efficiency due to the high oxidant/contaminant ratio (Beltrán et al. 1997). For photocatalytic ozonation, ozone being a better electron scavenger than oxygen makes the oxidation process to take place faster and more effectively. This is because O3 is more electrophilic than O2 towards electrons generated on the titanium dioxide surface (Hernández-Alonso et al. 2002).
Contaminant concentration
The contaminant degradation rate increases with an increase in its initial concentration to a certain level beyond which leads to a decrease of the degradation rate (Umar and Aziz 2013). This is because of reduction in light penetration into the solution as well as complete catalyst coverage leading to fewer photons reaching the catalyst surface (Nam et al. 2002). Photocatalysis occurs primarily on the surface of the catalyst; thus, the quantity of the contaminant adsorbed on the surface of the photocatalyst should be considered (Guettaï and Ait Amar 2005; Gaya and Abdullah 2008).
Photocatalyst concentration
The concentration of titanium dioxide particles affects the light penetration and the surface area for adsorption. As the catalyst concentration increases, the number of ·OH radicals generated increases (Mozia 2010). Beyond a certain catalyst concentration, solution turbidity impedes the penetration of the irradiation and the reaction rate decreases (Bahnemann et al. 2007).
Initial solution pH
The solution pH determines the surface charge of the photocatalyst and agglomeration of the catalyst particles. It also influences the production of ·OH radicals, since a higher concentration of hydroxyl ions (OH−) results in a higher production of ·OH radicals. However, pH also affects the electrostatic interactions between the semiconductor surface, solvent molecules, substrate and charged radicals formed (Ahmed et al. 2011). Titanium dioxide is amphoteric in nature, and it responds in different ways under acidic and alkaline conditions. Depending on the point of zero charge of the titanium dioxide, the surface of titanium dioxide will either be positively or negatively charged at different pH values (Bahnemann et al. 2007). The effectiveness of ozone is also pH dependent because pH affects ozone decomposition and chemical speciation. At high pH values, ozone reacts almost indiscriminately with all organic and inorganic compounds present in the reacting medium because of the formation of ·OH radicals, which are non-selective. At low pH values, molecular ozone is the dominant oxidation species (Poznyak et al. 2006); the concentration of dissolved ozone decreases with increase in pH (Sotelo et al. 1989).
Water matrix
The presence of natural organic matter in water and wastewater even at low concentrations is detrimental because it exerts a strong influence on reaction mechanisms and competes with the target micropollutants and microbes for the oxidants. The fast reaction of reactive oxygen species released during ozonation and photocatalysis with unsaturated bonds and aromatic rings present in organic matter molecules is demonstrated by a rapid decline of the UV254 during ozonation and photocatalysis (Westerhoff et al. 1999).
Mechanism of photocatalytic ozonation
The mechanism consists of photocatalytic oxidation (Langmuir–Hinshelwood kinetics) and oxidation by ozonation (Beltrán et al. 2009; Mena et al. 2012) and is provided in the literature (Mecha et al. 2016a). The production of ·OH radicals significantly improves the oxidation rate of photocatalytic ozonation compared to photocatalytic oxidation. This is due to the production of more ·OH radicals because O3 is more electrophilic than O2 towards photogenerated electrons (Hernández-Alonso et al. 2002); thus resulting in high mineralization and faster reaction kinetics (Li et al. 2003, 2005). Figure 2 illustrates the mechanism of photocatalytic ozonation.
Mechanism of photocatalytic ozonation. Electron–hole pairs are generated when the photocatalyst is illuminated by UV/solar light. Ozone scavenges the photogenerated charge carriers to form powerful hydroxyl and superoxide radicals on the photocatalyst surface which react with the pollutants and mineralize them to carbon dioxide and water. CB conduction band, VB valence band, hv irradiation energy, h+ holes, e− electrons
Application of photocatalytic ozonation in wastewater treatment
Degradation of organic pollutants
Table 2 gives a summary on the use of photolytic and photocatalytic ozonation processes for the degradation of organic contaminants. Most studies have monitored the total organic carbon reduction because the ·OH radicals produced by advanced oxidation processes react non-selectively, thus forming numerous intermediate compounds en route to complete mineralization. Because of this, total organic carbon is a better indicator of the treatment efficiency instead of focusing only on the parent compound (Malato et al. 2016). Mineralization efficiencies of up to 100% have been reported as shown in Table 2. The synergistic effect of photocatalytic ozonation obtained from various studies is also shown for cases where available. The synergy index (SI) was calculated using the equation:
where R is the percentage contaminant removal and the subscripts represent ozonation (Oz) and photocatalysis (Phot) (Mecha et al. 2016a).
However, very few studies have calculated the synergistic effect of combining two or more advanced oxidation processes. This information could be very useful in the practical implementation of these processes. The few that have calculated the synergy factors have reported values from 1.2 to 5.8 despite treating different contaminants and using different experimental conditions. The mineralization of biorecalcitrant organics also improves the biodegradability of the wastewater as reported (Espejo et al. 2015; Van Aken et al. 2015) and is summarized in Table 3. Biodegradability is evaluated using the ratios biological oxygen demand/chemical oxygen demand, biological oxygen demand/dissolved oxygen demand and biological oxygen demand/total oxygen demand (Alvares et al. 2001). An increase of the ratio indicates that the wastewater sample is becoming increasingly easier to treat by biological methods and thus the non-biodegradable compounds have been degraded to more biodegradable forms.
Disinfection of wastewater
Table 4 gives a summary on the use of photolytic and photocatalytic ozonation processes for disinfection of water and wastewater targeting a variety of microorganisms. Notably few studies have determined the synergistic effect of combining two or more advanced oxidation processes. However, the reported cases depict considerable synergy.
Evaluation of toxicity of treated wastewater
The degradation of contaminants into less harmful pollutants using highly reactive hydroxyl radicals distinguishes advanced oxidation processes from other wastewater treatment processes such as adsorption and membrane separation which transfer contaminants from the liquid phase (treated water) to the solid phase (sludge) (Sievers 2011). Photocatalytic ozonation is capable of breaking down organic pollutants and transforming them to mineral acids and carbon dioxide. Although destruction of contaminants is generally beneficial, the formation of by-products or transformation products that retain harmful biological activity is a possibility. Therefore, there is need to assess the toxicity evolution during this process to determine the safety of the treated wastewater and also to inform the implementation of suitable technologies (Linden and Mohseni 2014). Evaluation of toxicity is mainly done using biological tests (Žegura et al. 2009). Studies have demonstrated a reduction or even elimination of toxicity from wastewater treated by advanced oxidation processes. For instance, the acute toxicity of phenol and the intermediate compounds was reduced significantly after treatment using catalytic ozonation (Farzadkia et al. 2014); this was because of the degradation of phenol to aliphatic and low chain carboxylic acid products. Also, there were no compounds with oestrogenic effects observed after photocatalytic ozonation of wastewater (Moreira et al. 2016). The reduction of toxicity is attributed to the production of final oxidation products that are more hydrophilic, thus reducing their ability to penetrate cell membranes and cause damage to the cells (Huber et al. 2003; Escher et al. 2009). The attainment of a higher biodegradability and/or lower toxicity of the intermediate and final products compared with the parent compounds, is desirable benefits of applying photocatalytic ozonation for wastewater treatment. Table 5 shows the findings of toxicity assessment of wastewater treated using advanced oxidation processes including ozonation and photocatalysis.
Energy requirements
The efficiency of advanced oxidation processes mainly depends on factors such as contaminant type and concentration, water matrix and constituents, and reactor configuration and design (Linden and Mohseni 2014). Since these factors may vary widely and are difficult to control, meaningful comparison cannot be done based on them. Therefore, comparisons of advanced oxidation processes can be done based on the energy requirements. The advanced oxidation processes utilize a lot of electrical energy (Esplugas et al. 2002), electricity cost is a major operating cost (Bolton et al. 2001). To enable the estimation of energy consumed by different advanced oxidation processes, the International Union of Pure and Applied Chemistry proposed the use of figures of merit (Bolton et al. 2001; Miklos et al. 2018). Thus, for advanced oxidation processes based on electrical energy consumption, the electrical energy per order (EEO) is used, while for the solar-driven systems, the collector area per order (ACO) is used. The EEO is calculated as follows (Bolton et al. 2001):
where P is the power input, t is the treatment time, V is the volume of water treated, Ci and Cf are the initial and final concentrations of contaminant, respectively, and the factor 1000 converts g to kg (Cardoso et al. 2016).
However, due to the large expenses incurred when using electricity to run advanced oxidation processes, it is imperative to explore the use of less costly options. This has prompted accelerated research efforts on the use of the renewable sources such as natural sunlight. The development of visible light active photocatalysts enables the use of sunlight for photocatalytic processes which are environmental friendly and cost-effective (Tsydenova et al. 2015; Mecha et al. 2017b). Table 6 shows the energy consumption by advanced oxidation processes during wastewater treatment.
Recovery and reuse of photocatalyst
The recovery and reuse of photocatalysts in slurry reactors is a major concern for the large-scale utilization of photocatalytic processes in a sustainable way. This is necessary considering that the treatment of real wastewaters containing different types of contaminants may affect the catalyst activity and hence catalyst life (van Grieken et al. 2009b). Studies conducted in this area have showed that suspended photocatalysts can be recovered using a variety of ways such as filtration or centrifugation and reused multiple times without a significant decrease in performance. For example, Rupa et al. (2007) reported that silver-doped titanium dioxide could be reused at least three times, and Swarnakar et al. (2013) observed insignificant reduction in catalytic performance of titanium dioxide films that were reused five times. This shows that the photocatalysts are robust and stable against a variety of contaminants in wastewater. Hence with proper recovery, regeneration and reuse strategies, they can make photocatalytic ozonation sustainable. A summary of findings from previous studies is provided in Table 7.
Conclusion
The presence of biorecalcitrant organic pollutants in water sources has accelerated exploration of the use of advanced oxidation processes such as ozonation and photocatalysis. In this review, these processes were evaluated and their individual merits and demerits discussed. For instance, photocatalysis is limited by low oxidation rates arising from electron–hole recombination. On the other hand, ozonation suffers low mineralization rates attributed to the production of intermediate compounds that are ozone resistant. Potential ways of overcoming their individual drawbacks were explored. These include development of visible light active titanium dioxide photocatalyst to utilize solar light, thereby reducing energy costs and coupling of ozonation and photocatalysis to enhance the reaction kinetics. There is great potential of the photocatalytic ozonation process. In particular, solar-enhanced photocatalytic ozonation holds promise as an environmentally friendly technique for wastewater remediation. Additional benefits of using photocatalytic ozonation including (1) the use of a single reactor instead of two (reduced reactor costs and hence capital costs), (2) synergy between the two processes when used simultaneous as opposed to when they are employed separately and (3) potential reduction in reactor residence times are attractive benefits. The assessment of the recovery and reuse of photocatalysts, energy requirements and toxicity assessment of the treated wastewater are necessary as demonstrated in this study so as to make the process more sustainable. Based on the findings of the review, the following recommendations are proposed:
-
a.
Further studies on solar-powered photocatalytic processes to reduce dependence on electricity and decrease process costs.
-
b.
Most studies have been performed on laboratory scale. The information obtained in laboratory scale studies is not sufficient for large-scale operation. There is need to perform pilot scale studies on photocatalytic ozonation of wastewater to generate sufficient data especially on mass transfer limitation of reaction kinetics and mixing so as to guide the upscaling to large-scale treatment systems.
-
c.
The possibility of recovery and reuse of photocatalysts is very crucial as a way of reducing costs and also preventing secondary pollution of treated wastewater by photocatalyst particles. There is need to develop appropriate techniques suitable especially in large-scale applications.
-
d.
Studies demonstrated that synergy indeed exists between photocatalysis and ozonation when employed together. Based on the fact that process conditions play a significant role in this synergism, there is need to develop mathematical models that can be employed in the design of systems that maximize on synergy and performance effectiveness to make these processes economically competitive to the existing conventional processes.
-
e.
Given the impressive performance of photocatalytic ozonation in the degradation of recalcitrant organics in wastewater, it is necessary to explore the use of this process in related applications such as pretreatment of substrates for bioenergy production and treatment of biosolids to reduce soil pollution among others.
References
Aguinaco A, Beltrán FJ, García-Araya JF, Oropesa A (2012) Photocatalytic ozonation to remove the pharmaceutical diclofenac from water: influence of variables. Chem Eng J 189–190:275–282. https://doi.org/10.1016/j.cej.2012.02.072
Agustina TE, Ang HM, Vareek VK (2005) A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J Photochem Photobiol C 6(4):264–273. https://doi.org/10.1016/j.jphotochemrev.2005.12.003
Ahmed S, Rasul MG, Martens WN, Brown R, Hashib MA (2011) Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review. Water Air Soil Pollut 215(1):3–29. https://doi.org/10.1007/s11270-010-0456-3
Ahmed MB, Zhou JL, Ngo HH, Guo W, Thomaidis NS, Xu J (2017) Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review. J Hazard Mater 323(Part A):274–298. https://doi.org/10.1016/j.jhazmat.2016.04.045
Alvares ABC, Diaper C, Parsons SA (2001) Partial oxidation by ozone to remove recalcitrance from wastewaters—a review. Environ Technol 22(4):409–427. https://doi.org/10.1080/09593332208618273
Álvarez PM, Beltrán FJ, Jaramillo J, Pocostales P, Márquez G (2011) Comparison of various advanced treatment methods for municipal wastewater reclamation. World Acad Sci Eng Technol 55:653–654
Ambrus Z, Balázs N, Alapi T, Wittmann G, Sipos P, Dombi A, Mogyorósi K (2008) Synthesis, structure and photocatalytic properties of Fe(III)-doped TiO2 prepared from TiCl3. Appl Catal B 81(1–2):27–37. https://doi.org/10.1016/j.apcatb.2007.11.041
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53(1):51–59. https://doi.org/10.1016/S0920-5861(99)00102-9
Arabatzis IM, Stergiopoulos T, Bernard MC, Labou D, Neophytides SG, Falaras P (2003) Silver-modified titanium dioxide thin films for efficient photodegradation of methyl orange. Appl Catal B 42(2):187–201. https://doi.org/10.1016/S0926-3373(02)00233-3
Arslan-Alaton I (2007) Degradation of a commercial textile biocide with advanced oxidation processes and ozone. J Environ Manag 82(2):145–154. https://doi.org/10.1016/j.jenvman.2005.12.021
Asaithambi P, Saravanathamizhan R, Matheswaran M (2015) Comparison of treatment and energy efficiency of advanced oxidation processes for the distillery wastewater. Int J Environ Sci Technol 12(7):2213–2220. https://doi.org/10.1007/s13762-014-0589-9
Augugliaro V, Litter M, Palmisano L, Soria J (2006) The combination of heterogeneous photocatalysis with chemical and physical operations: a tool for improving the photoprocess performance. J Photochem Photobiol C 7(4):127–144. https://doi.org/10.1016/j.jphotochemrev.2006.12.001
Bahnemann W, Muneer M, Haque MM (2007) Titanium dioxide-mediated photocatalysed degradation of few selected organic pollutants in aqueous suspensions. Catal Today 124(3–4):133–148. https://doi.org/10.1016/j.cattod.2007.03.031
Bashiri H, Rafiee M (2014) Kinetic Monte Carlo simulation of 2,4,6-thrichloro phenol ozonation in the presence of ZnO nanocatalyst. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2014.11.001
Beltrán FJ, García-Araya JF, Álvarez P (1997) Impact of chemical oxidation on biological treatment of a primary municipal wastewater. 2. Effects of ozonation on kinetics of biological oxidation. Ozone Sci Eng 19:513–526
Beltrán FJ, Aguinaco A, García-Araya JF (2009) Mechanism and kinetics of sulfamethoxazole photocatalytic ozonation in water. Water Res 43(5):1359–1369. https://doi.org/10.1016/j.watres.2008.12.015
Bethi B, Sonawane SH, Bhanvase BA, Gumfekar SP (2016) Nanomaterials-based advanced oxidation processes for wastewater treatment: a review. Chem Eng Process 109:178–189. https://doi.org/10.1016/j.cep.2016.08.016
Bolton JR, Bircher KG, Tumas W, Tolman CA (2001) Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl Chem 73(4):627–637
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160(2–3):265–288. https://doi.org/10.1016/j.jhazmat.2008.03.045
Bustos Y, Vaca M, López R, Bandala E, Torres L, Rojas-Valencia N (2014) Disinfection of primary municipal wastewater effluents using continuous UV and ozone treatment. J Water Resour Prot 6:16–21. https://doi.org/10.4236/jwarp.2014.61003
Cao N, Yang M, Zhang Y, Hu J, Ike M, Hirotsuji J, Matsui H, Inoue D, Sei K (2009) Evaluation of wastewater reclamation technologies based on in vitro and in vivo bioassays. Sci Total Environ 407(5):1588–1597. https://doi.org/10.1016/j.scitotenv.2008.10.048
Cardoso JC, Bessegato GG, Boldrin Zanoni MV (2016) Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res 98:39–46. https://doi.org/10.1016/j.watres.2016.04.004
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177. https://doi.org/10.1016/j.progsolidstchem.2004.08.001
Cassano AE, Alfano OM (2000) Reaction engineering of suspended solid heterogeneous photocatalytic reactors. Catal Today 58(2–3):167–197. https://doi.org/10.1016/S0920-5861(00)00251-0
Castillo JH, Bueno A, Pelaez MA, Sanchez-Salas JL, Dionysiou DD, Bandala ER (2013) Solar water disinfection using NF-codoped TiO2 photocatalysis: estimation of scaling-up parameters. Int J Chem Reactor Eng 11(2):701–708. https://doi.org/10.1515/ijcre-2012-0051
Černigoj U (2007) Photodegradation of organic pollutants in aqueous solutions catalyzed by immobilized titanium dioxide: novel routes towards higher efficiency. Dissertation. University Of Nova Gorica, Graduate School
Chatterjee D, Dasgupta S (2005) Visible light induced photocatalytic degradation of organic pollutants. J Photochem Photobiol C 6(2–3):186–205. https://doi.org/10.1016/j.jphotochemrev.2005.09.001
Chin A, Berube PR (2005) Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res 39:2136–2144
Choi H, Al-Abed SR, Dionysiou DD, Stathatos E, Lianos P (2009) TiO2-based advanced oxidation nanotechnologies for water purification and reuse. In: Escobar IC, Schäfer AI (eds) Sustainable water for the future: sustainability science and engineering, vol 2. Elsevier, Amsterdam, pp 229–254
Demir F, Atguden A (2016) Experimental investigation on the microbial inactivation of domestic well drinking water using ozone under different treatment conditions. Ozone Sci Eng 38(1):25–35. https://doi.org/10.1080/01919512.2015.1074534
Devatkal SK, Jaiswal P, Kaur A, Juneja V (2016) Inactivation of Bacillus cereus and Salmonella enterica serovar typhimurium by Aqueous Ozone: modeling and UV–Vis spectroscopic analysis. Ozone Sci Eng 38(2):124–132. https://doi.org/10.1080/01919512.2015.1079119
Dewil R, Mantzavinos D, Poulios I, Rodrigo MA (2017) New perspectives for advanced oxidation processes. J Environ Manag 195:93–99. https://doi.org/10.1016/j.jenvman.2017.04.010
Diaz ME, Law SE, Frank JF (2001) Control of pathogenic microorganisms and turbidity in poultry-processing chiller water using UV-enhanced ozonation. Ozone Sci Eng 23(1):53–64. https://doi.org/10.1080/01919510108961988
Dong YM, Wang GL, Jiang PP, Zhang AM, Yue L, Zhang XM (2011) Simple preparation and catalytic properties of ZnO for ozonation degradation of phenol in water. Chin Chem Lett 22(2):209–212. https://doi.org/10.1016/j.cclet.2010.10.010
Escher BI, Baumgartner R, Lienert J, Fenner K (2009) Predicting the ecotoxicological effects of transformation products. In: Boxall ABA (ed) Transformation products of synthetic chemicals in the environment. Springer, Berlin, pp 205–244
Espejo A, Beltrán FJ, Rivas FJ, García-Araya JF, Gimeno O (2015) Iron-based catalysts for photocatalytic ozonation of some emerging pollutants of wastewater. J Environ Sci Health Part A 50(6):553–562. https://doi.org/10.1080/10934529.2015.994939
Esplugas S, Giménez J, Contreras S, Pascual E, Rodrı́guez M (2002) Comparison of different advanced oxidation processes for phenol degradation. Water Res 36(4):1034–1042. https://doi.org/10.1016/S0043-1354(01)00301-3
Farzadkia M, Shahamat YD, Nasseri S, Mahvi AH, Gholami M, Shahryari A (2014) Catalytic ozonation of phenolic wastewater: identification and toxicity of intermediates. J Eng 2014:1–10. https://doi.org/10.1155/2014/520929
Feilizadeh M, Mul G, Vossoughi M (2015) E. coli inactivation by visible light irradiation using a Fe–Cd/TiO2 photocatalyst: statistical analysis and optimization of operating parameters. Appl Catal B 168–169:441–447. https://doi.org/10.1016/j.apcatb.2014.12.034
Ferro G, Guarino F, Castiglione S, Rizzo L (2016) Antibiotic resistance spread potential in urban wastewater effluents disinfected by UV/H2O2 process. Sci Total Environ 560–561:29–35. https://doi.org/10.1016/j.scitotenv.2016.04.047
Fu P, Feng J, Yang H, Yang T (2016) Degradation of sodium n-butyl xanthate by vacuum UV-ozone (VUV/O3) in comparison with ozone and VUV photolysis. Process Saf Environ Prot 102:64–70. https://doi.org/10.1016/j.psep.2016.02.010
Fujishima A, Zhang X, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep 63:515–582. https://doi.org/10.1016/j.surfrep.2008.10.001
Gardoni D, Vailati A, Canziani R (2012) Decay of ozone in water: a review. Ozone Sci Eng 34(4):233–242. https://doi.org/10.1080/01919512.2012.686354
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C 9(1):1–12. https://doi.org/10.1016/j.jphotochemrev.2007.12.003
Gilmour CR (2012) Water treatment using advanced oxidation processes: application perspectives. University of Western Ontario, London
Giri RR, Ozaki H, Ishida T, Takanami R, Taniguchi S (2007) Synergy of ozonation and photocatalysis to mineralize low concentration 2,4-dichlorophenoxyacetic acid in aqueous solution. Chemosphere 66(9):1610–1617. https://doi.org/10.1016/j.chemosphere.2006.08.007
Glaze WH, Kang JW, Chapin DH (1987) The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng 9:335–342
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8(3–4):553–597. https://doi.org/10.1016/S1093-0191(03)00031-5
Gracia R, Cortés S, Sarasa J, Ormad P, Ovelleiro JL (2000) catalytic ozonation with supported titanium dioxide. The stability of catalyst in water. Ozone Sci Eng 22(2):185–193. https://doi.org/10.1080/01919510008547219
Gray NF (2014) Ozone disinfection. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 599–615
Guettaï N, Ait Amar H (2005) Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part I: parametric study. Desalination 185(1–3):427–437. https://doi.org/10.1016/j.desal.2005.04.048
Hernández-Alonso MD, Coronado JM, Javier Maira A, Soria J, Loddo V, Augugliaro V (2002) Ozone enhanced activity of aqueous titanium dioxide suspensions for photocatalytic oxidation of free cyanide ions. Appl Catal B 39(3):257–267. https://doi.org/10.1016/S0926-3373(02)00119-4
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95(1):69–96. https://doi.org/10.1021/cr00033a004
Hoigne J (1998) Chemistry of aqueous ozone, and transformation of pollutants by ozonation and advanced oxidation processes. In: Hubrec J (ed) The handbook of environmental chemistry quality and treatment of drinking water. Springer, Berlin, pp 83–141
Hsu Y-C, Chen J-H, Yang H-C (2007) Calcium enhanced COD removal for the ozonation of phenol solution. Water Res 41(1):71–78. https://doi.org/10.1016/j.watres.2006.09.012
Hu C, Lan Y, Qu J, Hu X, Wang A (2006) Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J Phys Chem 110(9):4066–4072. https://doi.org/10.1021/jp0564400
Huber MM, Canonica S, Park G-Y, von Gunten U (2003) Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ Sci Technol 37(5):1016–1024. https://doi.org/10.1021/es025896h
Jain B, Singh AK, Kim H, Lichtfouse E, Sharma VK (2018) Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ Chem Lett 16:947–967
Kabra K, Chaudhary R, Sawhney RL (2004) Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: a review. Ind Eng Chem Res 43(24):7683–7696. https://doi.org/10.1021/ie0498551
Kanakaraju D, Glass BD, Oelgemöller M (2014) Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ Chem Lett 12:27–47
Kasprzyk-Hordern B, Ziółek M, Nawrocki J (2003) Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal B 46:639–669. https://doi.org/10.1016/S0926-3373(03)00326-6
Katsoyiannis IA, Canonica S, von Gunten U (2011) Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res 45(13):3811–3822. https://doi.org/10.1016/j.watres.2011.04.038
Khanna A, Shetty VK (2014) Solar light induced photocatalytic degradation of Reactive Blue 220 (RB-220) dye with highly efficient Ag@TiO2 core–shell nanoparticles: a comparison with UV photocatalysis. Sol Energy 99:67–76. https://doi.org/10.1016/j.solener.2013.10.032
Khataee AR, Fathinia M (2013) Chapter 11—recent advances in photocatalytic processes by nanomaterials. In: Suib SL (ed) New and future developments in catalysis. Elsevier, Amsterdam, pp 267–288
Kim I, Tanaka H (2011) Energy consumption for PPCPs removal by O3 and O3/UV. Ozone Sci Eng 33:150–157
Kim S-E, Yamada H, Tsuno H (2004) Evaluation of estrogenicity for 17 β-estradiol decomposition during ozonation. Ozone Sci Eng 26(6):563–571. https://doi.org/10.1080/01919510490885370
Kositzi M, Poulios I, Malato S, Caceres J, Campos A (2004) Solar photocatalytic treatment of synthetic municipal wastewater. Water Res 38(5):1147–1154. https://doi.org/10.1016/j.watres.2003.11.024
Kumar J, Bansal A (2013) Photocatalysis by nanoparticles of titanium dioxide for drinking water purification: a conceptual and state-of-art review. Mater Sci Forum 764:130–150. https://doi.org/10.4028/www.scientific.net/MSF.764.130
Lazar MA, Varghese S, Nair SS (2012) Photocatalytic water treatment by titanium dioxide: recent updates. Catalysts 2:572–601. https://doi.org/10.3390/catal2040572
Lee H, Lee E, Lee C-H, Lee K (2011) Degradation of chlorotetracycline and bacterial disinfection in livestock wastewater by ozone-based advanced oxidation. J Ind Eng Chem 17(3):468–473. https://doi.org/10.1016/j.jiec.2011.05.006
Lekkerkerker-Teunissen K, Knol AH, Derks JG, Heringa MB, Houtman CJ, Hofman-Caris CHM, Beerendonk EF, Reus A, Verberk JQJC, Dijk JC (2013) Pilot plant results with three different types of UV lamps for advanced oxidation. Ozone Sci Eng 35(1):38–48. https://doi.org/10.1080/01919512.2013.721317
Li L, Zhu W, Zhang P, Chen Z, Han W (2003) Photocatalytic oxidation and ozonation of catechol over carbon-black-modified nano-TiO2 thin films supported on Al sheet. Water Res 37(15):3646–3651. https://doi.org/10.1016/S0043-1354(03)00269-0
Li L, Zhu W, Chen L, Zhang P, Chen Z (2005) Photocatalytic ozonation of dibutyl phthalate over TiO2 film. J Photochem Photobiol A 175(2–3):172–177. https://doi.org/10.1016/j.jphotochem.2005.01.020
Linden KG, Mohseni M (2014) 2.8—Advanced oxidation processes: applications in drinking water treatment A2. In: Satinder A (ed) Comprehensive water quality and purification. Elsevier, Waltham, pp 148–172
Ling Y, Liao G, Xie Y, Yin J, Huang J, Feng W, Li L (2016) Coupling photocatalysis with ozonation for enhanced degradation of Atenolol by Ag–TiO2 micro-tube. J Photochem Photobiol A 329:280–286. https://doi.org/10.1016/j.jphotochem.2016.07.007
Liu L, Liu Z, Bai H, Sun DD (2012) Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res 46(4):1101–1112. https://doi.org/10.1016/j.watres.2011.12.009
Macova M, Escher BI, Reungoat J, Carswell S, Chue KL, Keller J, Mueller JF (2010) Monitoring the biological activity of micropollutants during advanced wastewater treatment with ozonation and activated carbon filtration. Water Res 44(2):477–492. https://doi.org/10.1016/j.watres.2009.09.025
Magbanua BS, Savant G, Truax DD (2006) Combined ozone and ultraviolet inactivation of Escherichia coli. J Environ Sci Health Part A 41(6):1043–1055. https://doi.org/10.1080/10934520600620279
Malato S, Maldonado MI, Fernández-Ibáñez P, Oller I, Polo I, Sánchez-Moreno R (2016) Decontamination and disinfection of water by solar photocatalysis: the pilot plants of the Plataforma Solar de Almeria. Mater Sci Semicond Process 42(Part A):15–23. https://doi.org/10.1016/j.mssp.2015.07.017
Malik SN, Saratchandra T, Tembhekar PD, Padoley KV, Mudliar SL, Mudliar SN (2014) Wet air oxidation induced enhanced biodegradability of distillery effluent. J Environ Manag 136:132–138. https://doi.org/10.1016/j.jenvman.2014.01.026
Mano T, Nishimoto S, Kameshima Y, Miyake M (2011) Investigation of photocatalytic ozonation treatment of water over WO3 under visible light irradiation. J Ceram Soc Jpn 119(11):822–827
Mano T, Nishimoto S, Kameshima Y, Miyake M (2015) Water treatment efficacy of various metal oxide semiconductors for photocatalytic ozonation under UV and visible light irradiation. Chem Eng J 264:221–229. https://doi.org/10.1016/j.cej.2014.11.088
Martijn AJ, Kruithof JC (2012) UV and UV/H2O2 treatment: the silver bullet for by-product and genotoxicity formation in water production. Ozone Sci Eng 34:92–100. https://doi.org/10.1080/01919512.2012.649596
Matafonova G, Batoev V (2018) Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: a review. Water Res 132:177–189. https://doi.org/10.1016/j.watres.2017.12.079
Mecha CA (2017) Ultraviolet/solar radiation-ozonation coupled system for treatment of wastewater using metal doped titanium dioxide. Dissertation. Tshwane University of Technology
Mecha AC, Onyango MS, Ochieng A, Fourie CJS, Momba MNB (2016a) Synergistic effect of UV–Vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: a comparative study. J Catal 341:116–125. https://doi.org/10.1016/j.jcat.2016.06.015
Mecha AC, Onyango MS, Ochieng A, Jamil TS, Fourie CJS, Momba MNB (2016b) Uv and solar light photocatalytic removal of organic contaminants in municipal wastewater. Sep Sci Technol 51(10):1765–1778. https://doi.org/10.1080/01496395.2016.1178290
Mecha AC, Onyango MS, Ochieng A, Momba MN (2016c) Impact of ozonation in removing organic micro-pollutants in primary and secondary municipal wastewater: effect of process parameters. Water Sci Technol 74(3):756–765. https://doi.org/10.2166/wst.2016.276
Mecha AC, Onyango MS, Ochieng A, Momba MNB (2017a) Evaluation of synergy and bacterial regrowth in photocatalytic ozonation disinfection of municipal wastewater. Sci Total Environ 601–602:626–635. https://doi.org/10.1016/j.scitotenv.2017.05.204
Mecha AC, Onyango MS, Ochieng A, Momba MNB (2017b) Ultraviolet and solar photocatalytic ozonation of municipal wastewater: catalyst reuse, energy requirements and toxicity assessment. Chemosphere 186:669–676. https://doi.org/10.1016/j.chemosphere.2017.08.041
Mecha AC, Onyango MS, Ochieng A, Momba MN (2018) Inactivation of waterborne pathogens in municipal wastewater using ozone. In: Mujtaba IM, Majozi T, Amosa MK (eds) Water management: social and technological perspectives. CRC Press, Taylor and Francis Group, Boca Raton, pp 275–287
Mena E, Rey A, Acedo B, Beltrán FJ, Malato S (2012) On ozone-photocatalysis synergism in black-light induced reactions: oxidizing species production in photocatalytic ozonation versus heterogeneous photocatalysis. Chem Eng J 204–206:131–140. https://doi.org/10.1016/j.cej.2012.07.076
Meunier L, Canonica S, von Gunten U (2006) Implications of sequential use of UV and ozone for drinking water quality. Water Res 40(9):1864–1876. https://doi.org/10.1016/j.watres.2006.02.030
Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, Hübner U (2018) Evaluation of advanced oxidation processes for water and wastewater treatment: a critical review. Water Res 139:118–131. https://doi.org/10.1016/j.watres.2018.03.042
Miranda AC, Lepretti M, Rizzo L, Caputo I, Vaiano V, Sacco O, Lopes WS, Sannino D (2016) Surface water disinfection by chlorination and advanced oxidation processes: inactivation of an antibiotic resistant E. coli strain and cytotoxicity evaluation. Sci Total Environ 554–555:1–6. https://doi.org/10.1016/j.scitotenv.2016.02.189
Mital GS, Manoj T (2011) A review of TiO2 nanoparticles. Chin Sci Bull 56(16):1639–1657
Moreira NFF, Orge CA, Ribeiro AR, Faria JL, Nunes OC, Pereira MFR, Silva AMT (2015) Fast mineralization and detoxification of amoxicillin and diclofenac by photocatalytic ozonation and application to an urban wastewater. Water Res 87:87–96. https://doi.org/10.1016/j.watres.2015.08.059
Moreira NFF, Sousa JM, Macedo G, Ribeiro AR, Barreiros L, Pedrosa M, Faria JL, Pereira MFR, Castro-Silva S, Segundo MA, Manaia CM, Nunes OC, Silva AMT (2016) Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: micropollutants, antibiotic resistance genes and estrogenic activity. Water Res 94:10–22. https://doi.org/10.1016/j.watres.2016.02.003
Mozia S (2010) Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep Purif Technol 73(2):71–91. https://doi.org/10.1016/j.seppur.2010.03.021
Müller TS, Sun Z, Kumar G, Itoh K, Murabayashi M (1998) The combination of photocatalysis and ozonolysis as a new approach for cleaning 2,4-dichlorophenoxyaceticacid polluted water. Chemosphere 36(9):2043–2055. https://doi.org/10.1016/S0045-6535(97)10089-3
Nahar MS, Hasegawa K, Kagaya S (2006) Photocatalytic degradation of phenol by visible light-responsive iron-doped TiO2 and spontaneous sedimentation of the TiO2 particles. Chemosphere 65(11):1976–1982. https://doi.org/10.1016/j.chemosphere.2006.07.002
Nam T, Patrick D, Kaur BS (2015) Sonochemical techniques to degrade pharmaceutical organic pollutants. Environ Chem Lett 13:251–268
Nam W, Kim J, Han G (2002) Photocatalytic oxidation of methyl orange in a three-phase fluidized bed reactor. Chemosphere 47(9):1019–1024. https://doi.org/10.1016/S0045-6535(01)00327-7
Oh BS, Park SJ, Jung YJ, Park SY, Kang JW (2007) Disinfection and oxidation of sewage effluent water using ozone and UV technologies. Water Sci Technol 55(1–2):299–306. https://doi.org/10.2166/wst.2007.036
Parsons S (ed) (2004) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London
Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, Kontos AG, Dunlop PSM, Hamilton JWJ, Byrne JA, O’Shea K, Entezari MH, Dionysiou DD (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal 125:331–349. https://doi.org/10.1016/j.apcatb.2012.05.036
Petala M, Kokokiris L, Samaras P, Papadopoulos A, Zouboulis A (2009) Toxicological and ecotoxic impact of secondary and tertiary treated sewage effluents. Water Res 43(20):5063–5074. https://doi.org/10.1016/j.watres.2009.08.043
Pham T-D, Lee B-K (2014) Cu doped TiO2/GF for photocatalytic disinfection of Escherichia coli in bioaerosols under visible light irradiation: application and mechanism. Appl Surf Sci 296:15–23. https://doi.org/10.1016/j.apsusc.2014.01.006
Pirkanniemi K, Sillanpää M (2002) Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere 48(10):1047–1060. https://doi.org/10.1016/S0045-6535(02)00168-6
Postigo C, Richardson SD (2014) Transformation of pharmaceuticals during oxidation/disinfection processes in drinking water treatment. J Hazard Mater 279:461–475. https://doi.org/10.1016/j.jhazmat.2014.07.029
Poznyak T, Tapia R, Vivero J, Chairez I (2006) Effect of pH to the decomposition of aqueous phenols mixture by ozone. J Mexican Chem Soc 50(1):28–35
Rajeswari R, Kanmani S (2009a) A study on synergistic effect of photocatalytic ozonation for carbaryl degradation. Desalination 242(1–3):277–285. https://doi.org/10.1016/j.desal.2008.05.007
Rajeswari R, Kanmani S (2009b) TiO2-based heterogeneous photocatalytic treatment combined with ozonation for carbendazim degradation. Iran J Environ Health Sci Eng 6(2):61–66
Rayaroth MP, Aravind UK, Aravindakumar CT (2016) Degradation of pharmaceuticals by ultrasound-based advanced oxidation process. Environ Chem Lett 14:259–290
Reungoat J, Macova M, Escher BI, Carswell S, Mueller JF, Keller J (2010) Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water Res 44(2):625–637. https://doi.org/10.1016/j.watres.2009.09.048
Rizzo L (2011) Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res 45(15):4311–4340. https://doi.org/10.1016/j.watres.2011.05.035
Rizzo L, Malato S, Antakyali D, Beretsou VG, Đolić MB, Gernjak W, Heath E, Ivancev-Tumbas I, Karaolia P, Ribeiro ARL, Mascolo G, McArdell CS, Schaar H, Silva AMT, Fatta-Kassinos D (2019) Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci Total Environ 655:986–1008. https://doi.org/10.1016/j.scitotenv.2018.11.265
Rodríguez EM, Rey A, Mena E, Beltrán FJ (2019) Application of solar photocatalytic ozonation in water treatment using supported TiO2. Appl Catal B 254:237–245. https://doi.org/10.1016/j.apcatb.2019.04.095
Rodríguez-Chueca J, Ormad MP, Mosteo R, Ovelleiro JL (2015) Kinetic modeling of Escherichia coli and Enterococcus sp. inactivation in wastewater treatment by photo-Fenton and H2O2/UV–Vis processes. Chem Eng Sci 138:730–740. https://doi.org/10.1016/j.ces.2015.08.051
Rupa AV, Manikandan D, Divakar D, Sivakumar T (2007) Effect of deposition of Ag on TiO2 nanoparticles on the photodegradation of Reactive Yellow-17. J Hazard Mater 147(3):906–913. https://doi.org/10.1016/j.jhazmat.2007.01.107
Shinpon W, Fumihide S, Katsuyuki N (2002) A synergistic effect of photocatalysis and ozonation on decomposition of formic acid in an aqueous solution. Chem Eng J 87(2):261–271. https://doi.org/10.1016/S1385-8947(02)00016-5
Sievers M (2011) 4.13—Advanced oxidation processes A2. In: Peter W (ed) Treatise on water science. Elsevier, Oxford, pp 377–408
Silva CSCG (2008) Synthesis, spectroscopy and characterization of titanium dioxide based photocatalysts for the degradative oxidation of organic pollutants. Dissertation, Universidade do Porto
Silva DB, Cruz-Alcalde A, Sans C, Giménez J, Esplugas S (2019) Performance and kinetic modelling of photolytic and photocatalytic ozonation for enhanced micropollutants removal in municipal wastewaters. Appl Catal B 249:211–217. https://doi.org/10.1016/j.apcatb.2019.02.072
Singh S (2012) Ozone treatment of municipal wastewater effluent for oxidation of emerging contaminants and disinfection. Dissertation, University of Windsor
Sivagami K, Sakthivel KP, Nambi IM (2018) Advanced oxidation processes for the treatment of tannery wastewater. J Environ Chem Eng 6:3656–3663. https://doi.org/10.1016/j.jece.2017.06.004
Sobana N, Muruganadham M, Swaminathan M (2006) Nano-Ag particles doped TiO2 for efficient photodegradation of Direct azo dyes. J Mol Catal A 258(1–2):124–132. https://doi.org/10.1016/j.molcata.2006.05.013
Solís RR, Rivas FJ, Pérez-Bote JL, Gimeno O (2015) Photocatalytic ozonation of 4-chloro-2-methylphenoxyacetic acid and its reaction intermediate 4-chloro-2-methyl phenol. J Taiwan Inst Chem Eng 46:125–131. https://doi.org/10.1016/j.jtice.2014.09.010
Solís RR, Rivas FJ, Martínez-Piernas A, Agüera A (2016) Ozonation, photocatalysis and photocatalytic ozonation of diuron. Intermediates identification. Chem Eng J 292:72–81. https://doi.org/10.1016/j.cej.2016.02.005
Sotelo JL, Beltrán FJ, Benitez FJ, Beltrán-Heredia J (1989) Henry’s law constant for the ozone-water system. Water Res 23(10):1239–1246. https://doi.org/10.1016/0043-1354(89)90186-3
Sousa JM, Macedo G, Pedrosa M, Becerra-Castro C, Castro-Silva S, Pereira MFR, Silva AMT, Nunes OC, Manaia CM (2016) Ozonation and UV254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2016.03.096
Stalter D, Magdeburg A, Oehlmann J (2010) Comparative toxicity assessment of ozone and activated carbon treated sewage effluents using an in vivo test battery. Water Res 44(8):2610–2620. https://doi.org/10.1016/j.watres.2010.01.023
Suave J, José HJ, Moreira RFPM (2014) Degradation of polyvinylpyrrolidone by photocatalytic ozonation and evaluation of the influence of some operational parameters. Ozone Sci Eng 36(6):560–569. https://doi.org/10.1080/01919512.2014.894452
Suzuki H, Araki S, Yamamoto H (2015) Evaluation of advanced oxidation processes (AOP) using O3, UV, and TiO2 for the degradation of phenol in water. J Water Proc Eng 7:54–60. https://doi.org/10.1016/j.jwpe.2015.04.011
Swarnakar P, Kanel SR, Nepal D, Jiang Y, Jia H, Kerr L, Goltz MN, Levy J, Rakovan J (2013) Silver deposited titanium dioxide thin film for photocatalysis of organic compounds using natural light. Sol Energy 88:242–249. https://doi.org/10.1016/j.solener.2012.10.014
Tembhekar PD, Padoley KV, Mudliar SL, Mudliar SN (2015) Kinetics of wet air oxidation pretreatment and biodegradability enhancement of a complex industrial wastewater. J Environ Chem Eng 3(1):339–348. https://doi.org/10.1016/j.jece.2014.02.009
Teoh WY, Amal R, Mädler L, Pratsinis SE (2007) Flame sprayed visible light-active Fe–TiO2 for photomineralisation of oxalic acid. Catal Today 120(2):203–213. https://doi.org/10.1016/j.cattod.2006.07.049
Trapido M, Hirvonen A, Veressinina Y, Hentunen J, Munter R (1997) Ozonation, ozone/UV and UV/H2O2 degradation of chlorophenols. Ozone Sci Eng 19(1):75–96. https://doi.org/10.1080/01919519708547319
Tsydenova O, Batoev V, Batoeva A (2015) Solar-enhanced advanced oxidation processes for water treatment: simultaneous removal of pathogens and chemical pollutants. Int J Environ Res Public Health 12:9542–9561. https://doi.org/10.3390/ijerph120809542
Umar M, Aziz HA (2013) Photocatalytic degradation of organic pollutants in water. In: Rashed MN (ed) Organic pollutants—monitoring, risk and treatment. InTech, London, pp 195–208
Valério A, Wang J, Tong S, Souza AAUd, Hotza D, González SYG (2020) Synergetic effect of photocatalysis and ozonation for enhanced tetracycline degradation using highly macroporous photocatalytic supports. Chem Eng Process 149:107838. https://doi.org/10.1016/j.cep.2020.107838
Vamathevan V, Tse H, Amal R, Low G, McEvoy S (2001) Effects of Fe3+ and Ag+ ions on the photocatalytic degradation of sucrose in water. Catal Today 68(1–3):201–208. https://doi.org/10.1016/S0920-5861(01)00301-7
Vamathevan V, Amal R, Beydoun D, Low G, McEvoy S (2002) Photocatalytic oxidation of organics in water using pure and silver-modified titanium dioxide particles. J Photochem Photobiol A 148(1–3):233–245. https://doi.org/10.1016/S1010-6030(02)00049-7
Van Aken P, Van den Broeck R, Degrève J, Dewil R (2015) The effect of ozonation on the toxicity and biodegradability of 2,4-dichlorophenol-containing wastewater. Chem Eng J 280:728–736. https://doi.org/10.1016/j.cej.2015.06.019
van Grieken R, Marugán J, Sordo C, Martínez P, Pablos C (2009a) Photocatalytic inactivation of bacteria in water using suspended and immobilized silver–TiO2. Appl Catal B 93(1–2):112–118. https://doi.org/10.1016/j.apcatb.2009.09.019
van Grieken R, Marugán J, Sordo C, Pablos C (2009b) Comparison of the photocatalytic disinfection of E. coli suspensions in slurry, wall and fixed-bed reactors. Catal Today 144(1–2):48–54. https://doi.org/10.1016/j.cattod.2008.11.017
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol 42(3):251–325. https://doi.org/10.1080/10643389.2010.507698
Wang W-L, Wu Q-Y, Huang N, Xu Z-B, Lee M-Y, Hu H-Y (2018) Potential risks from UV/H2O2 oxidation and UV photocatalysis: a review of toxic, assimilable, and sensory-unpleasant transformation products. Water Res 141:109–125. https://doi.org/10.1016/j.watres.2018.05.005
Westerhoff P, Debroux J, Aiken G, Amy G (1999) Ozone-induced changes in natural organic matter (NOM) structure. Ozone Sci Eng 21:551–570
Wu JJ, Muruganandham M, Chang LT, Lee GJ, Batalova VN, Mokrousov GM (2011) Catalytic ozonation of oxalic acid using SrTiO3 catalyst. Ozone Sci Eng 33:74–79
Yamazaki S, Matsunaga S, Hori K (2001) Photocatalytic degradation of trichloroethylene in water using TiO2 pellets. Water Res 35(4):1022–1028. https://doi.org/10.1016/S0043-1354(00)00347-X
Zaleska A (2008) Doped-TiO2: a review. Recent Patents Eng 2:157–164
Zangeneh H, Zinatizadeh AAL, Habibi M, Akia M, Hasnain Isa M (2015) Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: a comparative review. J Ind Eng Chem 26:1–36. https://doi.org/10.1016/j.jiec.2014.10.043
Žegura B, Heath E, Černoša A, Filipič M (2009) Combination of in vitro bioassays for the determination of cytotoxic and genotoxic potential of wastewater, surface water and drinking water samples. Chemosphere 75(11):1453–1460. https://doi.org/10.1016/j.chemosphere.2009.02.041
Zhou H, Smith DW (2002) Advanced technologies in water and wastewater treatment. J Environ Eng Sci 1:247–264. https://doi.org/10.1139/S02-020
Zou L, Zhu B (2008) The synergistic effect of ozonation and photocatalysis on color removal from reused water. J Photochem Photobiol A 196(1):24–32. https://doi.org/10.1016/j.jphotochem.2007.11.008
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mecha, A.C., Chollom, M.N. Photocatalytic ozonation of wastewater: a review. Environ Chem Lett 18, 1491–1507 (2020). https://doi.org/10.1007/s10311-020-01020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01020-x