Abstract
Groundwater contamination with arsenic and fluoride has affected over 300 million people worldwide. Ingestion of arsenic and fluoride for extended period of time or at high concentration causes severe health effects. Arsenic can cause thickening and discoloration of the skin, cardiovascular disorders, cancer and skin lesions. Excessive intake of fluoride leads to dental and skeletal fluorosis and bone deformities. This report reviews the distribution of arsenic and fluoride contamination, their sources, mobilization and associated health risks. Remediation technologies to remove arsenic and fluoride are presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various natural and anthropogenic activities cause metal and non-metal contamination of different aquatic and terrestrial ecosystems. Agricultural and industrial based societies are being exposed daily to hazardous agricultural pesticides, herbicides, uncontrolled sewage discharge, toxic metals and other industrial chemicals (Husain et al. 2008; Bibi et al. 2008). Being toxic, arsenic and fluoride both are one of the biggest contributors to global water crisis and pose major health concerns resulting from drinking water (Bibi et al. 2015a). This issue has been aggravated due to increasing dependence on groundwater in many countries. High levels of arsenic and fluoride are simultaneously present in groundwater at numerous places throughout the world. The cause of co-occurrence of these contaminants in groundwater can be natural as well as anthropogenic. Drinking water containing high concentrations of fluoride and arsenic primarily from natural geogenic contamination is the major source of human exposure (Gebel 1999; Ruiz-Payan et al. 2005). Arsenic and fluoride release into environments by various natural process and human activities (Bibi et al. 2015a; Vithanage and Bhattacharya 2015; Rasool et al. 2016b). Arsenic occurs in numerous forms depending upon pH and redox potential of groundwater. On the other hand, high fluoride concentrations present in groundwater from calcium (Ca) poor aquifers and may also increase in groundwater where cation exchange of sodium (Na) for calcium takes place (Bibi et al. 2015b; Amna Ali et al. 2015; Ali et al. 2016).
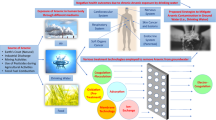
There is knowledge on the toxicity of fluoride and arsenic individually; however, very limited literature is available about the effects of combined exposure to these toxicants. An in situ experiment showed that Swiss albino male mice exposure to arsenic and fluoride led to a substantial depletion of blood δ-aminolevulinic acid dehydratase (ALAD) activity and glutathione (GSH) level (Mittal and Flora 2006). In another laboratory experiment, it was found that combined exposure to arsenic and fluoride considerably reduced the levels of brain biogenic amines (Flora and Mittal 2009). Arsenic and fluoride act as an unseen exterminates in water as they do not impart any odour, colour or taste. The present study provides a comprehensive overview about arsenic and fluoride contamination, toxicity, sources and existing removal technologies that are being used for the treatment. Moreover, problem associated with current methods and future research directions for removal technologies have also been discussed. This study will be very helpful to implement suitable and cost-effective removal technology for safe water supply to rural population in developing countries.
Arsenic contamination
Arsenic has been reported worldwide including Argentina (Smedley and Kinniburgh 2002), Australia (Appleyard et al. 2006), Bolivia (Archer et al. 2005), Chile (Romero et al. 2003), Finland (Kurttio et al. 1999), Greece (Kouras et al. 2007), Germany (Mkandawire and Dudel 2005), Ghana (Asante et al. 2007), Hungary (Rowland et al. 2011), Italy (Angelone et al. 2009), Mexico (Camacho et al. 2011), Romania (Rowland et al. 2011), Spain (García-Sánchez et al. 2005), Thailand (Williams et al. 1996) and USA (Schreiber et al. 2000). However, the arsenic-contaminated water via natural sources in various regions of East and South Asia is of bigger apprehension and affects the health of millions of people (Berg et al. 2001; Polya et al. 2005; Mukherjee et al. 2006). In East Asia it includes Cambodia (Luu et al. 2009), China (Xie et al. 2009), Mongolia (Guo et al. 2008), Taiwan (Liao et al. 2011) and Vietnam (Berg et al. 2001); and in South Asia Bangladesh (Chen et al. 2011), India (Kar et al. 2010), Nepal (Pokhrel et al. 2009) and Pakistan (Baig et al. 2012; Rasool et al. 2016a, b). Arsenic-contaminated groundwater affects the life of nearly 60 million people while arsenicosis has been reported among 700,000 people (Fewtrell et al. 2005). The predominant form of inorganic arsenic in aqueous oxic environments is arsenate [As(V) as H3AsO4, H2AsO−14, HAsO−24 and AsO−34], whereas arsenite [As(III) as H3AsO3 and H2AsO3 −] is more prevalent in anoxic environments (Oremland and Stolz 2003). Zero-valent (As0) and tri-valent (As3−) are rare in aquatic environments (Goldberg and Johnston 2001; Mandal and Suzuki 2002).
Sources of arsenic
The sources of arsenic contamination in water could be geogenic such as occurrence of arsenic in geological formations in the form of arsenic-bearing rocks and minerals as well as human-induced, i.e. agricultural and industrial, activities. The details are discussed below.
Geogenic source
Arsenic is present as a chief constituent of more than 200 minerals, together with elemental arsenic, sulphides, arsenides, oxides, arsenites and arsenates (Smedley and Kinniburgh 2002). Rittle et al. (1995) reported authigenic arsenopyrite in sediments, and microbial precipitation is responsible for the formation of orpiment (Newman et al. 1998). Many sources of arsenic contamination that are present naturally include geothermal, biogeochemical and geohydrological disbanding of arsenic compounds from arsenopyrite (Mondal et al. 2006).
Anthropogenic sources
Anthropogenic sources of arsenic contamination include processing of contaminated ores (Leist et al. 2000), ingredient of pesticides (McArthur et al. 2001), feed additives (Mondal et al. 2006), hazardous waste site (seepage) (Mondal et al. 2006) and arsenic-contaminated coal burning (McNeill and Edwards 1997; Abdullah et al. 2015). Geothermal waste, mining and industrial discharge are the main source of river pollution which is a chief drinking water source for large number of people (Smedley and Kinniburgh 2002). In California, acid seepage from Richmond mine located at Iron Mountain reported 850,000 g−l of arsenic with highest concentration reported so far (Nordstrom and Alpers 1999).
Mobilization
Arsenic concentration in groundwater is dependent on several factors like depth of well, characteristic and nature of aquifers whether confined or unconfined. Sahoo and Kim (2013) proved the fact that soils contaminated with arsenate and higher levels of silicate or phosphate may increase the mobility of arsenic(V) in subsurface (Sahoo and Kim 2013). In aqueous phase, mobilization of arsenic has been affected by differential adsorptive affinities of arsenite and arsenate to various mineral surfaces (i.e. alumina, ferrihydrite; Mondal et al. 2006). Various chemical, physical and microbial processes initiate the subsurface arsenic mobilization (Islam et al. 2004). Theories proposed to justify this mobilization include: (1) oxidation of arsenic-containing pyrites (Bhattacharjee et al. 2005), (2) iron oxide reduction release of arsenic(V) (Oremland and Stolz 2003), (3) reduction of iron oxides by allochthonous organic matter (from dissolved organics in recharging waters; Santini et al. 2002), (4) exchange of adsorbed arsenic(V) with fertilizer phosphates (Larios et al. 2013), (5) reduction by microbial oxidation of sedimentary carbon (Stüben et al. 2003), (6) displacement of arsenic by carbonate (Anawar et al. 2004).
Mobility of arsenic in the environment is a defining factor that determines the risk of exposure (Frankenberger and Arshad 2002). Arsenic exists most commonly in water as inorganic species arsenite (H3AsO3, H2AsO3 1−, HAsO3 2−) and arsenate (H3AsO4, H2AsO4 1−, HAsO4 2−, AsO4 3−), which dominate in reducing environments and at oxidizing conditions (Zhang et al. 2008). Both arsenite and arsenate are anionic species, but at typical pH values of 6–9 arsenite remains predominantly neutral. The neutral oxidation state results in increased remedial difficulties (Mohan and Pittman 2007). Reduction process releases 10–13 times more arsenic than oxidation (Kanel et al. 2005). However, the competition with organic anions for sorption sites is the main process for solid-phase release of arsenic while redox reactions perform only negligible role.
Health risk of arsenic
Prolonged exposure to 0.04 µg−kg−day or higher concentration of arsenic leads to gastrointestinal, haematological and other peripheral neuropathic effects (Brown and Ross 2002), while consumption of high concentration at once results in digestive system problems, i.e. stomach pain or vomiting, which lead to coma or death. Chronic poisoning with arsenic results in hypertension, cardiovascular and lung diseases, diabetes and different type of cancers (i.e. lungs, skin, liver, kidney, uterus Escobar et al. 2016). Drinking water contaminated with even low concentrations of 5–10 µg L−l can cause depigmentation, skin lesion and hyperkeratosis (Duarte et al. 2011). Brown and Ross (2002) investigated that chronic arsenic dose of 0.04 µg−kg−day or higher for 6 months to 3 years or slow dose of 0.01 µg−kg−day or higher for 5–15 years can cause hyperpigmentation. In 2001, United State Environmental Protection Agency (USEPA) replaced the 50 µg L−l old slandered with 10 µg L−l, while Pakistan Environmental Protection Agency (PAKEPA) still uses the old standard of 50 µg−l.
Removal of arsenic from water
Selection of removal technique depends on arsenic speciation, physio-chemical properties of water and concentration of SO−24, PO4 3− and Fe, etc. All methods are subjected on fundamental chemical processes that are applied disjointedly, concurrently or in series. All arsenic removal technologies require pH adjustment for optimized performance (Bissen and Frimmel 2003). Removal processes like sorption are predominantly sensitive to pH and function well at low pH (Wang et al. 2004). Arsenic removal is more efficient when it is present in pentavalent state as oxianions, i.e. H2AsO4 − and HAsO4 2−, at a pH range between 2 and 12 under oxidizing conditions, while the trivalent arsenic (H3AsO3) can be found at pH below 9.2, under reducing conditions (Wang et al. 2004). Due to this fact, many arsenic removal methods use oxidation step prior to other processes; however, this step is facilitated with other physical or chemical modifications for efficient arsenic removal. Diverse arsenic removal technologies are generally based on six principles: (1) oxidation and filtration, (2) co-precipitation: oxidation of arsenic(III) to arsenic(V) and then arsenic(V) removal by coagulation, sedimentation and filtration, (3) biological oxidation: microbial oxidation of arsenic(III) to arsenic(V) followed by removal through iron and manganese oxides, (4) adsorption, (5) ion exchange (by cation and anion exchange resins) and (6) membrane technology. For optimized performance, various arsenic treatment technologies require pH adjustment as it directly affects arsenic speciation in raw water (Bissen and Frimmel 2003). Removal processes like sorption are predominantly sensitive to pH and function well at low pH (Wang et al. 2004) because for most media types, a pH greater than 8.5 decreases the adsorption capacity of the media and consequently increases the replacement cost of single-use media.
Pre-treatment
A wide range of chemicals (chlorine, ozone bleaching powder, hydrogen peroxide or potassium permanganate) are used to oxidize arsenic(III) to arsenic(V) in different conditions. However, use of these chemicals can lead to undesirable by-products (Gallard and von Gunten 2002). Ultraviolet light was also not effective, because 7000 times the ultraviolet dose required for Escherichia Coli inactivation oxidized only 73 % arsenic(III) (Clifford and Ghurye 2002). Ultraviolet photooxidation shows promising conversion of arsenic(III) when water is spiked with sulphite (Clifford and Ghurye 2002). Dodd et al. (2006) studied arsenic(III) oxidation kinetics through chloramines, ozone and aqueous chlorine relation for drinking water treatment. In addition to chemical oxidation, arsenic oxidative process has also been catalysed by some species of bacteria that were applied in groundwater (Jain and Singh 2012). This technique can be applied generally under reducing aquifer conditions holding iron and manganese (Jain and Singh 2012). Iron- and manganese-oxidizing bacteria can transform arsenic(III) to arsenic(V) which is naturally present in groundwater. Arsenic can be removed by adsorption and co-precipitation. Prior to adsorption, soluble iron and arsenic(III) are oxidized and then arsenic(V) adsorbs onto iron hydroxide precipitates that are eventually filtered out of solution. Arsenic removal efficiency is determined by iron and arsenic ratio and preliminary iron concentration. In some cases, ferric coagulant is added initially for efficient arsenic removal, and this process is independent of pH in the range of 5.5–8.5 (Violante and Pigna 2009). Furthermore, at pH 4.0 more than in neutral or alkaline systems the formation of aluminium arsenate precipitates seemed to be favoured (Violante and Ricciardella 2006). However, high levels of orthophosphates, silicates and organic matter weaken the process as it increases the competition for sorption sites (Fields et al. 2000a, b). Numerous studies also described arsenic sorption on manganese oxides and chemical oxidation (Tournassat et al. 2002). Hug et al. (2001) investigated solar oxidation and developed a simple procedure for arsenic removal at neutral pH through locally accessible resources, without chemicals addition and pH adjustment, in order to cater with arsenic problem in Bangladesh drinking water. Furthermore, the formation of iron arsenate precipitates onto the surfaces of iron oxides seems to be favoured at low pH values (Violante and Gaudio 2007).
Reduction in arsenic concentration has also been observed due to water oxidation all through collection and storage in houses (Ahmed 2001). Investigation carried out in Bangladesh demonstrates that arsenic removal and sedimentation depend upon precipitating iron (Ahmed 2001). Study showed that arsenic content reduced by more than half through sedimentation of 380–480 µg L−l arsenic contaminated well water with CaCO3 and 8–12 µg L−l with iron but reduction up to desired level was not achieved (Ahmed 2001). Different studies reported 0–25 % reduction in arsenic concentration via sedimentation process. Generally, passive sedimentation has been suggested as abortive approach as it failed to achieve desired arsenic level (Kinniburgh and Kosmus 2002).
Biological removal
Microbial sorption, oxidation–reduction, complexation, co-precipitation and methylation–demethylation can reduce or mobilize arsenic contamination. Ex situ or in situ bioadsorption can be used; i.e. use of biofilm of either live or dead microbes (Kamran et al. 2014; bacteria, algae, aquatic macrophytes and biopolymers) for adsorption or co-precipitation of contaminants also ascertained to be useful (Hartley et al. 2009; Kamran et al. 2015). Iron bacteria facilitate both arsenic(III) and arsenic(V) adsorption and precipitation onto the biological flocks (Hartley et al. 2009; Wang and Zhao 2009). Various natural low-cost biological materials, i.e. sedges, waste biomass milled bones, sorghum and cellulose, have been investigated for remediation (Kamran et al. 2016a). Teixeira and Ciminelli (2005) investigated chicken feather biomass as adsorptive media due to high protein contents. Selected arsenic(III) adsorption at the rate of 270 µmol arsenic(III)/g of biomass took place at relatively low pH (Teixeira and Ciminelli 2005). Natural biogenic hydroxyapatite (HAPb) acquired from charred cow bones evidenced as favourable material for arsenic(V) removal from water in specific conditions (1000 µg L−l, 5 g L−l adsorbent, circumneutral pH, 24-h contact time) (Czerniczyniec et al. 2007). Phytofiltration is another emerging technique using plants to remove contaminants from water. Huang et al. (2004, 2015a, b) and Kamran et al. (2016b) studied arsenic uptake by two hydroponically cultured arsenic hyperaccumulating plants (Pteris vittata and Pteris creticacv. Mayii) to remove arsenic (20–500 µg L−l). Results show that P. vittata reduced arsenic concentration from 20 to 0.4 µg L−l and from 200 to 2.8 µg L−l in 24 h. Jasrotia et al. (2015) studied the potential of aquatic plant species for phytoremediation of arsenic. Water hyacinth (Eichhornia crassipes) and two algae (Chlorodesmis sp. and Cladophora sp.) Cladophora sp. was perceived to tolerate up to 6 mg L−1 of arsenic, whereas water hyacinth and Chlorodesmis sp. could persist under 2 and 4 mg L−1 arsenic, respectively. It was found that Cladophora sp. can reduce arsenic concentration from 6 to 0.1 mg L−l, while water hyacinth reduced only 20 % of arsenic. Thus, Cladophora sp. was proposed to be the best option for coinciding treatment of both arsenic-enriched sewage and brine.
A phytoremediation study was conducted to evaluate the arsenic uptake potential of two Cyperaceae species, Schoenoplectus americanus and Eleocharis macrostachya, collected near the towns of Chihuahua State, Mexico (Bundschuh et al. 2010). It was found that both species are able to survive at high arsenic levels and can be used for rhizofiltration because 97 % of the plants tolerate the arsenic with no visible effect on plant growth. Bundschuh et al. (2007) investigated dried macroalgae (Spyrogira spp.) for arsenic removal from acid mine drainage, and 80–90 % removal was accomplished within 4 days. Aquatic plant species, such as species Ranunculus trichophyllus, Ranunculus peltatus spp. saniculifolius, L. minor and Azolla caroliniana, and the leaves of Juncus effusus, also had very high potential for arsenic phytofiltration when they were introduced into constructed treatment wetlands or natural water bodies (Parmar and Singh 2015).
Another relatively emerging method is the biological oxidation of manganese and iron as a treatment method for arsenic removal. Groundwater contaminated with arsenic is mostly under reducing conditions and contains iron and manganese. Based on this fact the filters for removal of iron and manganese are populated with iron- and manganese-oxidizing bacteria, which can in turn oxidize arsenic(III) efficiently. Currently, the role of microbial processes in mineral oxide formation and removal of arsenic during water treatment is unknown. The formation of manganese(III/IV) oxides contributes to abiotic arsenic(III) oxidation and immobilization of arsenic(V) by sorption to ferric(III) oxides (Nitzsche et al. 2015a, b). Biological iron and manganese oxidation has also been applied for groundwater removal of arsenic without any oxidizing agents (Zouboulis and Katsoyiannis 2005). Rate of bacterial oxidation of arsenic(III) to arsenic(V) is considerably higher than manganese oxide-added abiotic oxidation (Katsoyiannis et al. 2004). Therefore, bacteria play vital role in arsenic(III) oxidation and reactive manganese oxide surfaces generation for dissolved arsenic(III) and arsenic(V) removal.
Co-precipitation
Co-precipitation has been widely practised in various pilot-scale applications to treat arsenic-contaminated water. Three processes are normally used for chemical precipitation (Chwirka et al. 2000; Sancha 2006): (1) lime softening, (2) gravity coagulation–filtration and (3) microfiltration. Lime softening solely for arsenic removal has limitations as it is not economical or cost-effective. However, lime softening when accomplished for removing hardness, this process can be further enhanced to remove arsenic. Additional lime is added in order to increase the pH above 10.5 and to remove arsenic. In this pH range magnesium hydroxide precipitates and arsenic can be removed by co-precipitation. However, arsenic co-precipitation by calcium carbonate (i.e. pH <0.5) is not very efficient (removal efficiency <10 %; Fields et al. 2000b).
Coagulation
The coagulation–filtration process is rapidly used technique using iron(III) for arsenic(V) removal in water treatment process on pilot scale. For effective arsenic removal, the coexisting arsenic(III) is commonly converted to arsenic(V) before removal. Research studies show more efficient removal of arsenic(III) with higher initial concentrations; however, no significant removal efficiency was observed for initial arsenic(III) concentrations in range of 10–100 μg−l. The process using iron(III) can safely meet the permissible limit of 10 μg L−l, only when the initial arsenic(III) concentration is less than 25 μg−l and the iron(III) amount more than 5 mg L−l. The efficient removal of arsenic(III) is limited due to the fact that arsenic(III) is usually present in uncharged H3AsO3 form under neutral pH conditions which is not adsorbed on iron oxy-hydroxides (FeOOH); the product of iron(III) hydrolysis (Ouzounis et al. 2015).
Coagulation–filtration includes the adsorption of arsenic to iron hydroxide. The process can trap arsenic(V) by particle agglomeration. However, pre-oxidation is required due to arsenic(III) removal efficiency and neutral charge at natural pH conditions (Pokhrel et al. 2005). Generally, water characteristics determine the efficiency and suitability of the system, i.e. natural organic matter, dosage and type of coagulant, pH and mixing intensity (Pokhrel et al. 2005). In general, under optimized conditions, coagulation–filtration systems can achieve over 90 % arsenic(V) removal and produce water with less than 5 µg−l of arsenic(V). Research shows that iron-based coagulants, i.e. ferric sulphate and ferric chloride, are more efficient in removing arsenic(V) as compared to aluminium-based coagulants (Pokhrel et al. 2005). Coagulation-based microfiltration uses the same coagulation process with modification of the granular media filtration step. However, the membrane needs to be backwashed periodically in order to remove solids and restore its hydraulic capacity (Thirunavukkarasu et al. 2003). New methods to remediate arsenic-contaminated water continue to be studied, particularly to fill the need for accessible methods that can significantly impact developing communities. A combination of cactus mucilage and ferric(Fe(III)) salt was investigated as a flocculation–coagulation system to remove arsenic(As) from water. Arsenic(V) solutions, ferric nitrate and mucilage suspensions were mixed and left to stand for various periods of time. At neutral pH, removal was dependent on iron(III) and mucilage concentration, and the age of the iron(III) solution. It was found that arsenic removal and settling rates were pH dependent; arsenic removal was between 52 (high pH) and 66 % (low pH) (Fox et al. 2016). Methylated arsenic is present everywhere in earth system (Hu et al. 2015a, b). So far, however, little information has been collected regarding their removal by coagulation. Methyl substitution during coagulation process has been used for monomethyl and dimethyl arsenate from drinking water (Hu et al. 2015a, b). By increasing the methyl group, negatively charged arsenic species decreased, which decreased the removal of methylated arsenic by coagulation. Hydroxide flocks adsorption was the main mechanism in coagulation. Additionally, traditional oxidants use and aid of coagulation revealed inadequate assistance for refining coagulation removal of dimethyl arsenate (Hu et al. 2015a, b).
Ion exchange
Ion exchange is physio-chemical method where exchange of ions took place between a solid resin and solution phase. The solid resin is typically a network of three-dimensional hydrocarbons holding many electrostatically bound ionizable groups. In treatment of drinking water, this technique is mostly applied for removing hardness and nitrates. This method can typically decrease arsenic concentrations from 50 to 10 µg L−l (Nguyen et al. 2009). However, its efficiency depends on the characteristics of untreated water and the contaminants. However, ion exchange is less common than precipitation–co-precipitation technology (Nguyen et al. 2009).
Zirconium oxide nanoparticles (HAIX–Zr) impregnated with hybrid anion exchange resins demonstrate high arsenic removal capacity. Non-hazardous and easy-to-transport, pre-calcined zirconium oxide has been used for the synthesis of hydrous zirconium oxide and tested for effective removal of arsenic(V) and arsenic(III). Over several cycles of exhaustion and regeneration, phosphate and silica competed for arsenic adsorption. Due to high regeneration ability of hydrous zirconium oxide nanoparticles (greater 90 %), hybrid anion exchange resins became more sustainable option for regeneration and reuse of hydrous zirconium oxide nanoparticles for effective reduction of arsenic concentration for numerous cycles. Dissimilar to other iron- or aluminium-based adsorbents, hydrous zirconium oxide nanoparticles are chemically stable at landfill conditions and could be disposed safely without leaching arsenic (Padungthon et al. 2015).
Membrane technology
Membrane technology eliminates an extensive variety of pollutants from water. But it also yields a higher volume of residues and is considered more costly as compared to other treatment technologies. Thus, adsorption precipitation–co-precipitation and ion exchange are more commonly used. Four chief membrane techniques include: reverse osmosis, microfiltration, ultrafiltration and nanofiltration. All the afore-mentioned processes are derived by pressure and depend on the size of particles to be passed through the membranes or by the pore size of membrane (Nguyen et al. 2009). The pore size determines the force required to drive fluid across the membrane; reverse osmosis and nanofiltration require higher pressure (50–150 psi), while ultrafiltration and microfiltration require relatively low pressure (5–100 psi) (Nguyen et al. 2009). Since arsenic species dissolved in water tend to have relatively low molecular weights, only nanofiltration and reverse osmosis membrane processes are likely to effectively treat dissolved arsenic (Nguyen et al. 2009). Reverse osmosis and nanofiltration primarily remove arsenic by categorization of size. A semipermeable membrane when exposed to a pressure gradient permits water to pass while retaining specific ions. Reverse osmosis membranes require higher driving pressures and are more selective then nanofiltration membranes. Arsenic rejection in reverse osmosis and nanofiltration is not sensitive to pH except that arsenic(III) is more efficiently rejected at pH greater 8 (Twidwell et al. 2005) because it is uncharged at low pH but anionic at higher pH. Jain and Singh (2012) investigated removal efficiency of arsenic using nanofiltration membranes in China by varying initial arsenic concentration, pH, natural organic matter and other ionic compounds. Results show that nanofiltration point-of-use (POU) systems were most suitable to treat arsenic-enriched groundwater in areas of suburban China. Effective arsenic(III) removal by an oxalic acid complex is revealed by hybrid distillation system of forward osmosis membrane. It is very efficient process as it draws solute along with high water fluxes and insignificant reverse fluxes in forward osmosis to determine the factors involved in arsenic(III) removal. Comparatively high water fluxes (28 LMH) for forward osmosis mode and under the pressure lagging osmosis was achieved by using 1000 ppm arsenic(III) solution as the feed at 60 °C. Removal of arsenic(III) up to 21.6 % and water recovery (forward osmosis mode) 48.3 % (pressure restarted osmosis mode) were also achieved in 2 h (Ge et al. 2016).
Adsorption technology
Adsorption technology has been extensively used to treat arsenic-contaminated groundwater. The technology can minimize arsenic concentrations to <10 µg L−l. Its efficiency is affected by a variety of untreated water characteristics and contaminants. The media used for adsorption is usually column packed. The contaminants get adsorbed as the contaminated water is passed through the column. When adsorption sites reach the threshold, the column must be regenerated or replaced with new media. Most commonly used adsorption media for arsenic removal includes activated alumina (AA), iron-coated sand, granular ferric hydroxide, indigenous cartridges and filters and other mixed adsorbents. The effectiveness of sportive media is dependent on the consumption of oxidizing agent that helps to adsorb arsenic.
Activated alumina
Activated alumina (AA) was the first adsorptive medium that was successfully used for arsenic removal from drinking water supplies (Ungureanu et al. 2015). It is a granular, porous material possessing good properties of sorption. Activated alumina grains have a high surface area for sorption and a usual diameter of 0.3–0.6 mm. It removes arsenic through microbial oxidation of arsenic(III) following a chemical adsorption step. A mixed culture of heterotrophic bacteria with high arsenic(III) oxidizing activity was obtained by acclimation to arsenic(III) from a soil sample that was free from contamination. The resultant mixed culture contained some genera of heterotrophic arsenic(III)-oxidizing and arsenic-tolerant bacteria: Haemophilus, Bacillus and Micrococcus. The isotherms of arsenic(III) and arsenic(V) adsorption on activated alumina indicate that bacterial oxidation of arsenic(III) to arsenic(V) proves to be a significant pre-treatment process for arsenic removal (Baig et al. 2015; Ike et al. 2008).
Industrial based adsorbents (IBS)
Adsorption on industrial based adsorbents is a promising technology for arsenic removal. Zero-valent iron, granular ferric hydroxide, and modified iron, iron-coated sand and iron oxide are commercially available as adsorbents. Main driving force behind this process is chemisorption which is an irreversible process (Emdadul et al. 2016). Iron-based sorbents are considered a promising solution for arsenic removal as compared to the performance of activated alumina (Hussam and Munir 2007). Cornejo et al. (2008) have proposed a method based on zero-valent iron, solar radiation and lemon juice as adsorbent materials. By using 1.3 g L−l of steel wool and one drop (ca. 0.04 mL) of lemon juice under solar radiation, arsenic removal attained up to 99.5 %. This method is very efficient and economical to treat arsenic-contaminated water. Ko et al. (2007) used sand coated with colloidal iron oxide for arsenic removal. Sylvester et al. (2007) used hybrid sorbent for arsenic adsorption by using nanoparticles of hydrous iron oxide while Chen et al. (2007) studied iron-impregnated activated carbons and found it very effective for arsenic removal. Iron oxide-coated Aspergillus niger biomass has also been used for arsenic removal (Pokhrel and Viraraghavan 2008). Thermodynamic study of the process demonstrated a spontaneous arsenic sorption on biomass by chemisorption process. Martin et al. (2007) synthesized iron(III) salt of mobilized ligands Octolig-21 which is available commercially, and it was found effective for arsenic removal from aqueous solutions.
Indigenous material
Various adsorbants are available for arsenic adsorption that have been developed from indiginous material. Iron ore, oxidizing iron, clay and cellulose-containing red soil have great capacity to adsorb arsenic, and some filters have been developed based on these materials, i.e. cellulose filters (Mandal 2015). A three-pitcher filter of brick chips and sand as filtering media was developed in Bangladesh, and baseline data collected after 1, 6 and 12 months demonstrate that due to inadequate maintenance this technology is only a short-term measure (Milton et al. 2007).
In recent years, various different adsorbents have been developed for arsenic removal. Hlavay and Polya (2005) characterized iron hydroxide-coated alumina for the treatment of arsenic-contaminated water. Hydrous iron oxide nanoparticles (Sylvester et al. 2007), chitosan (Chen and Chung 2006), modified fungal biomass (Pokhrel and Viraraghavan 2006), iron oxide minerals (Öztel et al. 2015a, b), activated neutralized red mud (Tor et al. 2009), iron-containing mesoporous carbon (Baikousi et al. 2015), natural haematite, magnetite and goethite (Dai et al. 2016) and various other materials have been tested as promising materials for arsenic removal. Coating natural biopolymer, chitosan, on ceramic alumina using a dip coating process has also been developed as a novel biosorbent (Boddu et al. 2008).
Agricultural wastes
Agricultural wastes are by-products, typically underused or unused for animal feed. Agricultural by-products such as rice husks were used for removal of arsenic from water, and it was found that uptake of arsenic is directly proportional to temperature change (Malik et al. 2009). Different studies have used untreated rice husk for aqueous arsenic remediation (Amin et al. 2006 more references). Arsenic removal of both arsenic(III) and arsenic(V) using rice husk column was attained using initial concentration of arsenic 100 g L−l with pH of 6.5 and 6.0, respectively. Blue Pine (Pinus wallichiana) wood shavings, walnut (Juglans regia) shell and chick pea testa were also used for arsenic remediation from aqueous solutions. Different conditions that affect the adsorption, such as pH, contact time, biosorbent dose, and temperature and adsorbate concentration, were studied. Blue Pine wood shavings proved a promising potential as a remediation material for arsenic removal from water samples. Walnut shell pieces also displayed good efficiency for biosorption (88 %; Saqib et al. 2013).
Geological materials
Geological materials are emergent remediation materials for house-level treatment in rural settlements, especially if the materials are locally available and can be collected by the population. Numerous natural iron- and aluminium-rich minerals such as haematite (a-Fe2O3), goethite (a-FeO(OH)), gibbsite (g-Al(OH)3) and soils or sediments containing these minerals (e.g. oxisols, laterite), indigenous limestone (Soyatal), iron-coated zeolites, clay minerals (montmorillonite, bentonite) were tested in either laboratory prepared or natural waters, and recognized as substitute adsorbents for small water volumes (Alvarez-Silva et al. 2009; Armienta et al. 2009). Iron/manganese oxide-based materials, like “greensand” and other several natural minerals, have also been inspected (Mohan and Pittman 2007). Ordinary sand filters can also be a viable arsenic removal option from groundwater up to around 400 µg L−l. This method has been ascertained very effective at household level in Vietnam (Luzi et al. 2004). Recent technologies used for arsenic removal are given in Table 1.
Fluoride
Fluorine is the 13th most abundant element on earth. It cannot exist separately in environment without conjoining with other substances to become fluoride. The chief source of fluoride for human intake is contaminated groundwater by geological sources (maximum concentrations reaching 30–50 mg L−l). Fluoride contamination level depends upon the nature of rocks and existence of fluoride-bearing minerals in groundwater. Fluoride concentrations in water are dependent on fluorite solubility in calcium-poor aquifers, and higher fluoride solubility should be expected in the groundwater. Fluoride is naturally present in soil, water, plants and animals in trace quantities. In groundwater, fluoride concentrations range from trace quantities to over 25 mg L−1 naturally. When fluoride is ingested orally it is taken up by body tissues, with long-term deposition in teeth and bones. With the increasing industrialization, water bodies with excessive concentration of fluoride are becoming a matter of great concern.
Sources of fluoride in groundwater
Geogenic sources
Chief source of fluoride in the groundwater is fluoride-bearing rocks, and weathering or leaching from these rocks contaminates the groundwater reserves (Handa 1975; Hem 1985). Highly reactive fluorine is naturally found as calcium fluoride (CaF2). It is an indispensable constituent in minerals like topaz, fluorite, fluorapatite, cryolite, phosphorite, theorapatite. Fluoride exhibits three forms, fluorospar or calcium fluoride (CaF2), apatite or rock phosphate (Ca3F (PO4)3) and cryolite (Na3AlF6). Fluoride concentration is five times more in granite than in basalt rocks. Likewise, higher concentration of fluoride is present in shale than sandstone and limestone. Higher concentration of fluoride is present in alkaline rocks (1200–8500 mg−kg) (Dey et al. 2004). In groundwater low calcium and high bicarbonate alkalinity favours high fluoride concentration. Fluoride-contaminated water is soft with high pH and hold huge amount of silica. Depending upon different variables, groundwater fluoride concentration ranged from <1.0 mg L−1 to more than 35.0 mg L−l.
Anthropogenic sources
Anthropogenic sources of fluoride contamination in groundwater include: (1) agricultural field run-off and infiltration due to use of fertilizers that contain high concentration of fluoride, (2) septic and sewage treatment system discharges, (3) seepage from industrial waste.
Health risk of fluoride
According to World Health Organisation report (WHO 2004) more than 200 million people around the globe rely on water containing fluoride concentrations that exceed the World Health Organisation guidelines of 1.5 mg L−l. Depending upon the amount of fluoride in drinking water and extent of uptake, impact of fluoride uptake can be beneficial or detrimental to mankind. In drinking water fluoride has a narrow beneficial concentration range for human health. Small quantities usually have beneficial effect on rate and occurrence of dental caries, mainly among children (Mahramanlioglu et al. 2002). On the other hand, excess intake can lead to numerous diseases, i.e. osteoporosis, arthritis, brittle bones, cancer, infertility, brain damage, alzheimer syndrome and thyroid disorder (Harrison 2005). Fluorosis is a common symptom of high-fluoride ingestion revealed by teeth mottling in mild cases and bone deformities and neurological damage in severe cases (Fan et al. 2003). Some studies suggest that fluoride may hamper the deoxyribonucleic acid (DNA) synthesis (Zhou et al. 2004). High concentrations of fluoride can also affect with carbohydrates, lipids, proteins, vitamins and mineral metabolism (Islam and Patel 2011). Fluoride toxicity can take place by numerous ways. If ingested, fluoride firstly affects the intestinal mucosa, and later in stomach it can form hydrofluoric acid, followed by gastrointestinal irritation (Islam and Patel 2011). It can also affect numerous other enzymes disrupting oxidative phosphorylation, glycolysis, coagulation and neurotransmission (Islam and Patel 2011). Individuals with kidney ailment have a highest vulnerability to accumulative lethal effects of fluoride (Xiong et al. 2007). In addition, lethal dose of fluoride at once has been reported to disrupt kidney function over short-term exposures both in humans and in animals (Xiong et al. 2007). Fluoride can affect brain and pineal gland functions (Xiong et al. 2007). Fluoride mainly accumulates in pineal gland within the body, at a concentration higher than either teeth or bone (Xiong et al. 2007). Fluoride exposure can also cause bladder cancer, predominantly among the workers exposed to high concentration of fluoride in the workplace (Xiong et al. 2007).
Technologies for fluoride removal
The traditional fluoride removal technologies from drinking water include liming along with fluorite precipitation. The precipitation and coagulation processes with iron(III) (Tressaud 2006), activated alumina (Ghorai and Pant 2005) and calcium (Yin et al. 2015a, b) have been studied extensively.
Ion exchange and coagulation
Ion exchange (Meenakshi and Viswanathan 2007; Popat et al. 1994) and reverse osmosis (Viswanathan and Meenakshi 2009) have been mainly used for fluoride removal from drinking water. Nevertheless, there are certain limitations for the application of these methods including high operational and maintenance costs, complex treatment procedure and generation of secondary pollution.
Aluminium chloride (AlCl3) and polymer aluminium chloride (PACl) coagulation behaviour towards fluoride had been investigated at different basicity. Coagulants attained optimum fluoride removal above pH 6–7, while polymer aluminium chloride revealed higher removal efficiency as compared to aluminium chloride (AlCl3) above pH 4–9. Fluoride removal by these coagulants increased with fluoride concentrations from 0 to 20 mg L−l. At high concentration of 80 mg L−l, removal efficiency of aluminium chloride decreases to as low as 13.1 % due to the formation of soluble aluminium fluoride (Al–F) complexes. On the other hand, this effect had not been showed by polymer aluminium chloride due to the stronger stability of aluminium species (Al13 or Alb). Polymer aluminium chloride with diverse aluminium species showed higher removal efficiency towards fluoride than aluminium chloride, especially at high fluoride concentration or under acidic and alkaline pH conditions (He et al. 2016a, b).
Toxic dissolved fluoride and calcium fluoride (CaF2) nanoparticle pollution is a serious environmental concern for the semiconductor industry. Coagulation combined with electroflotation technique is applied to simultaneous removal of suspended matter and fluoride. Under optimum conditions, the solid–liquid separation efficiency is about 97 % in terms of turbidity removal which corresponds to a residual turbidity of 4.4 nephelometric turbidity units (NTU) complying with the standard limit (5 NTU), while fluoride efficiency removal may reach 73 % corresponding to 10 mg L−l, which is below the environmental recommendations (Guzmán et al. 2016a, b). In general, coagulation methods are effective in defluoridation, but they are not successful in bringing fluoride concentration to desired levels (He et al. 2016a, b).
Membrane technologies
Membranes have advantage that they do not require additives, but their key disadvantage is that they are relatively expensive to install and operate. Moreover, they are prone to fouling, scaling or membrane degradation (Aoudj et al. 2015). In recent years, reverse osmosis membrane method has developed as an ideal substitute to provide safe drinking water without posing the complications related to other conventional methods. Efficiency of the process depends upon various factors, i.e. characteristics of raw water, temperature and systematic monitoring and maintenance. This method is highly effective for fluoride removal. Membranes act as strong impediment to suspended solids, inorganic and organic pollutants, pesticides, micropollutants and microorganisms (Maheshwari 2006a, b). But the key shortcoming of this technique is that it removes all the ions present in water. The process is competitively expensive than other methods (Maheshwari 2006a, b).
Nalgonda technique
In some developing countries, Nalgonda technique is commonly used for water defluoridation (Waghmare and Arfin 2015). In this technique alum, lime and bleaching powder added has been added to raw water followed by rapid mixing, flocculation, sedimentation, filtration and disinfection. Insoluble aluminium hydroxide flocks sediment along with co-precipitation of fluoride, while bleaching powder acts as disinfectant. However, high residual aluminium concentration (2–7 mg L−l) in the treated water than the set World Health Organisation standard (0.2 mg L−l; Ayoob et al. 2008b; Maheshwari 2006a, b) is the main disadvantage of this process.
Adsorption
Among several processes for fluoride removal from water, adsorption offers most acceptable results and seems to be a most striking method for fluoride removal in terms of design simplicity, cost and operation (Mohapatra et al. 2009). Activated alumina has been used for years among the researchers for removal of fluoride (Craig et al. 2015). Farrah et al. (1987) studied aluminium hydroxide (Al(OH)3), aluminium oxide (Al2O3) for fluoride adsorption for pH range between 3 and 8. Results indicate that at pH less than 6 nearly all amorphous gel gets dissolved and aluminium fluoride (AlF) complexes were formed. The quantity of substrate transformed into aluminium fluoride improved with weakening pH and augmenting preliminary fluoride concentration. Ku and Chiou (2002) deliberated the influence of pH on adsorption of fluoride, and highest fluoride removal (16.3 µg−g) was established which ranged between pH 5 and 7. On the basis of low activation energy values, conclusion was made that fluoride removal occurred due to non-specific adsorption. Activated alumina surface was modified by researchers in order to increase its efficiency. La(III) and Y(III) were impregnated on aluminium in order to alter alumina (Tokunaga et al. 1997). Comparisons were made between impregnated alumina and original alumina under a variety of conditions. The removal efficiency of modified alumina was found highest for fluoride than phosphate, arsenate and selenite. Broad research has been conducted for defluoridation of water using diverse calcium salts because calcium has great association with fluoride. Turner et al. (2005) carried out defluoridation studies using calcite, and batch studies were performed. Using atomic force microscopy (AFM) and X-ray photoelectron spectroscopy (XPS) and other potential measurements confirmed that combined action of surface adsorption and precipitation reactions was responsible for fluoride adsorption from water and degree of adsorption was based on calcite surface area (Islam and Patel 2007). These adsorbents have extensively studied for defluoridation as iron had strong attraction for fluoride. Mostly adsorbents used for defluoridation need pH because they are unstable at extreme pH values. Thus, wastewater from polishing industries contains high fluoride and stands as a major environmental problem. Due to its magnetic properties and stability at lower pH, schwertmannite is being used for the defluoridation of polluted wastewater (Eskandarpour et al. 2008).
Nanotechnology
Nanotechnology is an emerging and promising technology in a variety of fields. Use of nanoparticles as adsorbents for the treatment of water has also gained broad consideration in past few years. Due to their smaller sizes, huge surface area, elevated mechanical strength and incredible electrical conductivities carbon nanotubes (CNTs) have gained enormous interest and became potential adsorbents. Li et al. (2001) applied carbon nanotubes as supportive media to deposit aluminium oxide (Al2O3) and discovered the prospect of carbon nanotubes for defluoridation of drinking water. Isotherms data indicated that best defluoridation results were achieved in 5–9 pH range. Defluoridation of water was performed using activated carbon nanotubes which were developed by xylene decomposition in the presence of ferrocene catalyst (Li et al. 2003). Wang et al. (2009) carried out defluoridation by using aluminium hydroxide (nanoscale in comparison with traditional methods makes these nanoparticles more suitable media). Zhao et al. (2010) combined the advantages of aluminium hydroxide (Al(OH)3) and magnetic nanoparticles to fabricate nanosized adsorbents with high surface area, high affinity towards fluoride and good magnetic separability, to develop a new kind of magnetic fluoride adsorbent.
Natural materials
Natural materials which are easily accessible in huge quantity are being investigated for F-contaminated water (Biswas et al. 2010). Studies have also been carried out on fluoride removal with the help of low-quality coal (Borah and Dey 2009). Acid-modified raw laterite has also been evaluated for defluoridation of water (Maiti et al. 2011). Alteration of bentonite clay using lanthanum, magnesium and manganese was also carried out for its adsorption and defluoridation activity (Kamble et al. 2009). In order to achieve effective fluoride removal, low-cost bentonite clay was modified chemically by MgCl2 (Thakre et al. 2010).
Biosorption
Various biosorbents have been developed for fluoride removal. Among a variety of biosorbents available for defluoridation, wide attention has been given to derivatives of chitin and chitosan because of their cost-effectiveness and higher concentration of –NH- and OH functional groups they showed considerable adsorption capacity for different of water pollutants. These show significant adsorption potential for the removal of various aquatic pollutants. A comparison of adsorption efficiency for defluoridation was made between chitin, chitosan and 20 % La-chitosan adsorbents (Kamble et al. 2007). Fluoride uptake comparison was made between distilled water and contaminated actual water, and results indicate that application was high in distilled water than field water due to competing ions effect and high pH. Yao et al. (2009) have tested modified chitosan for defluoridation of water. Optimum pH for defluoridation was 7.0, and under acidic conditions chitosan was found unstable. Several plant-based biosorbents such as dried orange juice residue, tamarind (Tamarindus indica) fruit shell carbon, Morringa indica-based activated carbon have been studied very recently by various investigators and reported for fluoride removal from water with different degrees of success. Neem leaf powder (NLP) developed from the mature leaves of neem (Azadirachta indica) trees has also been shown to be an active biosorbent for removal of fluoride from water (Bharali and Bhattacharyya 2015a, b).
Waste materials
Industrialization produces enormous quantity of waste in the form of by-products. To transform these materials into cheap adsorbents will be the most appropriate use of these waste products in order to treat wastewater. A variety of treated and untreated industrial by-products are evaluated for defluoridation of water. The ability of fly ash (a thermal power plant waste) to remove fluoride from water and wastewaters was studied (Husain et al. 2015). The residue of industrial waste which is produced during process of alum in thermal power plants was evaluated by Chaturvedi et al. for defluoridation of water (Chaturvedi et al. 1990). High fluoride removal efficiency was observed at high temperature and low fluoride concentration under acidic conditions. The highest removal efficiency was observed by using Langmuir isotherm of 20.3 mg−g (Chaturvedi et al. 1990). Marble powder, brick powder and hydrated cement had been studied for the removal of fluoride from drinking water (Bibi et al. 2015a, b).
Only few researchers have evaluated construction materials as adsorbents for the treatment of fluoride-contaminated water. Yadav et al. (2006) evaluated and compared brick powder for fluoride removal from water and compared with analytical grade activated charcoal. Removal % of brick powder was found 56.8 % for a pH range of 6.0–8.0. While for activated charcoal, removal efficiency decreased by increasing pH. Under optimum pH interaction between metal and fluoride ions takes place. Other competing ions did not interfere with the defluoridation process. By increasing the contact time from 15 to 20 min, the defluoridation efficiency increased up to 54.4 per cent by brick powder and up to 80 per cent with activated carbon. Comparison between two adsorbents revealed that brick powder had better adsorption efficiency and it is cost-effective. Concrete (gas), a material used for construction had also proved very effective adsorbent for defluoridation (Oguz 2005). Hydrated cement had also been tested as a potential adsorbent for defluoridation. The potential of hydrated cement (HC) for the removal of excess fluoride from aqueous solution has also been tested (Kagne et al. 2008). The kinetics of defluoridation efficiency from water by alumina cement granules (ALC) has also been examined (Ayoob et al. 2008a). A comparison of fluoride removal efficiency of ALC from synthetic water and natural ground water was also studied by Ayoob and Gupta (2009).
Agricultural waste
The use of agricultural waste products for treatment of water is very cost-effective option because of their easy availability and cost-effectiveness. Parmar et al. (2006) used aluminium- and calcium chloride (Al/CaCl3)-treated corn cobs powder for defluoridation of water. Al-/Ca-treated cobs showed excellent results. Mohan et al. (2008) used different agricultural materials including coconut shell, shell fibres and rice husk in order to remove pollutants, i.e. fluoride. Carbon of coconut shell proved best adsorbent. Sivabalan et al. (2003) studied fluoride adsorption by using palm seed coat charcoal. Adsorption process depends upon pH, and adsorption efficiencies were found higher in range of 4–8. Zirconium-treated coconut shell carbon was examined to treat water contaminated by fluoride (Sathish et al. 2007). Researchers have also investigated the potential of zirconium-impregnated cashew nut shell carbon and compared its performance with that of cashew nut shell carbon for fluoride removal from aqueous solutions (Alagumuthu and Rajan 2010).
Six principles (biological oxidation, oxidation–filtration, co-precipitation, adsorption, ion exchange and membrane technology) are the basis of different technologies available for the removal of arsenic and fluoride from contaminated water. Every technique has its own merits and demerits. Typical treatment efficiencies for processes operated under normal conditions are provided in Tables 1 and 2. Figure 1 provides the comparison for different materials that have been used recently for arsenic and fluoride removal, and their adsorption capacities have been compared. In case of arsenic adsorption capacities of nickel- and iron-based materials are much higher than other materials, while for fluoride, aluminium-based materials showed tremendous adsorption capacity towards fluoride.
Conclusion
Drinking water contamination by arsenic and fluoride is a problem just about anywhere in the world, predominantly in developing countries of Asia. These contaminants are generally present in geological formations, so remediation technologies are the only option to diminish the effect. Traditional treatment processes such as coagulation–filtration, lime softening, iron/manganese oxidation and membrane filtration have been used in water treatment plants for the removal of these contaminants. Ion exchange, filtration and adsorption have been employed at domestic level. Advanced technologies, i.e. biological treatment, reactive membrane, phytoremediation, are also used for water treatment of arsenic contamination. Yet, various techniques are still at experimental stage and some have not been established at full scale. It is suggested that combination of ion exchange, filtration and adsorption as a low-cost chemical treatment with bioremediation could be advantageous for the decontamination of drinking water. Arsenic and fluoride alleviation approach should be adopted according to the specific geographic and morphological characteristics and socio-economic conditions of the area. All removal technologies discussed above have advantages and disadvantages, so suitable technology for specific situation should be opted. Technologies should be modified on pilot-scale implementation to effectively remove these contaminants by reducing operational and maintenance cost in a user-friendly manner. In many affected areas, arsenic and fluoride removal method could be the only alternative for safe water supply. So it is strongly recommended that efficient technologies that are found effective and safe should be indorsed for arsenic and fluoride removal in affected areas to evade ingestion of these toxins via drinking water.
References
Abdullah M, Fasola M, Muhammad A, Malik SA, Boston N, Bokhari H, Kamran MA, Shafqat MN, Alamdar A, Khan M, Ali N, Eqani SAMAS (2015) Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosphere 119:553–561
Ahmed MF (2001) An overview of arsenic removal technologies in Bangladesh and India. In: Proceedings of BUET-UNU international workshop on technologies for arsenic removal from drinking water, Dhaka, pp 5–7
Ajisha MAT, Rajagopal K (2015) Fluoride removal study using pyrolyzed Delonix regia pod, an unconventional adsorbent. J Environ Sci Technol 12:223–236
Alagumuthu G, Rajan M (2010) Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem Eng 158:451–457
Ali I, Alothman ZA, Sanagi MM (2015) Green synthesis of iron nano-impregnated adsorbent for fast removal of fluoride from water. J Mol Liq 211:457–465
Ali S, Thakur SK, Sarkar A, Shekhar S (2016) Worldwide contamination of water by fluoride. Environ Chem Lett 14:291–315
Alvarez-Silva M, Uribe-Salas A, Nava-Alonso F, Pérez-Garibay R (2009) Adsorption of As(V) onto goethite: experimental statistical optimization. Natural Arsenic in Groundwater of Latin America. In: Bundschuh J, Bhattacharya P (eds) Arsenic in the environment, vol 1. pp 527–534
Amin MN, Kaneco S, Kitagawa T, Begum A, Katsumata H, Suzuki T (2006) Removal of arsenic in aqueous solutions by adsorption onto waste rice husk. Ind Eng Chem Res 45:8105–8110
Amna Ali N, Masood S, Mukhtar T, Kamran MA, Rafique M, Munis MFH, Chaudhary HJ (2015) Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linum usitatissimum in association with Glomus intraradices. Environ Monit Assess 187:1–11
Anawar HM, Akai J, Sakugawa H (2004) Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere 54:753–762
Angelone M, Cremisini C, Piscopo V, Proposito M, Spaziani F (2009) Influence of hydrostratigraphy and structural setting on the arsenic occurrence in groundwater of the Cimino-Vico volcanic area (central Italy). Hydrogeology 17:901–914
Aoudj S, Khelifa A, Drouiche N, Belkada R, Miroud D (2015) Simultaneous removal of chromium(VI) and fluoride by electrocoagulation–electroflotation: application of a hybrid Fe–Al anode. Chem Eng 267:153–162
Appleyard S, Angeloni J, Watkins R (2006) Arsenic-rich groundwater in an urban area experiencing drought and increasing population density, Perth, Australia. Appl Geochem 21:83–97
Archer J, Hudson-Edwards K, Preston D, Howarth R, Linge K (2005) Aqueous exposure and uptake of arsenic by riverside communities affected by mining contamination in the Río Pilcomayo basin, Bolivia. Mineral Mag 69:719–736
Armienta M, Micete S, Flores-Valverde E (2009) Feasibility of arsenic removal from contaminated water using indigenous limestone. Natural Arsenic in Groundwater of Latin America. In: Bundschuh J, Bhattacharya P (eds) Arsenic in the environment, vol 1. p 505e510
Asante KA, Agusa T, Subramanian A, Ansa-Asare OD, Biney CA, Tanabe S (2007) Contamination status of arsenic and other trace elements in drinking water and residents from Tarkwa, a historic mining township in Ghana. Chemosphere 66:1513–1522
Ayoob S, Gupta A (2009) Performance evaluation of alumina cement granules in removing fluoride from natural and synthetic waters. Chem Eng 150:485–491
Ayoob S, Gupta A, Bhakat P, Bhat VT (2008a) Investigations on the kinetics and mechanisms of sorptive removal of fluoride from water using alumina cement granules. Chem Eng 140:6–14
Ayoob S, Gupta A, Bhat VT (2008b) A conceptual overview on sustainable technologies for the defluoridation of drinking water. Crit Rev Environ Sci Technol 38:401–470
Baig JA, Kazi TG, Shah AQ, Kandhro GA, Afridi HI, Khan S (2012) Arsenic speciation and other parameters of surface and ground water samples of Jamshoro, Pakistan. Int J Environ Anal Chem 92:28–42
Baig SA, Sheng T, Sun C, Xue X, Tan L, Xu X (2014) Arsenic removal from aqueous solutions using Fe3O4-HBC composite: effect of calcination on adsorbents performance. PloS one 9(6):e100704. doi:10.1371/journal.pone.0100704
Baig SA, Sheng T, Hu Y, Xu J, Xu X (2015) Arsenic removal from natural water using low cost granulated adsorbents: a review. CLEAN Soil Air Water 43:13–26
Baikousi M, Georgiou Y, Daikopoulos C, Bourlinos AB, Filip J, Zbořil R (2015) Synthesis and characterization of robust zero valent iron/mesoporous carbon composites and their applications in arsenic removal. Carbon 93:636–647
Berg M, Tran HC, Nguyen TC, Pham HV, Schertenleib R, Giger W (2001) Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environ Sci Technol 35:2621–2626
Bharali RK, Bhattacharyya KG (2015a) Biosorption of fluoride on Neem (Azadirachta indica) leaf powder. J Environ Chem Eng 3:662–669
Bharali RK, Bhattacharyya KG (2015b) Biosorption of fluoride on Neem (Azadirachta indica) leaf powder. J Environ Chem Eng 3:662–669
Bhattacharjee S, Chakravarty S, Maity S, Dureja V, Gupta K (2005) Metal contents in the groundwater of Sahebgunj district, Jharkhand, India, with special reference to arsenic. Chemosphere 58:1203–1217
Bhaumik M, Noubactep C, Gupta VK, McCrindle RI, Maity A (2015) Polyaniline/Fe 0 composite nanofibers: an excellent adsorbent for the removal of arsenic from aqueous solutions. Chem Eng 271:135–146
Bibi S, Husain SZ, Malik RN (2008) Pollen analysis and heavy metals detection in honey samples from seven selected countries. Pak J Bot 40:507–516
Bibi S, Farooqi A, Hussain K, Haider N (2015a) Evaluation of industrial based adsorbents for simultaneous removal of arsenic and fluoride from drinking water. J Clean Prod 87:882–896
Bibi S, Farooqi A, Ramzan M, Javed A (2015b) Health risk of arsenic in the alluvial aquifers of Lahore and Raiwind, Punjab Province, Pakistan: an investigation for safer well water. Toxicol Environ Chem 97:888–907
Bissen M, Frimmel FH (2003) Arsenic—a review. Part I: occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 31:9–18
Biswas K, Gupta K, Goswami A, Ghosh UC (2010) Fluoride removal efficiency from aqueous solution by synthetic iron(III)–aluminum(III)–chromium(III) ternary mixed oxide. Desalination 255:44–51
Boddu VM, Abburi K, Talbott JL, Smith ED, Haasch R (2008) Removal of arsenic(III) and arsenic(V) from aqueous medium using chitosan-coated biosorbent. Water Res 42:633–642
Borah L, Dey N (2009) Removal of fluoride from low TDS water using low grade coal. Ind J Chem Technol 16:361
Brown KG, Ross GL (2002) Arsenic, drinking water, and health: a position paper of the American Council on Science and Health. Regul Toxicol Pharmacol 36:162–174
Buamah R, Oduro C, Sadik M (2016) Fluoride removal from drinking water using regenerated aluminum oxide coated media. J Environ Chem Eng 4:250–258
Bundschuh J, García M, Alvarez M (2007) Arsenic and heavy metal removal by phytofiltration and biogenic sulfide precipitation a comparative study from Poopó Lake basin, Bolivia. Abstract vol 3rd international groundwater conference IGC-2007, water, environment and agriculture–present problems and future challenges, p152
Bundschuh J, Litter M, Ciminelli VS, Morgada ME, Cornejo L, Hoyos SG (2010) Emerging mitigation needs and sustainable options for solving the arsenic problems of rural and isolated urban areas in Latin America: a critical analysis. Water Res 44:5828–5845
Cai H, Chen G, Peng CY, Zhang Z, Dong Y, Shang G (2015) Removal of fluoride from drinking water using tea waste loaded with Al/Fe oxides: a novel, safe and efficient biosorbent. Appl Surf Sci 328:34–44
Camacho LM, Gutiérrez M, Alarcón-Herrera MT, Villalba MdL, Deng S (2011) Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere 83:211–225
Chaturvedi A, Yadava K, Pathak K, Singh V (1990) Defluoridation of water by adsorption on fly ash. Water Air Soil Pollut 49:51–61
Chen CC, Chung YC (2006) Arsenic removal using a biopolymer chitosan sorbent. J Environ Sci Health A 41:645–658
Chen W, Parette R, Zou J, Cannon FS, Dempsey BA (2007) Arsenic removal by iron-modified activated carbon. Water Res 41:1851–1858
Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T (2011) Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ Br Med 342:2431
Chen ML, Sun Y, Huo CB, Liu C, Wang JH (2015) Akaganeite decorated graphene oxide composite for arsenic adsorption/removal and its proconcentration at ultra-trace level. Chemosphere 130:52–58
Chen L, Zhang KS, He JY, Xu WH, Huang XJ, Liu JH (2016) Enhanced fluoride removal from water by sulphate doped hydroxyapatite hierarchical hollow microspheres. Chem Eng 285:616–624
Chwirka J, Thompson BM, Stomp JM III (2000) Removing arsenic from groundwater. J Am Water Works Assoc 92:79–88
Çiftçi TD, Henden E (2015) Nickel/nickel boride nanoparticles coated resin: a novel adsorbent for arsenic(III) and arsenic(V) removal. Powder Technol 269:470–480
Clifford DA, Ghurye G (2002) Metal-oxide adsorption, ion exchange, and coagulation-microfiltration for arsenic removal from water. Marcel Dekker, New York
Cornejo L, Lienqueo H, Arenas M, Acarapi J, Contreras D, Yanez J (2008) In field arsenic removal from natural water by zero-valent iron assisted by solar radiation. Environ Pollut 156:827–831
Craig L, Stillings LL, Decker DL, Thomas JM (2015) Comparing activated alumina with indigenous laterite and bauxite as potential sorbents for removing fluoride from drinking water in Ghana. Appl Geochem 56:50–66
Cui J, Jing C, Che D, Zhang J, Duan S (2015) Groundwater arsenic removal by coagulation using ferric(III) sulfate and polyferric sulfate: a comparative and mechanistic study. J Environ Sci 32:42–53
Czerniczyniec M, Farías S, Magallanes J, Cicerone D (2007) Arsenic(V) adsorption onto biogenic hydroxyapatite: solution composition effects. Water Air Soil Pollut 180:75–82
Dai M, Xia L, Song S, Peng C, Lopez-Valdivieso A (2016) Adsorption of As(V) inside the pores of porous hematite in water. J Hazard Mater 307:312–317
Dash SS, Sahu MK, Sahu E, Patel RK (2015) Fluoride removal from aqueous solutions using cerium loaded mesoporous zirconium phosphate. New J Chem 39:7300–7308
Dechnik J, Janiak C, De S (2016) Aluminium fumarate metal-organic framework: a super adsorbent for fluoride from water. J Hazard Mater 303:10–20
Deng H, Yu X (2015) Fluoride removal from drinking water by zirconium-impregnated fibrous protein. Desalin Water Treat 54:1594–1603
Dey S, Goswami S, Ghosh UC (2004) Hydrous ferric oxide (HFO) a scavenger for fluoride from contaminated water. Water Air Soil Pollut 158:311–323
Dodd MC, Vu ND, Ammann A, Le VC, Kissner R, Pham HV (2006) Kinetics and mechanistic aspects of As(III) oxidation by aqueous chlorine, chloramines, and ozone: relevance to drinking water treatment. Environ Sci Technol 40:3285–3292
Dong S, Wang Y (2016) Characterization and adsorption properties of a lanthanum-loaded magnetic cationic hydrogel composite for fluoride removal. Water Res 88:852–860
Duarte AA, Oliveira SL, Amorim MT (2011) Arsenic removal from drinking water by advanced filtration processes. In: XIV IWRA World Water Congress. IWRA-CD Rom
Elwakeel KZ, Guibal E (2015) Arsenic(V) sorption using chitosan/Cu (OH)2 and chitosan/CuO composite sorbents. Carbohydr Polym 134:190–204
Emdadul HM, Sabur MA, Mahamud-Ul-Hoque M, Safiullah S (2016) Competition of various ions present in shallow aquifer water in respect of arsenic removal by hydrated ferric oxide. Asian J Water Environ Pollut 12:25–33
Escobar MO, Hue N, Cutler W (2016) Recent developments on arsenic: contamination and remediation. Recent Res Dev Bioenerg 4:1–32
Eskandarpour A, Onyango MS, Ochieng A, Asai S (2008) Removal of fluoride ions from aqueous solution at low pH using schwertmannite. J Hazard Mater 152:571–579
Fan X, Parker D, Smith M (2003) Adsorption kinetics of fluoride on low cost materials. Water Res 37:4929–4937
Fan L, Zhang S, Zhang X, Zhou H, Lu Z, Wang S (2015) Removal of arsenic from simulation wastewater using nano-iron/oyster shell composites. J Environ Manag 156:109–114
Fang F, Jia Y, Wu PY, Zhang QY, Jiang YP, Zhou SS (2015) Facile one-pot preparation of goethite/parabutlerite nanocomposites and their removal properties and mechanism toward As(V) ions. Appl Surf Sci 324:355–362
Farrah H, Slavek J, Pickering WF (1987) Fluoride interactions with hydrous aluminum oxides and alumina. Soil Res 25:55–69
Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr (2005) Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5:42–52
Fields K, Chen AS, Wang L, Sorg TJ, Cincinnati O, Columbus O (2000a) Arsenic removal from drinking water by iron removal plants. National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency
Fields KA, Chen AS, Wang L (2000b) Arsenic removal from drinking water by coagulation/filtration and lime softening plants. National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency
Flora S, Mittal M (2009) Co-exposure to arsenic and fluoride on oxidative stress, glutathione linked enzymes, biogenic amines and DNA damage in mouse brain. J Neurol Sci 285:198–205
Fox DI, de Lima Stebbins DM, Alcantar N (2016) Combining ferric salt and cactus mucilage for arsenic removal from water. Environ Sci Technol 50(5):2507–2513
Frankenberger JW Jr, Arshad M (2002) Volatilization of arsenic. Environ Chem Arsen 363–380
Gai W-Z, Deng Z-Y, Shi Y (2015) Fluoride removal from water using high-activity aluminum hydroxide prepared by the ultrasonic method. RSC Adv 5:84223–84231
Gallard H, von Gunten U (2002) Chlorination of natural organic matter: kinetics of chlorination and of THM formation. Water Res 36:65–74
García-Sánchez A, Moyano A, Mayorga P (2005) High arsenic contents in groundwater of central Spain. Environ Geol 47:847–854
Ge Q, Han G, Chung T-S (2016) Effective As(III) removal by a multi-charged hydroacid complex draw solute facilitated forward osmosis-membrane distillation (FO-MD) processes. Environ Sci Technol 50(5):2363–2370
Gebel TW (1999) Arsenic and drinking water contamination. Science 283:1458
Ghorai S, Pant K (2005) Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep Purif Technol 42:265–271
Gogoi S, Dutta RK (2016) Fluoride removal by hydrothermally modified limestone powder using phosphoric acid. J Environ Chem Eng 4:1040–1049
Goldberg S, Johnston CT (2001) Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J Colloid Interface Sci 234:204–216
Guo H, Yang S, Tang X, Li Y, Shen Z (2008) Groundwater geochemistry and its implications for arsenic mobilization in shallow aquifers of the Hetao Basin, Inner Mongolia. Sci Total Environ 393:131–144
Guo L, Ye P, Wang J, Fu F, Wu Z (2015a) Three-dimensional Fe3O4-graphene macroscopic composites for arsenic and arsenate removal. J Hazard Mater 298:28–35
Guo Z, Dong D, Hua X, Zhang L, Zhu S, Lan X, Liang D (2015b) Cr and As decrease lindane sorption on river solids. Environ Chem Lett 13(1):111–116
Guzmán A, Nava JL, Coreño O, Rodríguez I, Gutiérrez S (2016a) Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor. Chemosphere 144:2113–2120
Guzmán A, Nava JL, Coreño O, Rodríguez I, Gutiérrez S (2016b) Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor. Chemosphere 144:2113–2120
Handa BK (1975) Geochemistry and genesis of fluoride‐containing ground waters in india. Ground Water 13:275–281
Harrison PT (2005) Fluoride in water: a UK perspective. J Fluor Chem 126:1448–1456
Hartley W, Dickinson NM, Riby P, Lepp NW (2009) Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut 157:2654–2662
He J, Zhang K, Wu S, Cai X, Chen K, Li Y (2016a) Performance of novel hydroxyapatite nanowires in treatment of fluoride contaminated water. J Hazard Mater 303:119–130
He Z, Lan H, Gong W, Liu R, Gao Y, Liu H (2016b) Coagulation behaviors of aluminum salts towards fluoride: significance of aluminum speciation and transformation. Separat Purifi Technol 165:137–144
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, vol 2254. Department of the Interior, US Geological Survey
Hlavay J, Polyák K (2005) Determination of surface properties of iron hydroxide-coated alumina adsorbent prepared for removal of arsenic from drinking water. J Colloid Interface Sci 284:71–77
Hu C, Chen Q, Liu H, Qu J (2015a) Coagulation of methylated arsenic from drinking water: influence of methyl substitution. J Hazard Mater 293:97–104
Hu X, Ding Z, Zimmerman AR, Wang S, Gao B (2015b) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Huang JW, Poynton CY, Kochian LV, Elless MP (2004) Phytofiltration of arsenic from drinking water using arsenic-hyperaccumulating ferns. Environ Sci Technol 38:3412–3417
Huang PP, Cao CY, Wei F, Sun YB, Song WG (2015a) MgAl layered double hydroxides with chloride and carbonate ions as interlayer anions for removal of arsenic and fluoride ions in water. RSC Adv 5:10412–10417
Huang Y, Miyauchi K, Inoue C, Endo G (2015b) Development of suitable hydroponics system for phytoremediation of arsenic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci Biotechnol Biochem 80(3):614–618
Hug SJ, Canonica L, Wegelin M, Gechter D, von Gunten U (2001) Solar oxidation and removal of arsenic at circumneutral pH in iron containing waters. Environ Sci Technol 35:2114–2121
Husain SZ, Malik RN, Javaid M, Bibi S (2008) Ethonobotanical properties and uses of medicinal plants of Morgah biodiversity park, Rawalpindi. Pak J Bot 40(189):7–1911
Husain M, Chavan FI, Abhale B (2015) Use of Maize husk fly ash as an adsorbent for removal of fluoride. Int Res J Eng Technol
Hussam A, Munir AK (2007) A simple and effective arsenic filter based on composite iron matrix: development and deployment studies for groundwater of Bangladesh. J Environ Sci Health A 42:1869–1878
Ike M, Miyazaki T, Yamamoto N, Sei K, Soda S (2008) Removal of arsenic from groundwater by arsenite-oxidizing bacteria. Water Sci Technol 58:1095–1100
Islam M, Patel R (2007) Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. J Hazard Mater 143:303–310
Islam M, Patel R (2011) Thermal activation of basic oxygen furnace slag and evaluation of its fluoride removal efficiency. Chem Eng 169:68–77
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D (2004) Role of metal reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Ismail ZZ, AbdelKareem HN (2015) Sustainable approach for recycling waste lamb and chicken bones for fluoride removal from water followed by reusing fluoride-bearing waste in concrete. Waste Manag 45:66–75
Jain C, Singh R (2012) Technological options for the removal of arsenic with special reference to South East Asia. J Environ Manag 107:1–18
Jasrotia S, Kansal A, Mehra A (2015) Performance of aquatic plant species for phytoremediation of arsenic-contaminated water. Appl Water Sci. doi:10.1007/s13201-015-0300-4
Jiang JQ (2015) The role of coagulation in water treatment. Curr Opin Chem Eng 8:36–44
Jiang L, Xiao S, Chen J (2015) Removal behavior and mechanism of Co(II) on the surface of Fe–Mn binary oxide adsorbent. Coll Surf A Physicochem Eng Asp 479:1–10
Jin H, Ji Z, Yuan J, Li J, Liu M, Xu C (2015a) Research on removal of fluoride in aqueous solution by alumina-modified expanded graphite composite. J Alloy Compd 620:361–367
Jin Z, Jia Y, Luo T, Kong L-T, Sun B, Shen W (2015b) Efficient removal of fluoride by hierarchical MgO microspheres: Performance and mechanism study. Appl Surf Sci 357:1080–1088
Kagne S, Jagtap S, Dhawade P, Kamble S, Devotta S, Rayalu S (2008) Hydrated cement: a promising adsorbent for the removal of fluoride from aqueous solution. J Hazard Mater 154:88–95
Kamble SP, Jagtap S, Labhsetwar NK, Thakare D, Godfrey S, Devotta S (2007) Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem Eng 129:173–180
Kamble SP, Dixit P, Rayalu SS, Labhsetwar NK (2009) Defluoridation of drinking water using chemically modified bentonite clay. Desalination 249:687–693
Kameda T, Oba J, Yoshioka T (2015) Kinetics and equilibrium studies on Mg–Al oxide for removal of fluoride in aqueous solution and its use in recycling. J Environ Manag 156:252–256
Kamran MA, Mufti R, Mubariz N, Syed JH, Bano A, Javed MT, Chaudhary HJ (2014) The potential of the flora from different regions of Pakistan in phytoremediation: a review. Environ Sci Pollut Res 21:801–812
Kamran MA, Syed JH, Eqani SAMAS, Munis MFH, Chaudhary HJ (2015) Effect of plant growth-promoting rhizobacteria inoculation on cadmium (Cd) uptake by Eruca sativa. Environ Sci Pollut Res 22:9275–9283
Kamran MA, Eqani SAMAS, Bibi S, Xu RK, Monis MFH, Katsoyiannis A, Bokhari H, Chaudhary HJ (2016a) Bioaccumulation of nickel by E. sativa and role of plant growth promoting rhizobacteria (PGPRs) under nickel stress. Ecotoxicol Environ Saf 126:256–263
Kamran MA, Bibi S, Xu RK, Hussain S, Mehmood K, Chaudhary HJ (2016b) Phyto-extraction of chromium and influence of plant growth promoting bacteria to enhance plant growth. J Geochem Explor. doi:10.1016/j.gexplo.2016.09.005
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39:1291–1298
Kang D, Yu X, Tong S, Ge M, Zuo J, Cao C (2013) Performance and mechanism of Mg/Fe layered double hydroxides for fluoride and arsenate removal from aqueous solution. Chem Eng 228:731–740
Kang D, Tong S, Yu X, Ge M (2015) Template-free synthesis of 3D hierarchical amorphous aluminum oxide microspheres with broccoli-like structure and their application in fluoride removal. RSC Adv 5:19159–19165
Kanyora A, Kinyanjui T, Kariuki S, Njogu M (2015) Fluoride removal capacity of regenerated bone char in treatment of drinking water. Asian J Nat Appl Sci 4:1
Kar S, Maity JP, Jean J-S, Liu C-C, Nath B, Yang H-J (2010) Arsenic-enriched aquifers: Occurrences and mobilization of arsenic in groundwater of Ganges Delta Plain, Barasat, West Bengal, India. Appl Geochem 25:1805–1814
Katsoyiannis IA, Zouboulis AI, Jekel M (2004) Kinetics of bacterial As(III) oxidation and subsequent As(V) removal by sorption onto biogenic manganese oxides during groundwater treatment. Ind Eng Chem Res 43:486–493
Katsoyiannis IA, Voegelin A, Zouboulis AI, Hug SJ (2015) Enhanced As(III) oxidation and removal by combined use of zero valent iron and hydrogen peroxide in aerated waters at neutral pH values. J Hazard Mater 297:1–7
Kaygusuz H, Uzaşçı S, Erim FB (2015) Removal of fluoride from aqueous solution using aluminum alginate beads. CLEAN Soil Air Water 43:724–730
Kinniburgh DG, Kosmus W (2002) Arsenic contamination in groundwater: some analytical considerations. Talanta 58:165–180
Ko I, Davis AP, Kim JY, Kim KW (2007) Arsenic removal by a colloidal iron oxide coated sand. J Environ Eng 133:891–898
Kobya M, Ozyonar F, Demirbas E, Sik E, Oncel M (2015) Arsenic removal from groundwater of Sivas-Şarkişla Plain, Turkey by electrocoagulation process: comparing with iron plate and ball electrodes. J Environ Chem Eng 3:1096–1106
Kouras A, Katsoyiannis I, Voutsa D (2007) Distribution of arsenic in groundwater in the area of Chalkidiki, Northern Greece. J Hazard Mater 147:890–899
Ku Y, Chiou HM (2002) The adsorption of fluoride ion from aqueous solution by activated alumina. Water Air Soil Pollut 133:349–361
Kurttio P, Pukkala E, Kahelin H, Auvinen A, Pekkanen J (1999) Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ Health Perspect 107:705
Larios R, Fernández-Martínez R, Silva V, Rucandio I (2013) Chemical availability of arsenic and heavy metals in sediments from abandoned cinnabar mine tailings. Environ Earth Sci 68:535–546
Lee H, Kim D, Kim J, Ji MK, Han YS, Park YT (2015) As(III) and As(V) removal from the aqueous phase via adsorption onto acid mine drainage sludge (AMDS) alginate beads and goethite alginate beads. J Hazard Mater 292:146–154
Leist M, Casey R, Caridi D (2000) The management of arsenic wastes: problems and prospects. J Hazard Mater 76:125–138
Lescano MR, Passalía C, Zalazar CS, Brandi RJ (2015) Arsenic sorption onto titanium dioxide, granular ferric hydroxide and activated alumina: batch and dynamic studies. J Environ Sci Health A 50:424–431
Li Y-H, Wang S, Cao A, Zhao D, Zhang X, Xu C (2001) Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chem Phys Lett 350:412–416
Li Y-H, Wang S, Zhang X, Wei J, Xu C, Luan Z (2003) Adsorption of fluoride from water by aligned carbon nanotubes. Mater Res Bull 38:469–476
Liao VHC, Chu YJ, Su YC, Hsiao SY, Wei CC, Liu CW (2011) Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123:20–29
Liu R, Zhu L, He Z, Lan H, Liu H, Qu J (2015) Simultaneous removal of arsenic and fluoride by freshly-prepared aluminum hydroxide. Coll Surf A Physicochem Eng Asp 466:147–153
Luu TTG, Sthiannopkao S, Kim KW (2009) Arsenic and other trace elements contamination in groundwater and a risk assessment study for the residents in the Kandal Province of Cambodia. Environ Int 35:455–460
Luzi S, Berg M, Pham T, Pham H, Schertenleib R (2004) Household sand filters for arsenic removal. Swiss Federal Institute for Environmental Science and Technology (EAWAG), Duebendorf, Switzerland
Maheshwari R (2006a) Fluoride in drinking water and its removal. J Hazard Mater 137:456–463
Maheshwari R (2006b) Fluoride in drinking water and its removal. J Hazard Mater 137(1):456–463
Mahramanlioglu M, Kizilcikli I, Bicer I (2002) Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J Fluor Chem 115:41–47
Maiti A, Basu JK, De S (2011) Chemical treated laterite as promising fluoride adsorbent for aqueous system and kinetic modeling. Desalination 265:28–36
Malakootian M, Javdan M, Iranmaneshc F (2015) Fluoride removal study from aqueous solutions using Jajarm bauxite: case study on Koohbanan water. Fluoride 48:113–122
Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168:1–12
Mandal S (2015) Development of new adsorbent materials for the removal of arsenic (III) and chromium (VI) from water and its mathematical modelling (Doctoral dissertation)
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Mariappan R, Vairamuthu R, Ganapathy A (2015) Use of chemically activated cotton nut shell carbon for the removal of fluoride contaminated drinking water: kinetics evaluation. Chin J Chem Eng 23:710–721
Martin DF, O’Donnell L, Martin BB, Alldredge R (2007) Removal of aqueous arsenic using iron attached to immobilized ligands (IMLIGs). J Environ Sci Health A 42:97–102
McArthur J, Ravenscroft P, Safiulla S, Thirlwall M (2001) Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour Res 37:109–117
McNeill LS, Edwards M (1997) Arsenic removal during precipitative softening. J Environ Eng 123:453–460
Meenakshi S, Viswanathan N (2007) Identification of selective ion-exchange resin for fluoride sorption. J Colloid Interface Sci 308:438–450
Mehta D, Mondal P, George S (2016) Utilization of marble waste powder as a novel adsorbent for removal of fluoride ions from aqueous solution. J Environ Chem Eng 4(1):932–942
Melidis P (2015) Fluoride removal from aluminium finishing wastewater by hydroxyapatite. Environ Process 2:205–213
Mendoza-Castillo DI, Rojas-Mayorga CK, García-Martínez IP, Pérez-Cruz MA, Hernández-Montoya V, Bonilla-Petriciolet A (2015) Removal of heavy metals and arsenic from aqueous solution using textile wastes from denim industry. Int J Environ Sci Technol 12:1657–1668
Milton AH, Smith W, Dear K, Ng J, Sim M, Ranmuthugala G (2007) A Randomised intervention trial to assess two arsenic mitigation options in Bangladesh. J Environ Sci Health A 42:1897–1908
Min LL, Yuan ZH, Zhong LB, Liu Q, Wu RX, Zheng YM (2015) Preparation of chitosan based electrospun nanofiber membrane and its adsorptive removal of arsenate from aqueous solution. Chem Eng 267:132–141
Mittal M, Flora S (2006) Effects of individual and combined exposure to sodium arsenite and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem Biol Interact 162:128–139
Mkandawire M, Dudel EG (2005) Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci Total Environ 336:81–89
Mohan D, Pittman CU Jr (2007) Arsenic removal from water/wastewater using adsorbents: a critical review. J Hazard Mater 142:1–53
Mohan D, Singh KP, Singh VK (2008) Wastewater treatment using low cost activated carbons derived from agricultural by products a case study. J Hazard Mater 152:1045–1053
Mohapatra M, Anand S, Mishra B, Giles DE, Singh P (2009) Review of fluoride removal from drinking water. J Environ Manag 91:67–77
Mohseni-Bandpi A, Kakavandi B, Kalantary RR, Azari A, Keramati A (2015) Development of a novel magnetite–chitosan composite for the removal of fluoride from drinking water: adsorption modeling and optimization. RSC Adv 5:73279–73289
Mondal P, Majumder C, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137:464–479
Mondal NK, Bhaumik R, Datta JK (2015) Removal of fluoride by aluminum impregnated coconut fiber from synthetic fluoride solution and natural water. Alex Eng 54:1273–1284
Morillo D, Uheida A, Pérez G, Muhammed M, Valiente M (2015) Arsenate removal with 3-mercaptopropanoic acid-coated superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci 438:227–234
Mukherjee A, Sengupta MK, Hossain MA, Ahamed S, Das B, Nayak B (2006) Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr 24:142–163
Mwangi CK, Mwangi IW, Wanjau RN, Swaleh S, Ram M, Ngila JC (2016) Remediation of fluoride laden water by complexation with triethylamine modified maize tassels
Newman DK, Ahmann D, Morel FM (1998) A brief review of microbial arsenate respiration. Geomicrobiol J 15:255–268
Nguyen CM, Bang S, Cho J, Kim KW (2009) Performance and mechanism of arsenic removal from water by a nanofiltration membrane. Desalination 245:82–94
Nitzsche KS, Lan VM, Trang PTK, Viet PH, Berg M, Voegelin A (2015a) Arsenic removal from drinking water by a household sand filter in Vietnam: effect of filter usage practices on arsenic removal efficiency and microbiological water quality. Sci Total Environ 502:526–536
Nitzsche KS, Weigold P, Lösekann-Behrens T, Kappler A, Behrens S (2015b) Microbial community composition of a household sand filter used for arsenic, iron, and manganese removal from groundwater in Vietnam. Chemosphere 138:47–59
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci 96:3455–3462
Oguz E (2005) Adsorption of fluoride on gas concrete materials. J Hazard Mater 117:227–233
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300:939–944
Ouzounis K, Katsoyiannis I, Zouboulis A, Mitrakas M (2015) Is the coagulation-filtration process with Fe(III) efficient for As(III) removal from groundwaters. Sep Sci Technol 50:1587–1592
Öztel MD, Akbal F, Altaş L (2015a) Arsenite removal by adsorption onto iron oxide-coated pumice and sepiolite. Environ Earth Sci 73:4461–4471
Öztel MD, Akbal F, Altaş L (2015b) Arsenite removal by adsorption onto iron oxide-coated pumice and sepiolite. Environ Earth Sci 73:4461–4471
Padungthon S, German M, Wiriyathamcharoen S, SenGupta AK (2015) Polymeric anion exchanger supported hydrated Zr(IV) oxide nanoparticles: a reusable hybrid sorbent for selective trace arsenic removal. React Funct Polym 93:84–94
Parmar S, Singh V (2015) Phytoremediation approaches for heavy metal pollution: a review. J Plant Sci Res 2:139
Parmar HS, Patel JB, Sudhakar P, Koshy V (2006) Removal of fluoride from water with powdered corn cobs. J Environ Sci Eng 48:135–138
Pokhrel D, Viraraghavan T (2006) Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res 40:549–552
Pokhrel D, Viraraghavan T (2008) Arsenic removal from an aqueous solution by modified A. niger biomass: batch kinetic and isotherm studies. J Hazard Mater 150:818–825
Pokhrel D, Viraraghavan T, Braul L (2005) Evaluation of treatment systems for the removal of arsenic from groundwater. Pract Period Hazard Toxic Radioact Waste Manag 9:152–157
Pokhrel D, Bhandari B, Viraraghavan T (2009) Arsenic contamination of groundwater in the Terai region of Nepal: an overview of health concerns and treatment options. Environ Int 35:157–161
Polya D, Gault A, Diebe N, Feldman P, Rosenboom J, Gilligan E (2005) Arsenic hazard in shallow Cambodian groundwaters. Mineral Mag 69:807–823
Popat K, Anand P, Dasare B (1994) Selective removal of fluoride ions from water by the aluminium form of the aminomethylphosphonic acid-type ion exchanger. React Polym 23:23–32
Prabhu SM, Meenakshi S (2015) A dendrimer-like hyper branched chitosan beads toward fluoride adsorption from water. Int J Biol Macromol 78:280–286
Qi J, Zhang G, Li H (2015) Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Biores Technol 193:243–249
Qiao J, Cui Z, Sun Y, Hu Q, Guan X (2014) Simultaneous removal of arsenate and fluoride from water by Al–Fe (hydr) oxides. Front Environ Sci Eng 8:169–179
Rasool A, Xiao T, Farooqi A, Shafeeque M, Liu Y, Kamran MA, Katsoyiannis IA, Eqani SAMAS (2016a) Quality of tube well water intended for irrigation and human consumption with special emphasis on arsenic contamination at the area of Punjab, Pakistan. Environ Geochem Health 1–17
Rasool A, Farooqi A, Xiao T, Masood S, Kamran MA (2016b) Elevated levels of arsenic and trace metals in drinking water of Tehsil Mailsi, Punjab, Pakistan. J Geochem Explor 169:89–99
Ravančić ME, Habuda-Stanić M (2015) Equilibrium and kinetics studies for the adsorption of fluoride onto commercial activated carbons using fluoride ion-selective electrode. Int Electrochem Sci 10:8137–8149
Riahi F, Bagherzadeh M, Hadizadeh Z (2015) Modification of Fe3O4 superparamagnetic nanoparticles with zirconium oxide; preparation, characterization and its application toward fluoride removal. RSC Adv 5:72058–72068
Rittle KA, Drever JI, Colberg PJ (1995) Precipitation of arsenic during bacterial sulfate reduction. Geomicrobiol J 13:1–11
Rojas-Mayorga CK, Silvestre-Albero J, Aguayo-Villarreal IA, Mendoza-Castillo D, Bonilla-Petriciolet A (2015) A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater 209:38–44
Romero L, Alonso H, Campano P, Fanfani L, Cidu R, Dadea C (2003) Arsenic enrichment in waters and sediments of the Rio Loa (Second Region, Chile). Appl Geochem 18:1399–1416
Rowland HA, Omoregie EO, Millot R, Jimenez C, Mertens J, Baciu C (2011) Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Appl Geochem 26:1–17
Ruiz-Payan A, Ortiz M, Duarte-Gardea M (2005) Determination of fluoride in drinking water and in urine of adolescents living in three counties in Northern Chihuahua Mexico using a fluoride ion selective electrode. Microchemical 81:19–22
Sahoo PK, Kim K (2013) A review of the arsenic concentration in paddy rice from the perspective of geoscience. Geoscience 17(1):107–122
Sancha AM (2006) Review of coagulation technology for removal of arsenic: case of Chile. J Health Popul Nutr 24:267
Sánchez J, Butter B, Rivas BL, Basáez L, Santander P (2015) Electrochemical oxidation and removal of arsenic using water-soluble polymers. J Appl Electrochem 45:151–159
Santini JM, Sly LI, Wen A, Comrie D, Wulf-Durand PD, Macy JM (2002) New arsenite-oxidizing bacteria isolated from australian gold mining environments-phylogenetic relationships. Geomicrobiology 19:67–76
Saqib ANS, Waseem A, Khan AF, Mahmood Q, Khan A, Habib A et al (2013) Arsenic bioremediation by low cost materials derived from Blue Pine (Pinus wallichiana) and Walnut (Juglans regia). Ecol Eng 51:88–94
Sathish RS, Raju N, Raju G, Nageswara Rao G, Kumar KA, Janardhana C (2007) Equilibrium and kinetic studies for fluoride adsorption from water on zirconium impregnated coconut shell carbon. Sep Sci Technol 42:769–788
Schreiber M, Simo J, Freiberg P (2000) Stratigraphic and geochemical controls on naturally occurring arsenic in groundwater, eastern Wisconsin, USA. Hydrogeology 8:161–176
Sehaqui H, Mautner A, de Larraya UP, Pfenninger N, Tingaut P, Zimmermann T (2016) Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohydr Polym 135:334–340
Sevcenco A-M, Paravidino M, Vrouwenvelder JS, Wolterbeek HT, van Loosdrecht MC, Hagen WR (2015) Phosphate and arsenate removal efficiency by thermostable ferritin enzyme from Pyrococcus furiosus using radioisotopes. Water Res 76:181–186
Shih YJ, Huang RL, Huang YH (2015) Adsorptive removal of arsenic using a novel akhtenskite coated waste goethite. J Clean Prod 87:897–905
Singh TP, Majumder C (2015) Removal of fluoride from industrial waste water by using living plant (Ipomoea aquatica). Meteorites 28:30
Sivabalan R, Rengaraj S, Arabindoo B, Murugesan V (2003) Cashewnut sheath carbon: a new sorbent for defluoridation of water. Ind J Chem Technol 10:217–222
Sklari S, Pagana A, Nalbandian L, Zaspalis V (2015) Ceramic membrane materials and process for the removal of As(iii)/As(v) ions from water. J Water Process Eng 5:42–47
Smedley P, Kinniburgh D (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Song K, Kim W, Suh CY, Shin D, Ko KS, Ha K (2013) Magnetic iron oxide nanoparticles prepared by electrical wire explosion for arsenic removal. Powder Technol 246:572–574
Stüben D, Berner Z, Chandrasekharam D, Karmakar J (2003) Arsenic enrichment in groundwater of West Bengal, India: geochemical evidence for mobilization of As under reducing conditions. Appl Geochem 18:1417–1434
Suriyaraj S, Bhattacharyya A, Selvakumar R (2015) Hybrid Al2O3/bio-TiO2 nanocomposite impregnated thermoplastic polyurethane (TPU) nanofibrous membrane for fluoride removal from aqueous solutions. RSC Adv 5:26905–26912
Sylvester P, Westerhoff P, Möller T, Badruzzaman M, Boyd O (2007) A hybrid sorbent utilizing nanoparticles of hydrous iron oxide for arsenic removal from drinking water. Environ Eng Sci 24:104–112
Taleb K, Markovski J, Milosavljević M, Marinović-Cincović M, Rusmirović J, Ristić M (2015) Efficient arsenic removal by cross-linked macroporous polymer impregnated with hydrous iron oxide: material performance. Chem Eng 279:66–78
Tang D, Zhang G (2016) Efficient removal of fluoride by hierarchical Ce–Fe bimetal oxides adsorbent: thermodynamics, kinetics and mechanism. Chem Eng 283:721–729
Teimouri A, Nasab SG, Habibollahi S, Fazel-Najafabadi M, Chermahini AN (2015) Synthesis and characterization of a chitosan/montmorillonite/ZrO2 nanocomposite and its application as an adsorbent for removal of fluoride. RSC Adv 5:6771–6781
Teixeira MC, Ciminelli VS (2005) Development of a biosorbent for arsenite: structural modeling based on X-ray spectroscopy. Environ Sci Technol 39:895–900
Thakre D, Rayalu S, Kawade R, Meshram S, Subrt J, Labhsetwar N (2010) Magnesium incorporated bentonite clay for defluoridation of drinking water. J Hazard Mater 180:122–130
Thirunavukkarasu O, Viraraghavan T, Subramanian K (2003) Arsenic removal from drinking water using granular ferric hydroxide. Water Sa 29:161–170
Tokunaga S, Wasay SA, Park SW (1997) Removal of arsenic (V) ion from aqueous solutions by lanthanum compounds. Water Sci Technol 35:71–78
Tor A, Danaoglu N, Arslan G, Cengeloglu Y (2009) Removal of fluoride from water by using granular red mud: batch and column studies. J Hazard Mater 164:271–278
Tournassat C, Charlet L, Bosbach D, Manceau A (2002) Arsenic(III) oxidation by birnessite and precipitation of manganese(II) arsenate. Environ Sci Technol 36:493–500
Tressaud A (2006) Fluorine and the environment: agrochemicals, archaeology, green chemistry and water, vol 2. Elsevier, Amsterdam
Trois C, Cibati A (2015) South African sands as a low cost alternative solution for arsenic removal from industrial effluents in permeable reactive barriers: column tests. Chem Eng 259:981–989
Turner BD, Binning P, Stipp S (2005) Fluoride removal by calcite: evidence for fluorite precipitation and surface adsorption. Environ Sci Technol 39:9561–9568
Twidwell L, Robins R, Hohn J (2005) The removal of arsenic from aqueous solution by coprecipitation with iron(III). In: Reddy RG, Ramachandran V (eds) Proceedings arsenic metallurgy: fundamentals and applications, pp 3–24
Ungureanu G, Santos S, Boaventura R, Botelho C (2015) Arsenic and antimony in water and wastewater: overview of removal techniques with special reference to latest advances in adsorption. J Environ Manag 151:326–342
Van Vinh N, Zafar M, Behera S, Park H-S (2015) Arsenic(III) removal from aqueous solution by raw and zinc-loaded pine cone biochar: equilibrium, kinetics, and thermodynamics studies. Int J Environ Sci Technol 12:1283–1294
Vences-Alvarez E, Velazquez-Jimenez LH, Chazaro-Ruiz LF, Diaz-Flores PE, Rangel-Mendez JR (2015) Fluoride removal in water by a hybrid adsorbent lanthanum–carbon. J Colloid Interface Sci 455:194–202
Violante A, Gaudio SD (2007) Coprecipitation of arsenate with metal oxides. 2. Nature, mineralogy, and reactivity of iron(III) precipitates. Environ Sci Technol 41:8275–8280
Violante A, Pigna M (2009) Coprecipitation of arsenate with metal oxides. 3. Nature, mineralogy, and reactivity of iron(III): aluminum precipitates. Environ Sci Technol 43:1515–1521
Violante A, Ricciardella M (2006) Coprecipitation of arsenate with metal oxides: nature, mineralogy, and reactivity of aluminum precipitates. Environ Sci Technol 40:4961–4967
Viswanathan N, Meenakshi S (2009) Role of metal ion incorporation in ion exchange resin on the selectivity of fluoride. J Hazard Mater 162:920–930
Vithanage M, Bhattacharya P (2015) Fluoride in the environment: sources, distribution and defluoridation. Environ Chem Lett 13:131–147
Vivek Vardhan C, Srimurali M (2016) Defluoridation of drinking water using a novel sorbent: lanthanum-impregnated green sand. Desalin Water Treat 57:202–212
Vu TA, Le GH, Dao CD, Dang LQ, Nguyen KT, Nguyen QK (2015) Arsenic removal from aqueous solutions by adsorption using novel MIL-53 (Fe) as a highly efficient adsorbent. RSC Adv 5:5261–5268
Waghmare SS, Arfin T (2015) Fluoride removal from water by various techniques: review. Int J Innov Sci Eng Technol 2:560–571
Waghmare SS, Arfin T, Manwar N, Lataye DH, Labhsetwar N, Rayalu S (2015) Preparation and characterization of polyalthia longifolia based alumina as a novel adsorbent for removing fluoride from drinking water. Asian J Adv Basic Sci 4:12–24
Wang S, Zhao X (2009) On the potential of biological treatment for arsenic contaminated soils and groundwater. J Environ Manag 90:2367–2376
Wang L, Condit WE, Chen AS, Sorg TJ (2004) Technology selection and system design US EPA arsenic removal technology demonstration program round 1: National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency
Wang SG, Ma Y, Shi YJ, Gong WX (2009) Defluoridation performance and mechanism of nano-scale aluminum oxide hydroxide in aqueous solution. J Chem Technol Biotechnol 84:1043–1050
Wang J, Kang D, Yu X, Ge M, Chen Y (2015a) Synthesis and characterization of Mg–Fe–La trimetal composite as an adsorbent for fluoride removal. Chem Eng 264:506–513
Wang S, Gao B, Zimmerman AR, Li Y, Ma L, Harris WG (2015b) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Biores Technol 175:391–395
Wen Z, Dai C, Zhu Y, Zhang Y (2015) Arsenate removal from aqueous solutions using magnetic mesoporous iron manganese bimetal oxides. RSC Adv 5:4058–4068
Williams M, Fordyce F, Paijitprapapon A, Charoenchaisri P (1996) Arsenic contamination in surface drainage and groundwater in part of the southeast Asian tin belt, Nakhon Si Thammarat Province, southern Thailand. Environ Geol 27:16–33
Xie X, Ellis A, Wang Y, Xie Z, Duan M, Su C (2009) Geochemistry of redox-sensitive elements and sulfur isotopes in the high arsenic groundwater system of Datong Basin, China. Sci Total Environ 407:3823–3835
Xiong X, Liu J, He W, Xia T, He P, Chen X (2007) Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res 103:112–116
Yadav AK, Kaushik C, Haritash AK, Kansal A, Rani N (2006) Defluoridation of groundwater using brick powder as an adsorbent. J Hazard Mater 128:289–293
Yao R, Meng F, Zhang L, Ma D, Wang M (2009) Defluoridation of water using neodymium-modified chitosan. J Hazard Mater 165:454–460
Yin H, Kong M, Tang W (2015a) Removal of fluoride from contaminated water using natural calcium-rich attapulgite as a low-cost adsorbent. Water Air Soil Pollut 226:1–11
Yin H, Kong M, Tang W (2015b) Removal of fluoride from contaminated water using natural calcium-rich attapulgite as a low-cost adsorbent. Water Air Soil Pollut 226:1–11
Yu L, Ma Y, Ong CN, Xie J, Liu Y (2015) Rapid adsorption removal of arsenate by hydrous cerium oxide–graphene composite. RSC Adv 5:64983–64990
Zazouli MA, Mahvi AH, Mahdavi Y, Balarakd D, Sari T (2015) Isothermic and kinetic modeling of fluoride removal from water by means of the natural biosorbents sorghum and canola. Fluoride 48:37–44
Zehhaf A, Benyoucef A, Quijada C, Taleb S, Morallón E (2015) Algerian natural montmorillonites for arsenic(III) removal in aqueous solution. Int J Environ Sci Technol 12:595–602
Zhang J, Zhu YG, Zeng DL, Cheng WD, Qian Q, Duan GL (2008) Mapping quantitative trait loci associated with arsenic accumulation in rice (Oryza sativa). New Phytol 177:350–356
Zhao X, Wang J, Wu F, Wang T, Cai Y, Shi Y (2010) Removal of fluoride from aqueous media by Fe3O4@ Al (OH)3 magnetic nanoparticles. J Hazard Mater 173:102–109
Zhou Y, Yu C, Shan Y (2004) Adsorption of fluoride from aqueous solution on La3+ impregnated cross-linked gelatin. Sep Purif Technol 36:89–94
Zhu J, Lin X, Wu P, Zhou Q, Luo X (2015) Fluoride removal from aqueous solution by Al(III)–Zr(IV) binary oxide adsorbent. Appl Surf Sci 357:91–100
Zouboulis AI, Katsoyiannis IA (2005) Recent advances in the bioremediation of arsenic-contaminated groundwaters. Environ Int 31:213–219
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bibi, S., Kamran, M.A., Sultana, J. et al. Occurrence and methods to remove arsenic and fluoride contamination in water. Environ Chem Lett 15, 125–149 (2017). https://doi.org/10.1007/s10311-016-0590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-016-0590-2