Abstract
Groundwater is consumed by a large number of people as their primary source of drinking water globally. Among all the countries worldwide, nations in South Asia, particularly India and Bangladesh, have severe problem of groundwater arsenic (As) contamination so are on our primary focus in this study. The objective of this review study is to provide a viewpoint about the source of As, the effect of As on human health and agriculture, and available treatment technologies for the removal of As from water. The source of As can be either natural or anthropogenic and exposure mediums can either be air, drinking water, or food. As-polluted groundwater may lead to a reduction in crop yield and quality as As enters the food chain and disrupts it. Chronic As exposure through drinking water is highly associated with the disruption of many internal systems and organs in the human body including cardiovascular, respiratory, nervous, and endocrine systems, soft organs, and skin. We have critically reviewed a complete spectrum of the available ex situ technologies for As removal including oxidation, coagulation–flocculation, adsorption, ion exchange, and membrane process. Along with that, pros and cons of different techniques have also been scrutinized on the basis of past literatures reported. Among all the conventional techniques, coagulation is the most efficient technique, and considering the advanced and emerging techniques, electrocoagulation is the most prominent option to be adopted. At last, we have proposed some mitigation strategies to be followed with few long and short-term ideas which can be adopted to overcome this epidemic.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 3% of the water available on earth is freshwater, of which 83% is locked in icecaps and glaciers, leaving just 17% accessible in the form of utilizable freshwater, and only 30% of this utilizable freshwater is groundwater. Deforestation, mining, industrialization, urbanization, over-exploitation and direct anthropogenic contamination are significant modifications of the surface land cover which has set groundwater under threat. The statistics show that about 14% of the world’s population is unable to obtain clean drinking water and 32% is unable to use a suitable sanitation facility. Around 5 million people die due to water-borne diseases (i.e., typhoid, cholera, hepatitis A, etc.) in a year, and it is projected that the majority of the global citizens by 2025 are to be expected to reside in nations where water scarcity is mild to high (Azizullah et al., 2011).
The existence of heavy metals in groundwater has been a growing matter of concern as it causes a deleterious impact on human health. Numerous metals are vital for individuals in small amounts, but in excess, they can pollute water and lead to serious health complications for human beings (Azizullah et al., 2011; Raza et al., 2017). Heavy metals are chemical elements having a specific density greater than 5 g/ml, toxic at low concentrations, found in the earth's crust as naturally occurring elements that cannot be degraded in any way and include cadmium (Cd), mercury (Hg), thallium (Tl), chromium (Cr), arsenic (As), and lead (Pb) (Sharma et al., 2020). Arsenic has been classified as a category I cancer-causing substance among human beings by the International Agency of Research on Cancer (IARC), indicating that there is ample evidence of carcinogenicity in humans (Martinez et al., 2011). Arsenic is not present as a free element in nature but found as a compound of different elements like hydrogen, carbon, oxygen, chlorine, sulfur, lead, gold, mercury, and iron (Fazal et al., 2001). In numerous nations throughout the world, and especially in Asia, arsenic is considered a hazard to public health (Azizullah et al., 2011; Haque et al., 2008). Currently, 107 nations have recorded medium to excessive levels of As in groundwater, putting 296 million people at possible risk from As ingestion through various sources of exposure (Chakrabarti et al., 2019; Ghosh et al., 2019). In India, groundwater of a total of 718 districts is contaminated with one or more than one contaminant including nitrate, fluoride, Fe, As, Pb, Cr, and Cd across different states and the states like Punjab, Tamil Nadu, Telangana, Uttar Pradesh, Haryana, and West Bengal have most polluted groundwater across the country. To assess the level and severity of As contamination in groundwater, a thorough analysis performed in Nadia, West Bengal (India), found that all blocks under investigation contained As concentration more than 50 µg/l with the maximum of 3200 µg/l and they discovered As concentrations of more than 10 and 50 µg/l in 51.4% and 17.3% of tube wells, respectively, after analyzing the samples collected from different tube wells (Rahman et al., 2014).
According to a study (Arain et al., 2009; Azizullah et al., 2011), one-third of people in an investigated area had rugged skin with black spots and arsenical skin lesions on some of the body parts, due to exposure to greater As levels and other conditions such as starvation, poverty, a lack of good medical services, and the prevalence of other pollutants functioning in coordination with As surplus. One more related research was carried out in which 285 subjects were sampled, off these two-third population suffered from long-term As toxicity. They found a close connection among drinking water As concentration and blood and hair samples of diseased patients with exposed skin. They suffered from several conditions such as respiratory disorders, stomach disorders, and fatigue. There were no such issues with people who drank municipal water having low As concentration (Azizullah et al., 2011; Kazi et al., 2009). A study carried out by Rahman et al. (2014) investigated several samples from different villagers and concluded that many villagers had arsenical skin lesions, and majority of the sampled concentration exceeded the toxic level.
The method of treating contaminated groundwater by eliminating toxic chemicals or turning them into non-dangerous chemicals is known as groundwater remediation (Concetta Tomei & Daugulis, 2013; Sharma et al., 2020). There are various traditional physical and chemical methods available for As elimination from groundwater, both in the laboratory and in the field such as adsorption, membrane-based technology, chemical precipitation, and ion exchange (Pal et al., 2009). Other non-traditional and alternative treatment technologies includes electrocoagulation (EC), advanced oxidation processes (AOPs), ultraviolet (UV) oxidation, microbial oxidation, bioelectrochemical systems, and many more. Due to high technological performance, low operating cost, low maintenance, and less energy demand, permeable reactive barrier (PRB) is considered as most favorable in situ remediation technique and is one of the revolutionary methods. This is an environment-friendly remediation technology for contaminated groundwater, which works by deployment of a porous barricade that carries reactive chemicals to capture and treat pollutants as the groundwater moves through it (Luo et al., 2016; Obiri-Nyarko et al., 2014; Park et al., 2002; Robertson et al., 2008). An experiment was carried out by Lee et al. (2009) to assess long-term degradation of As compounds with the help of Zero Valent Ion (ZVI) using the PRB technique in which they took samples at different intervals of time for 360 days. They showed that the rate of total As removal reduced by 45% and 39% under aerobic and anoxic conditions, respectively, due to saturation and concluded their experiment by suggesting that for effective As elimination, the reactive substance should be changed regularly.
Humans are exposed to As throughout their entire life span which is carcinogenic. Arsenic can get into the human body through various ways, typically, by drinking water or via dietary sources. Arsenic can act as a poisonous agent and therapeutic for human beings (Bhardwaj et al., 2018). Long-term exposure to As can be fatal to the human body, so studying the source of As and treating it appropriately can cure long-term exposure to the human body. The study provides complete review of As pollution and its negative health effects in the human body, treatment facilities, and mitigation strategies.
According to the authors' best knowledge, there is presently no literature available that contains comprehensive information on As, starting with the source of pollution, human health and agricultural implications, remediation techniques, and recommended mitigation measures all in one article. The study's main goal is to present a complete overview of work on As pollution, from its sources to its solutions, in a structured manner. One of our main focus is to aware researchers worldwide about the As contamination and also to emphasize solutions at the same time and that’s why mitigation strategies are one of the key focuses in this article that has not been addressed on such scale yet in addition with its origin to its end.
This investigation concentrates on India as a geography, with very slight focus on few neighboring nations. This article provides an intriguing overview of As-polluted groundwater, its impact on healthcare and agriculture, and highlights a good number of investigations on treatment processes that have been carried out in the field as well as countermeasures, helping readers to build a solid understanding.
Sources of arsenic contamination
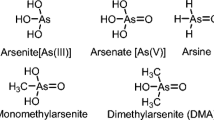
Arsenic is a trace metalloid substance found in the earth's crust. Arsenic-contaminated groundwater is attributed to one or more natural and anthropogenic causes. In nature, the presence of As in water bodies was reported as low as a few mg/l, and levels in soils can range from 0.01 mg/kg to some hundred mg/kg and it is found in nature in various oxidation states like + 5, + 3, 0 and − 3 which are known as arsenate, arsenite, arsenic, and arsine, respectively (Bissen & Frimmel, 2003; Mitra et al., 2017). In addition, As can be seen in the form of sulfides and oxides in nature. Anthropogenic source of water and soil arsenic pollution includes the inappropriate production, utilization, and discard of As-containing industrial chemicals, widespread use of As-containing pesticides and phosphate fertilizers, emissions and drainage from smelting and mining of As-containing minerals, fossil fuel combustion, bacterial decomposition of natural or synthetic organic chemicals, etc. (Bissen & Frimmel, 2003; Henke, 2009). In the environment, there are more than 150 different As-containing minerals in which As is mostly found in sulfide minerals, among them three composites including Realgar or arsenic disulfide (As2S2), orpiment or arsenic trisulfide (As2S3), and arsenopyrite or ferrous arsenic sulfide (FeAsS) are known as the arsenic ore because they are readily available compared to other As composites with higher As concentration (Fazal et al., 2001). Arsenopyrite is the most common As ore mineral, and it is commonly accepted that Arsenopyrite, along with the other major As sulfide minerals realgar and orpiment, is only produced under elevated temperature conditions on the earth's surface. According to the chemical constituents, As is divided into two types—organic (less toxic) and inorganic (more toxic), in which inorganic As is further distinguished by two types—trivalent and pentavalent, and among them trivalent As is considered to be more toxic and more difficult to remove from water than pentavalent As (Fazal et al., 2001). Because of the greater retention stability of As species in the corresponding ore, there is less likelihood of As contamination in groundwater and surface water (Pal et al., 2009). Kumar et al. (2016) calculated the safe level of As in drinking water and different food ingredients to assess the possible health risk in As-polluted areas in Bihar, India. This study discovered that drinking water contributed to two-third of the overall estimated daily dietary consumption of 169 µg As per day, with the rest coming from the food intake. Since crude oil is extracted from the underground reservoirs, their end products (i.e., diesel, gasoline, kerosene, jet fuel, etc.) also contain small amounts of As. An experimental study carried out by De Jesus et al. (2012) showed that the presence of As in crude oil samples was about 0.214 mg/kg (maximum out of 7 samples). So, combustion products of crude oil may lead to emission of As in the atmosphere. Coal also contains a small quantity of arsenic as it is a fossil resource and can be mined either on the surface or underground due to which the combustion of coal can result in the release of As into the atmosphere. Ordinary bituminous and lignite coals have mean As concentrations of 9.0 and 7.4 ppm at the global level, respectively, and the global highest As concentration is 50 and 49 ppm, respectively (Pandey et al., 2011; Yudovich & Ketris, 2005). Moreover, if we consider this in the Indian scenario, the As content of Indian bituminous coal ranges from 22.3 to 62.5 ppm (Pandey et al., 2011). Fly ash is the by-product of the coal-based thermal power plant, generated by the combustion of coal, which represents a possible anthropogenic source of As threats. Arsenic levels in fly ash usually vary from 2 to 440 mg/kg, but they might reach 1000 mg/kg based on the composition of the coal used and the method of combustion (Pandey et al., 2011).
Impacts of As on human health
Many citizens around the globe use groundwater as a means of drinking water. According to IS 10500 (2012), the acceptable limit for the total As content in the drinking water is set as a maximum of 0.01 mg/l, and some relaxation has been given in the case of the absence of an alternative source of drinking water in which the permissible limit for the total As content is set as a maximum of 0.05 mg/l. Additionally, the provisional guideline value given by World Health Organization (WHO) for As in drinking water is also 0.01 mg/l (or 10 µg/l). Toxicity and carcinogenic effects of As are determined by the oxidation states and chemical forms in which it exists (Hong et al., 2014; Rasheed et al., 2016). Arsenic can enter the body via several pathways, including inhalation, ingestion, and dermal interaction. Depending on the time of exposure, the effect of As is classified into two categories, acute and chronic effects. Humans who are exposed to As for extended periods face chronic health issues (Hong et al., 2014; Rasheed et al., 2016). While acute toxicity is usually reversible and happens when more than a sufficient amount of As is consumed in a short period, chronic toxicity occurs when As is ingested by polluted drinking water for a prolonged period. The most usual health concern correlated with As toxicity is arsenical keratosis and skin cancer (Jha et al., 2017). Acute exposure to As has no consequences if the dosage is smaller than 0.03 mg/kg of body weight/day, little consequence if the dosage is in between 0.03 and 0.3 mg/kg of body weight/day, produces consequences of illness if the dosage is in between 0.3 and 0.5 mg/kg of body weight/day, and fatal if the dosage is more than 1 mg/kg of body weight/day (Jha et al., 2017). According to Farmer and Johnson (1990), the human body holds between 40 and 60% of the As consumed in drinking water, so the more As-containing water we ingest, the higher is the consequent risk. In areas where people used to eat fish as a source of food, they could have been exposed to As from the fish, and in that situation, the magnitude of the issue and possible health effects are determined by measuring the bioaccumulation factor of an analyte, which correlates the concentration of the analyte under examination in fish and water bodies. An experiment conducted by Kar et al. (2011) investigated three major As species namely arsenite, arsenate, and dimethyl arsenic in a total of 9 samples. The samples were collected from the three different body parts (tissue, bone, and head) of two types of freshwater fish (shrimp and tilapia) from three distinct ponds of Taiwan. The results showed maximum bioaccumulation factor of 22, and out of three As species, dimethyl arsenic was found to be present in a higher amount compared to the inorganic As species (arsenite and arsenate). This may be dangerous to people of the surrounding areas who eat fish regularly and are more likely exposed to As by ingestion. A review conducted by Bhowmick et al. (2018) and Guha Mazumder and Dasgupta (2011) concluded the fact that high levels of As in the environment can have both carcinogenic and non-carcinogenic effects. Respiratory illness, gastrointestinal affliction, renal failure, cardiovascular disorder, neurotoxicity, negative hematological consequences, diabetes mellitus, adverse birth complications, and neonate mortality are examples of non-carcinogenic impacts. An experimental study carried out by Mazumder et al. (2012) shows that in respondents residing in As-contaminated areas, there is a coordination between chronic As toxicity and the probability of development of hypertension. Chronic As toxicity has been directly linked to the occurrence of cardiovascular diseases (Henke, 2009; Navas-Acien et al., 2005; Wang et al., 2007). A correlation between As exposure and skin cancer has been studied by many researchers. One of the study shows that UV-caused skin cancers have been directly linked with exposure to trivalent arsenite (Hong et al., 2014; Rossman et al., 2004). Furthermore, several researchers have examined the dose-dependent correlation between As ingestion and the prevalence of lung cancer (Ferreccio et al., 2000; Smith et al., 2006). In a research carried out in Taiwan, individuals who had drunk high-arsenic-polluted drinking water for the past 5 decades had an increased death rate; lower As levels of drinking water resulted in lower lung cancer death rates (Chiu et al., 2004; Hong et al., 2014). A study carried out by Chen et al. (2004) found better correlation between tobacco smoking and exposure to As, which shows that tobacco smoking coupled with As toxicity raises the probability of lung cancer, and vice versa. A new investigation in the Yatenga province, Burkina Faso, tested 240 people from 20 different villages for arsenical skin lesions in which As levels of tube-well water ranged from 1 to 124 µg/l. The concentration of As was higher than the WHO recommendation value of 10 µg/l in more than 50% of the tube wells. Among the tested people, melanosis and keratosis were found in 29.3% and 46.3% of the population respectively during clinical tests; prevalence of skin lesions was linked to As concentrations in tube-well water (Chakrabarti et al., 2019). Long-term exposure to As may have negative impacts on the result of pregnancy such as premature abortion, neonatal death, miscarriage or stillbirth, and lower birth weight (Bhowmick et al., 2018; Quansah et al., 2015). “Arsenicosis” is a disease that occurs as a result of prolonged As toxicity which appears in the form of skin lesions including melanosis, keratosis, and leucomelanosis (Shaji et al., 2021). Arsenicosis can be divided into four phases in which the first phase is called the preclinical phase because it occurs when the patient has no visible signs but As is found in urine or body tissues (Choong et al., 2007). The second phase usually needs nearly about half to 1 decade of As exposure and is referred to as a clinical phase. At this phase, the skin has a variety of consequences among which melanosis (skin pigmentation) is perhaps the most usual symptom and keratosis (skin hardening) is a more severe symptom. The third phase is known by complications that include damage to inner organs, and the last phase is known as a malignancy that comprises cancers or tumors that damages different organs or skin. An investigation found that As exposed people had a higher chance of evolving diabetes in their preliminary phase of young age, and the condition is much worse for young adults having arsenicosis, with a frequency of more than double in comparison with the young adults having no exposure (Khan et al., 2016).
Figure 1 shows the destructive human health outcomes because of the presence of As in drinking water for a prolonged period, whereas no medicines have been discovered which can treat chronic As poisoning. Only unpolluted drinking water, healthy food, and physical activities (i.e., exercise) can be utilized to combat this epidemic (Chakraborti et al., 2013). Figure 2 shows long-term exposure of As on different organs and system of human body.
Impact of As on agriculture sector
The majority of the crops (i.e., wheat, rice, pulses, sugarcane, etc.) and different types of vegetation grown in developed countries are highly vulnerable to As-contaminated water (Gong et al., 2020). The region close to the Indo-Gangetic delta and West Bengal (India) are having a major impact on As enrichment where As is washed up and carried throughout the Ganga river and finally ends up deposited in the sedimentary basin (Sharma et al., 2020). It further settles down to the groundwater bed and contaminates groundwater, which is further used for irrigation and drinking purposes (Pal et al., 2009). The contaminated groundwater leads to a decrease in crop productivity and quality (food chain contamination) especially of rice, as it is grown with irrigation water, thus resulting in accumulation by ten times more than other cereals. Some of the literature shows that As accumulation is highest in roots followed by stem, leaf and at last grains of the crops (Kundu et al., 2013; Senanayake & Mukherji, 2014). Crops that grow below the surface (i.e., carrot, potatoes) are highly exposed to As-contaminated groundwater, and the crops like rice have a high tendency to absorb As and transport through the food chain. Arsenic concentration in plants varied from 0.01 to 5.0 mg/kg worldwide (Mandal & Suzuki, 2002; Pal et al., 2009). Consumption of milled rice (staple food) is estimated at around 480 million tons annually (Muthayya et al., 2014). Water consumed by rice crops is around 750 mm which mostly comes from groundwater, rain and surface water (Aryal, 2013). Arsenic contaminated water gets accumulated on the outer bran layer surrounding the endosperm of the rice. Although cooking rice at boiling temperature can also reduce As content absorbed by rice grain (Menon et al., 2021). 75–90% of nutrient depletion is caused due to accumulation of As in bran (Upadhyay et al., 2019). WHO’s permissible limit for As concentration in rice is 0.3 mg/kg (Gu et al., 2020). The inflated toxicity of the soil not only result in As enrichment of the outer layer of the soil but also reflected in the roots and stem of the plant (Abedin et al., 2002). Wheat crops exposed to As-contaminated water also behaved similar to rice accumulation. Root development, biomass, germination and cell expansion, nutrients and uptake of water, respiration process, and photosynthesis showed significant variation under As stress which may lead to lower yield and productivity (Saeed et al., 2021). Its concentration on the wheat grain was found between 0.01 and 0.19 mg/kg (Bhattacharya et al., 2010). As can easily enter our food chain through our food and can lead to various diseases and health problems (Rai et al., 2019). Figure 3 shows fate and transport of arsenic in human food chain.
Treatment technologies for As remediation
In recent years, comprehensive research has been carried out on As elimination methodologies from groundwater through experiments in laboratory and field. Chemistry and presence of different constituents in the As-polluted water are the two most important considerations in deciding the removal of As (Nicomel et al., 2015; Singh et al., 2015). To remove the pollutant, scientists and researchers use a variety of approaches and processes with an emphasis on separation techniques including adsorption, membrane-based technology, coagulation–flocculation, and ion exchange (Chatterjee et al., 2017). Since As can’t be degraded, it can either be transformed from an aqueous phase to a non-aqueous phase (i.e., solid phase as the most suitable phase) or removed from the water directly by other processes. Only after transforming arsenite to arsenate, it can be removed efficiently (Nidheesh & Singh, 2017). Technologies for As-contaminated groundwater treatment are considered more efficient with the use of a two-step process involving initial arsenite to arsenate oxidation, accompanied by an arsenate removal method (Nicomel et al., 2015; Pous et al., 2015). Additionally, toxicity of arsenite makes the pre-oxidation step essential, which is much greater than arsenate. The oxidation method involves different chemical oxidants including Ozone (O3), Peroxy-monosulfate (PMS), hydrogen peroxide (H2O2), peroxy-disulfate (PDS), potassium permanganate (KMnO4), etc. It is not mandatory that an approach or a process that works well in one location also works well in others and the selection of the technology is also an essential factor because every approach or process get their own advantages and disadvantages. Various treatment technologies for the removal of As from groundwater are depicted in Fig. 4.
Oxidation
A study conducted by Gude et al. (2018) showed that pre-oxidation dosage decreases arsenite and increases arsenate concentration before passing through the sand filter for further treatment. This method was used in the Netherlands to achieve 1 µg/l of arsenite in drinking water by pre-oxidation treatment followed by the sand filtration. In this experimental study, Natural Groundwater was used for treatment to remove arsenite. The flow rate used for all experiments was set to 1 ml/min. Manganese was used as an oxidizing agent at 8 mg/l of concentration. The concentration of arsenite was 135 µg/l which decreases to 9 µg/l after treatment. Complete removal of arsenite by oxidation was achieved within 22 days in the fresh sand filter.
As can also be removed by oxidation using solar energy. The experiment was executed by O’Farrell et al. (2016) with the aim to reduce the arsenate level to 10 µg/l by using solar energy. The sample used in the experiment was prepared in the laboratory. The initial concentration of arsenate in the sample was 1000 µg/l. The solar reactor (compound parabolic collector) consists of 10 parallel borosilicate glass tubes connected with opaque plastic fitting to form a continuous flow in the pipe. The detention time for oxidation was 8 h. Fe was also added at 10 mg/l to get optimum results. The use of UV radiation was found to be a minimum of 7.5 kJUV/l. The coagulants used in the experiment were alum and Moringa oleifera (drumstick tree). Activated Moringa oleifera for As removal through adsorption mechanism was studied by Sumathi and Alagumuthu (2014). The concentration of both coagulants was 20 mg/l. The flocculant added to the sample was potato starch, and the concentration for the same was 20 mg/l. After treatment, the concentration of arsenate was reduced to 10 µg/l.
An experiment was performed by Bora et al. (2016) to remove arsenite from the solution prepared in the laboratory having a concentration of 100 µg/l. During the experiments, the initial pH was adjusted to 8.3. The oxidant used for treatment was KMnO4. The KMnO4 solution was added in varying amount from 5 to 100% of Fe2+. The following values: 80%, 85%, 90%, 93%, 95% of KMnO4, are equivalent to 18.85 mg/l, 20 mg/l, 21 mg/l, 21.88 mg/l, 22.16 mg/l of initial KMnO4 concentration, respectively, where initial concentration of Fe2+ was 25 mg/l. The source of Fe2+ was ferric sulfate, and FeCl3 was added as a coagulant. The experiment showed that dose of KMnO4 about 80–95% gives better results for removal. The study found that arsenite concentration reduces to 2 µg/l after the oxidation–coagulation process at optimized pH of 8.3. The decrement in Fe concentration was also noted after the oxidation–coagulation process.
An investigation was carried out to oxidize As(III) by using H2O2 as a catalyst and adsorb As(V) in the hybrid oxidation and adsorption process (Su et al., 2021). The initial concentration of the synthetic As(III) sample was 20 mg/l and dose of MnL (Mn-doped lanthanum oxycarbonate with 5.26% Mn), and H2O2 were 200 mg/l and 0.534 mM correspondingly. Experiments were carried out at room temperature (25 ± 2 °C) and different pH of 3, 5, 7, 9, and 11. In H2O2–MnL combined system, highest removal efficiency of 88% was observed at pH 7, and pH of 5 and 9, around 70% As(III) was removed within 6 h. As a result, Mn-doped La2O2CO3 was proved to be efficient in combination with H2O2 and also can be used as a catalyst for oxidation of As(III) at a very wide range of pH (5–9), and As(III) can be reduced up to 10 µg/l. The wide working range of pH considerably expands the range of applications for this oxidant. In this pH range, concentration of As(V) increased for first 30 min and then reduced moderately confirms that As was removed by both the oxidation of As(III) and adsorption of As(V).
Coagulation
Due to the low-cost and high-efficiency treatment, removal of As from the contaminated groundwater using adsorption and coagulations is more promising. Coagulations treatment of contaminated water involves adding coagulant agents (Fe3+ and Al3+) which helps in formation of positively charged flocs (Tolkou & Zouboulis, 2020). The coagulation process with neutral pH value in high As water is the best low costing and efficient treatment technique (Song et al., 2006). The study was performed using a few samples of the underground mine drainage system. Using ferric ions as a coagulant, authors were able to remove 99% of the As from the acidic water, whereas the removal of As was not so promising in alkaline water. 100 mg/l of ferric sulfate was used as a coagulant agent. Addition 2.5 gm/l coarse calcite as a coagulant increased the removing efficiency of As in alkaline water by 20–30% resulting in 99% removal from the residual As concentration of 5 mg/l at pH 6.
A study was conducted by Khan et al. (2002) in which they collected As-contaminated groundwater samples from 28 different tube wells. Samples were stirred at 50 rpm for 180 s before mixing with coagulant agents (i.e., ferric chloride and alum) under various pH conditions (pH range of 4–9). Addition of 200 ppm of ferric chloride at pH 7–8 led to 91% of As removal, whereas inclusion of 25–50 ppm alum to 81% As removal. The study concluded ferric chloride was a better coagulant agent than alum due to ion inclination in the ferrous compound (Bina et al., 2013).
Bina et al. (2013) focused on removal of arsenite and arsenate species in groundwater. Arsenite has more toxicity rate than arsenate due to a slower excretion rate (Kuivenhoven & Mason, 2019). During initial experiments, FeCl3 (60 mg/l) at pH 7 was used as a coagulant for the coagulation process. The removal efficiency was found to be better for arsenate than arsenite. Addition of chitosan (0.2–2 mg/l) in the mixer as a coagulant agents resulted in more removal efficiency and less dosage of FeCl3. Nearly 90–100% and 60–80% removal efficiency were found for Arsenate and arsenite, respectively. Initial concentration plays a significant role in the As removal. The less initial concentration tends toward better removal efficiency (Hao et al., 2018).
An experiment was carried out by Oza et al. (2020) to remove total As from the simulated As-containing water by combined EC and ultrasonic processes. The sequence is sonication treatment followed by EC process. In the EC method, highly pure iron electrodes were employed. Firstly, sonication process was carried out with initial concentration of 0.29 mg/l, initial pH of 7.8, ultrasound frequency of 40 kHz, and 2 h of treatment time. After that EC process was conducted at adjusted initial pH of 8.2, current density of 20.83 A/m2, and 1 h of treatment time. This combination technique was effective in eliminating 98.44% of total As. While employing EC reactor alone with current density of 41.66 A/m2, pH of 8.4, and initial As concentration of 0.5 mg/l, the optimum treatment time found was 15 min for removing 98.09% of total As.
Adsorption
An activated alumina-based As removal unit was used by Jalil and Ahmed (2001) for the removal of arsenate. Arsenate removal efficiency was found better than arsenite removal efficiency. The water samples were collected from the different tube wells in Bangladesh, and 65% of the sample collected were having 2–10 mg/l of iron depending upon the location. This iron plays a significant role in the performance of the alumina column. Iron oxidized at less pH producing insoluble iron resulting in Fe(OH)3 through hydrolysis. The activated alumina adsorption was able to remove As concentration less than 50 ppb at a pH level of 5.5 with the removal efficiency of 97%. Dissolved iron in groundwater increased the performance efficiency of the treatment resulting in more removal efficiency of As (Bhattacharya et al., 2015).
A study conducted by Bang et al. (2005) shows the removal of As using ZVI and the effects of dissolved oxygen and pH in the process. Experiment results of arsenite and arsenate were obtained under oxic and anoxic conditions. An arsenate at 9 h of mixing in oxic condition at pH level 6 showed 86% removal efficiency, whereas removal efficiency drops down to only 4% at anoxic condition. Similar results were obtained in arsenite with the removal efficiency of 99.8% and < 9% in oxic and anoxic conditions, respectively. The rate of iron corrosion and As removal can be increased by the presence of dissolved oxygen and low pH (Rezaei & Vione, 2018).
Another study was performed by Li et al. (2010) on a low arsenic sample water, using a ferrous-based red mud sludge as an adsorbent for removal of As from the water. Positively charged nanoparticles on the surface of Fe3O4 from red mud sludge act as an adsorbent agent for As removal (Wang & Liu, 2021). 2 × 10–4 mol/l ferrous and 7.7 × 10–2 g/l red mud were mixed with the initial concentration of 16–1.4 mg/l arsenate for 24 h—resulting in 91.2% As removal efficiency. The pH was maintained at 8 during the experiment. Increased FeCl2 content in red mud from 10 to 30% increased removal efficiency while reducing the pH of the solution by 6.4.
To remove higher concentrations of As, an Mg and Al layered double hydroxide (LDH) modified by sulfur species (LDH-S) was developed by Huang et al. (2022). Mg/Al molar ratio was set at 2:1. In a continuous flow configuration with initial As(V) concentration of 20 ppm, solution was passed along the column, which contained 300 mg of adsorbent. The LDH (2:1)-S adsorbent decreased As(V) concentrations to less than ppb levels within 120 min, which, when compared to the WHO recommendation, demonstrates the adsorbent's capabilities in real-world scenario. At the mentioned molar ratio, maximum adsorption of As(V) was observed as 40.8 mg/g at room temperature having BET surface area of 39.2 m2/g, adsorbent dosage of 50 mg, initial As(V) concentration of 100 ppm (sample volume of 30 ml), pH of 9, and contact time of 16 h. Effect of presence of different anions and cations on As removal indicated that PO43− significant effect on As(V) adsorption, and Cl−, Br−, and I− had negligible effect on As(V) adsorption at the same conditions mentioned above except initial As(V) concentration of 50 ppm.
Membrane filtration
A study was performed by Jiang et al. (2016) for the removal of As from groundwater using three different nanofiltration membranes. All the membranes were made from polyamide having a film thickness of 0.14 mm, 0.158 mm, 0.151 mm, respectively. The size of the membrane used in the experiment was 15, 55, and 100 mm2. The initial pH was maintained at 7.42. The initial concentration of arsenate in the sample was 30 µg/l. The flow rate maintained during the experiment was 1 l/min at a pressure of 10 bar. The arsenate concentration decreases to 10 µg/l using the NF-3A membrane after the treatment.
Four different membranes were used in the experiment carried out by Yu et al. (2013) to remove arsenate. In the laboratory, the sample was artificially developed. The effective membrane area was 139 cm2. The water permeability of membrane ESNA-1LF was 2.4 × 10−8 m3/s m2 kPa and the MgSO4 rejection capacity of membranes was 97%. The sample had 200 µg/l of arsenate initially. During the experiment, the pH and temperature were maintained at 7.5–8 and 21 °C, respectively. The flow rate was maintained at 2 l/min. This study found that membrane ESNA-1LF had the highest arsenate removal efficiency of 94%.
Recently, experiments by Boussouga et al. (2021) were performed in the laboratory to remove arsenate using nanofiltration. Two different membranes were used in the experiment namely semi-aromatic-based polyamide and fully aromatic polyamide. The pore radius and permeability of the membrane (NF-90) were 0.29 ± 0.02 nm and 8 ± 2 l/h/bar, respectively. The sample had an initial arsenate concentration of 250 µg/l. The temperature was set at 25 °C during the experiment. With pH of 2, the high rejection capacity of arsenate was observed at higher salinity. Additionally, MgSO4 rejection was > 97%. This study shows that filtration with NF-90 can remove arsenate to achieve the WHO standard of 10 µg/l at pH of 2.
In a study, samples were taken from various groundwater sources of Sila Massif (Italy) for the experiment of arsenite removal by nanofiltration and reverse osmosis (Figoli et al., 2020). The membrane used in the experiment was made from polyamide-TFC. The pH, flow rate and temperature were maintained at 6.7, 9 l/min and 16 °C. The duration of the experiment was 1 h. The flux/psi ratio of both membranes was 22/100 and 39/100, respectively. The efficiency of the membranes was tested by passing the sample at different pressures (3, 7, 11 and 15 bars) to the membrane. After testing, samples having 59–118 µg/l arsenic concentration have reduced to 10 µg/l at 15 bars following the filtration process.
In an integrated system, using nanofiltration coagulation has been developed by Pal et al. (2014). The water sample had 180 mg/l arsenite at pH level 5. Firstly, pre-oxidation using a KMnO4 oxidizing agent is used for the conversion of arsenite to arsenate. The coagulant agent used was ferric salt. The rate of coagulant added in treatment was 250 mg/l. The flux of the membrane was 144–145 l/m2/h. After pre-oxidation, the nanofiltration process was conducted by membrane NF-1 (Sepro membrane). After various tests, the result concluded that 4 mg/l of KMnO4 pre-oxidation agent is optimum to convert 98–99% of arsenite to arsenate from groundwater, and nanofiltration removed 93–95% of arsenic from groundwater.
Another study for As(III) removal was carried out by Ma et al. (2021), in which an ultrafiltration (UF) membrane consisting of hydrophilic nickel carbon nanotube layer as cathode was prepared. A 6-µm-thick CNT layer was deposited onto the UF membrane using a pressure deposition process. The concentration of As(III) in raw water was spiked to 750 µg/l, in both synthetic and real tap water. Membranes utilized in the investigation were compressed at 689 kPa for minimum of 12 h before operation. As a result, it can be concluded that while treating synthetic water at flux of 8.9 l/m2 h, initial pH of 6.57, 7 kPa pressure, and 1.29 l/m2 h/kPa of permeance, 93% of As(III) rejection was achieved. Consequently, while treating real tap water at flux of 8.9 and 9.1 l/m2 h, initial pH of 7.69 and 7.69, 6.89 and 28 kPa pressure, and 1.29 and 0.32 l/m2 h/kPa of permeance, 73% and 93% of As(III) rejection was obtained.
Ion exchange
Kinetic studies on arsenite and arsenate removal by Cl and OH-exchangeable form of anion exchange resins had been carried out by Rao et al. (2015). The sample used in the experiment was synthetically prepared in the laboratory. The initial concentration of arsenate in the sample was 500 µg/l. The pH and temperature of samples was set at 7.5–8 and 28 °C. About 94% of arsenate removal by the OH-exchangeable form of anion exchanger was noticed within the first 60 min of reaction, whereas the maximum removal was 97% after 270 min of reaction. The performance of the Cl− exchangeable form of anion was noticed to be inferior as only 81% removal took place after 60 min. Although arsenate removal was good, and arsenite removal was less than 21% by either of the resins, even after 270 min of reaction. By using an OH-exchangeable form of anion exchanger, the arsenate can be efficiently removed up to standards as per WHO guidelines for As concentration in drinking water.
An examination by Issa et al. (2011) was conducted for the removal of arsenic in Serbia. The samples were prepared in the laboratory using deionized water. In this experiment, both organic (monomethyl arsenic acid and dimethyl arsenic acid) and inorganic As (as arsenate and arsenite) were separated. The resin used in the experiment was (1) strong base anion exchange, Lewatit Monoplus M 500 having an average bead size of 0.61 mm, (2) hybrid-iron resin having an average bead size of 0.35 mm, and (3) hybrid-silver chloride resin prepared in the laboratory having average bead size of 0.56 mm. The diameter and length of the column in which As was removed were 2 cm and 20 cm, respectively. In a batch experiment with hybrid AgCl resin, at pH 9, around 50 µg arsenate and arsenite adsorption per gram of resin were observed when the initial total As concentration, resin dosage, temperature, and time were 500 µg/l, 1 gm, 20 °C, and 2 h, respectively. Authors observed that this resin has a low adsorption capacity for both arsenate (80 µg/gm) and arsenite (85 µg/gm) in a continuous flow system having a flow rate of 1.25 ml/min compared to other resins, but when they tested real water sample, they found that resin was stable and had higher As separation efficiency. Optimum conditions of flowrate and resin dosage were observed at 1.25–1.66 ml/min and 6 gm, respectively, in a continuous experiment. The authors concluded that hybrid AgCl resin was efficient enough to separate organic (passed through the resin) and inorganic As (retained by the resin).
A polymer-bridged nanoparticle fiber was developed by An et al. (2011) for the removal of arsenate from water by ion exchange. The mean particle size of starch-bridged nanoparticles was 26.6 ± 4.8 nm. The sample was prepared in the laboratory using deionized water. Magnetite and starch concentrations were 1.7 g/l and 0.049 wt%, respectively, to remove 280 mg/l of arsenate up to 100% at pH 6.5 in 1 h. It was found that starched nanoparticles remove arsenic 5 times more than bare magnetic particles. The researchers noted maximum arsenate adsorption at pH 4–6 using starched magnetite nanoparticles (in which optimum starch and Fe concentrations were taken). The maximum adsorption capacity of arsenate was 248 mg/g at pH 5 in the presence of 6% NaCl.
A hybrid anion exchange membrane was used in an experiment, executed by Oehmen et al. (2011), to remove arsenic from groundwater. The membranes used in experiments were four different anion exchange membranes, each having size of 11.3 cm2. The samples were prepared in the laboratory. In this experiment, the sample was added in flow-cell at the flow rate of 46 ml/h. Three hours of residence time was provided in the experiment. The pH of the sample was maintained at 7.8–8.0. The concentration of arsenic as total arsenic in a sample was reduced from 57 to 3 µg/l by using AR204-UZRA anion exchange membrane.
Summary of different As removal technologies with main attention on optimal conditions along with their removal efficiency is listed in Table 1.
The merits and demerits of the treatment approaches mentioned earlier have been condensed based on the thorough review of published article in Table 2.
While conventional methods are inefficient to remove As from groundwater, chemical coagulation with the popular coagulants like iron and aluminum salts is certainly efficient and cost-effective and is being utilized more than other methods. The chemical coagulation method has been observed as an effective arsenate removal compared to arsenite. EC has been regarded as an innovative and advanced remediation technique for As-polluted groundwater. As reported by Can et al. (2014), Kobya et al. (2015), and Song et al. (2016), removal efficiency of the EC process was above 99% for As within the maximum time of 30 min with the help of Fe electrodes and/or Fe-Al hybrid electrodes. The dissolved oxygen present in the water helps to transform metals into their hydroxides, due to which arsenite is oxidized to arsenate and hence, both are eliminated from the water with very high removal efficiency over a brief period.
Proposed ideas for mitigation
-
The primary goal and purpose of a mitigation plan is to identify the As-contaminated sources and vulnerable populations and to provide a sufficient amount of uncontaminated drinking water to them.
-
To address the issue of groundwater As toxicity, proper As monitoring of the contaminated region (i.e., state or country) is considered an important first measure that can be included in the action plan, with the development of a well-organized Geographic Information System (GIS) directory being suggested in particular to understand the geographical transportation of As (Nath, 2018).
-
Many study results showed that the amount of As concentration in shallow aquifers is higher than in deep aquifers. To avoid As contaminants, it is recommended that groundwater from deep aquifers should be prioritized for drinking purposes (Shankar et al., 2014).
-
In order to secure agricultural activities, the rising threat of As pollution of land and harvested yield needs to be investigated as soon as possible to make lifelong changes in cultivation and irrigation activities and to limit the utilization of groundwater (Nath, 2018).
-
Local government authorities should develop a color code to distinguish between As-contaminated and non-contaminated groundwater to let the local illiterate people know which sources contain safe drinking water and which does not. Various provisions should be made by local bodies to utilize different sources of As-contaminated groundwater for different purposes according to the degree of As contamination (Das et al., 2009).
-
Rainwater harvesting should be adopted by the endangered populations as a replacement of As-contaminated groundwater after proper chemical and disinfection treatment (Shankar et al., 2014).
-
Thorough maintenance and frequent evaluation of the treated water quality of the groundwater decontamination plants and/or local water treatment plants should be assessed regularly within the affected region by the regulatory authorities.
-
Well-organized and coherent examination of the people diagnosed with arsenicosis should be carried out by the respective medical authorities. It is their responsibility to let the affected people of the nearby region know about the indication of chronic As toxicity (Das et al., 2009).
-
It is suggested that the respective government should prompt a rule which entails the proper examination of the province and regulatory approval for a well to be established (Chakraborti et al., 2013).
-
No medicines which can treat chronic As poisoning have so far been discovered. Only unpolluted drinking water, healthy food, and physical activities (i.e., exercise) can be utilized to combat this epidemic (Chakraborti et al., 2013).
-
Conventional surface water is a prodigious source of As-free water. After treating the surface water, it can be distributed through underground pipes or transport vehicles to the As-affected rural regions. NGOs and other organizations should be appointed for transportation and other expenditure (Zaved Kaiser Khan, 2012).
-
Stakeholders have inadequate coordination with each other as they are the leading organization for the treatment and distribution of As-free water (Milton et al., 2012). Stakeholders include NGOs, institutes, donor agencies, government and social activists (Khan & Yang, 2014).
-
An appropriate household study should be undertaken to determine the existing water consumption patterns, as well as the alternatives favored by consumers who require an alternative treatment option. Tube wells were by far the most viable treatment option among the society since they were inexpensive to maintain and simple to operate (Hossain et al., 2015).
-
People should be aware of the future aspects and catastrophic effects of consuming As-rich water. The negative health impacts and economic impacts of exposure to high As containment should be exploited publicly (Milton et al., 2012).
-
Artificial water recharge is one of the typical mitigation programs for As dilution. As natural replenishment of As is a very slow process; artificial water recharge can be used to increase the pace of replenishment. It can also increase the efficiency of various treatment proposes like filtration, absorption, coagulation as the initial concentration of As will be decreased due to the replenishment.
-
Arsenic absorption through the skin is minimal; thus, bathing washing hands or working in the field with arsenic-containment water poses less risk to human health. This poses a high risk to arsenic-contaminated drinking water, and thus mitigation strategies should be more focused on reducing the consumption of arsenic-rich drinking water. Paddy and rice are the staple food in India. Arsenic contamination in these crops is usually typical in India and Bangladesh delta regions. Arsenic accumulation in rice and paddy can be mitigated through agronomics and crop hybrid strategies. Engineered plants with genetic modification can tolerate more As and reduce arsenic uptake for improved food safety (Zhao et al., 2010). In situ treatment process is a well-known mitigation method used in Europe where an aquifer is exposed to atmospheric O2. This helps metal in the aquifer oxidize and reduces toxic contamination (Kumar, 2015). Rain-Water Harvesting is one of the most common mitigation methods from avoiding drinking arsenic-contaminated water. Biovolatilization of arsenic by efficient bacteria in a culture medium can be used to achieve arsenic-free water (Majumder et al., 2013). Long-term mitigation measures for future generations are to make them aware by giving them the proper education and training. Well Switching is successfully implemented in villages in Bangladesh, where wells are labeled “safe” and “unsafe” based on their arsenic contamination (Jameel et al., 2021).
-
Besides different mitigation processes, treatment processes are more reliable for the arsenic removal rate as the cost–benefit analysis report shows more benefits toward the treatment process than the mitigation process. Mitigation cost exceeds treatment cost, and the removal rate also shows significantly more in treatment.
The mitigation measures can further be classified as long-term and short-term which are discussed in Table 3. Any one of them can be applied on the field based on the purpose and required timespan.
Challenges and future scope
The biggest challenge in the field of As decontamination is to ensure an effective solution that produces less amount of slurry and has the lowest running expenses. In comparison with other treatment methods, the EC is the most efficient As eliminating technique which produces an extremely low amount of slurry. One of the limitations of EC is the passivation/fouling of the electrode surface which dramatically reduces ionic movement between the anode and the cathode that may lead to the reduced removal efficiency after a certain operational period. Passivation negatively affects mass transfer during the process. Surface chemistry, of course, has a critical involvement in the overall operation. van Genuchten et al. (2016) published a paper that included an outstanding clarification of this phenomenon throughout As elimination by EC in which they compared electrodes utilized in lab and field experiments to analyze the surface films developed during As elimination process. To overcome such a situation, there are different approaches through which we can minimize the passivation of the electrodes (Barek, 2021; Hanssen et al., 2016). One of the simplest steps to conquer this problem is to change the electrodes periodically and/or schedule periodic post-treatment of the electrodes to remove the deposition whenever needed. These measures increase the operating and maintenance cost, but, on the other hand, it helps to achieve higher consistent removal efficiency. According to the experiments conducted by Eyvaz et al. (2014), applying the alternating pulse current method not only helps to prevent electrode passivation but also helps in Chemical Oxygen Demand (COD), turbidity, color removal. Moreover, it also helps to reduce operating costs in comparison with the DC mode. At the same time, the amount of sludge formation also increases. Although the passivation film generated on the electrode surface during operation can’t be regulated, it can be reduced to certain magnitude by switching polarity after every experiment (Syam Babu et al., 2021). Surface modifications of an electrode and modern electrode materials can be used that are less susceptible to passivation. Rotating disk electrodes have the potential capability to enhance the mass transfer process by washing out the passivation-inducing products from the surface of the electrodes which helps to reduce their accumulation on the electrode surface (Chen et al., 2020; Zanotto et al., 2019). Additionally, EC operation demands the utilization of energy that raises running costs. To optimize electricity costs, the energy produced from clean and sustainable renewable resources (i.e., solar energy) should be investigated. When looking at the EC process, residual sludge handling and disposal is also a significant factor to address. A study was carried out by Syam Babu et al. (2021) to reuse the As-containing As-Fe sludge as a binding agent in concrete with the help of solidification and stabilization processes resulted into small change in concrete’s compressive strength. In the same study, leachability studies of As-containing sludge and concrete samples were investigated, and negligible arsenic leaching was found. Low-cost emerging technologies have been used as a promising solution for As removal like laterite-based As removal, use of red mud as an adsorbent, or use of nanomembrane as a filtration technique. Although arsenic is considered a toxic element in many of the studied papers, As and its compounds are used in medicine for the treatment of skin ulcers, psoriasis, diabetes, joint diseases, syphilis (also known as sexually transmitted infection—cured by drug called Arsphenamine or Salvarsan), etc. as well as long-term promyelocytic leukemia, a type of white blood cell cancer (Kulik-Kupka et al., 2016; Santacroce et al., 2021). Dissolved As is a type that is left in the treatment process after the removal of As from water. This dissolved As can be used to obtain arsenous acid that helps to get sodium arsenate, sodium arsenite, copper pyroarsenite, calcium arsenite, and calcium arsenate (i.e., different salts of arsenous acid) which are further used as pesticides and herbicides in agriculture (Bencko & Foong, 2017). Treatment of As-contaminated water is one of the most expensive mitigation strategy. In semi-arid regions, long-term As remediation options like rainwater harvesting and deep aquifers are not reliable. Thus, government established Arsenic Removal Unit (ARU) according to the population and requirements of that regions is preferred (Bhardwaj et al., 2018).
Conclusion
The main source of As poisoning for the individuals is its presence in drinking water and/or food tests substantially beyond the allowed levels. According to the reports, the prolonged exposure to As-contaminated drinking water has high chances of developing chronic diseases including cancer of the lung, skin, kidney, bladder, and liver, arsenicosis, tuberculosis, impaired intellectual functions, diabetes, and cardiovascular diseases including high blood pressure. The arsenic toxicity in the crops highly depends upon the position of the crops grown either above or below the surface. The typical treatment process of As-contaminated water involves two stages in which oxidation stage is considered as a pre-treatment step. Because of the feasible energy consumption, economical operating cost, limited sludge production, and high removal efficiency, EC is the best available technology to remove As from water. To mitigate this global issue, we have put forward some plans of action to be followed. Some of the low-cost efforts are to be put into mitigation of As involving rainwater harvesting, artificial groundwater recharge, finding As-free water from deep aquifers, etc. Due to less rainfall in dry regions, switching to deep aquifers may solve the As-contaminated water issue as rainwater harvesting won't mitigate the problem. Furthermore, following the post-treatment operation, individuals can consume shallow depth water.
Availability of data and material
All relevant data and material are presented in the main paper.
Abbreviations
- AOPs:
-
Advanced oxidation process
- As:
-
Arsenic
- BET:
-
Brunauer–Emmett–Teller
- COD:
-
Chemical oxygen demand
- DC:
-
Direct current
- EC:
-
Electrocoagulations
- GIS:
-
Geographic information system
- IS:
-
Indian standard
- IARC:
-
International agency of research on cancer
- PRB:
-
Permeable reactive barrier
- PDS:
-
Peroxy-disulphate
- PMS:
-
Peroxy-monosulfate
- UV:
-
Ultraviolet
- WHO:
-
World Health Organization
- ZVI:
-
Zero valent iron
References
Abedin, M. J., Cotter-Howells, J., & Meharg, A. A. (2002). Arsenic uptake and accumulation in rice (Oryza sativa L.) irrigated with contaminated water. Plant and Soil, 240(2), 311–319.
An, B., Liang, Q., & Zhao, D. (2011). Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Research, 45(5), 1961–1972. https://doi.org/10.1016/j.watres.2011.01.004
Arain, M. B., Kazi, T. G., Baig, J. A., Jamali, M. K., Afridi, H. I., Shah, A. Q., Jalbani, N., & Sarfraz, R. A. (2009). Determination of arsenic levels in lake water, sediment, and foodstuff from selected area of Sindh, Pakistan: Estimation of daily dietary intake. Food and Chemical Toxicology, 47(1), 242–248. https://doi.org/10.1016/j.fct.2008.11.009
Arsenic removal through coagulation and flocculation from contaminated water in Macedonia. (n.d.).
Aryal, S. (2013). Rainfall and water requirement of rice during growing period. Journal of Agriculture and Environment, 13, 1–4. https://doi.org/10.3126/aej.v13i0.7576
Azizullah, A., Khattak, M. N. K., Richter, P., & Häder, D. P. (2011). Water pollution in Pakistan and its impact on public health—A review. Environment International, 37(2), 479–497. https://doi.org/10.1016/j.envint.2010.10.007
Bang, S., Korfiatis, G. P., & Meng, X. (2005). Removal of arsenic from water by zero-valent iron. Journal of Hazardous Materials, 121(1–3), 61–67. https://doi.org/10.1016/j.jhazmat.2005.01.030
Barek, J. (2021). How to improve the performance of electrochemical sensors via minimization of electrode passivation. Chemosensors, 9(1), 1–15. https://doi.org/10.3390/chemosensors9010012
Bencko, V., & Foong, F. Y. L. (2017). The history of arsenical pesticides and health risks related to the use of Agent Blue. Annals of Agricultural and Environmental Medicine, 24(2), 312–316. https://doi.org/10.26444/aaem/74715
Bhardwaj, A., Rajput, R., & Misra, K. (2018). Status of arsenic remediation in India. In S. Ahuja (Ed.), Advances in water purification techniques: Meeting the needs of developed and developing countries (pp. 219–258). Elsevier. https://doi.org/10.1016/B978-0-12-814790-0.00009-0
Bhattacharya, P., Mukherjee, A., & Mukherjee, A. B. (2015). Groundwater arsenic in India: Source, distribution, effects and alternate safe drinking water sources☆. In J.O. Nriagu (Ed.), Reference module in earth systems and environmental sciences. Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.09342-8
Bhattacharya, P., Samal, A. C., Majumdar, J., & Santra, S. C. (2010). Arsenic contamination in rice, wheat, pulses, and vegetables: A study in an arsenic affected area of West Bengal, India. Water, Air, and Soil Pollution, 213(1–4), 3–13. https://doi.org/10.1007/s11270-010-0361-9
Bhowmick, S., Pramanik, S., Singh, P., Mondal, P., Chatterjee, D., & Nriagu, J. (2018). Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Science of the Total Environment, 612, 148–169. https://doi.org/10.1016/j.scitotenv.2017.08.216
Bina, B., Ebrahimi, A., Hesami, F., & Amin, M. (2013). Arsenic removal by coagulation using ferric chloride and chitosan from water. International Journal of Environmental Health Engineering, 2(1), 17. https://doi.org/10.4103/2277-9183.110170
Bissen, M., & Frimmel, F. (2003). Arsenic—A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochimica Et Hydrobiologica, 31, 9–18.
Bora, A. J., Gogoi, S., Baruah, G., & Dutta, R. K. (2016). Utilization of co-existing iron in arsenic removal from groundwater by oxidation–coagulation at optimized pH. Journal of Environmental Chemical Engineering, 4(3), 2683–2691. https://doi.org/10.1016/j.jece.2016.05.012
Boussouga, Y. A., Frey, H., & Schäfer, A. I. (2021). Removal of arsenic(V) by nanofiltration: Impact of water salinity, pH and organic matter. Journal of Membrane Science, 618, 118631. https://doi.org/10.1016/j.memsci.2020.118631
Can, B. Z., Boncukcuoglu, R., Yilmaz, A. E., & Fil, B. A. (2014). Effect of some operational parameters on the Arsenic removal by electrocoagulation using iron electrodes. Journal of Environmental Health Science and Engineering, 12(1), 1–10. https://doi.org/10.1186/2052-336X-12-95
Chakrabarti, D., Singh, S. K., Rashid, M. H., & Rahman, M. M. (2019). Arsenic: Occurrence in groundwater. In J. Nriagu (Ed.), Encyclopedia of environmental health (2nd ed.). Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.10634-7
Chakraborti, D., Rahman, M. M., Das, B., Nayak, B., Pal, A., Sengupta, M. K., Hossain, M. A., Ahamed, S., Sahu, M., Saha, K. C., Mukherjee, S. C., Pati, S., Dutta, R. N., & Quamruzzaman, Q. (2013). Groundwater arsenic contamination in Ganga–Meghna–Brahmaputra plain, its health effects and an approach for mitigation. Environmental Earth Sciences, 70(5), 1993–2008. https://doi.org/10.1007/s12665-013-2699-y
Chatterjee, S., Chetia, M., Voronina, A., & Gupta, D. K. (2017). Prospects of combating arsenic: Physico-chemical Aspects. In D. K. Gupta & S. Chatterjee (Eds.), Arsenic contamination in the environment (pp. 103–121). Springer. https://doi.org/10.1007/978-3-319-54356-7_5
Chen, C. L., Hsu, L. I., Chiou, H. Y., Hsueh, Y. M., Chen, S. Y., Wu, M. M., & Chen, C. J. (2004). Ingested arsenic, cigarette smoking, and lung cancer risk: A follow-up study in arseniasis-endemic areas in Taiwan. Journal of the American Medical Association, 292(24), 2984–2990. https://doi.org/10.1001/jama.292.24.2984
Chen, W., Xu, M. L., Li, M. F., Wei, Z., Cai, J., & Chen, Y. X. (2020). Quantifying intrinsic kinetics of electrochemical reaction controlled by mass transfer of multiple species under rotating disk electrode configuration. Journal of Electroanalytical Chemistry, 872, 114042. https://doi.org/10.1016/j.jelechem.2020.114042
Chiu, H. F., Ho, S. C., & Yang, C. Y. (2004). Lung cancer mortality reduction after installation of tap-water supply system in an arseniasis-endemic area in Southwestern Taiwan. Lung Cancer, 46(3), 265–270. https://doi.org/10.1016/j.lungcan.2004.05.012
Choong, T. S. Y., Chuah, T. G., Robiah, Y., Gregory Koay, F. L., & Azni, I. (2007). Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination, 217(1–3), 139–166. https://doi.org/10.1016/j.desal.2007.01.015
Concetta Tomei, M., & Daugulis, A. J. (2013). Ex situ bioremediation of contaminated soils: An overview of conventional and innovative technologies. Critical Reviews in Environmental Science and Technology, 43(20), 2107–2139. https://doi.org/10.1080/10643389.2012.672056
Das, B., Rahman, M. M., Nayak, B., Pal, A., Chowdhury, U. K., Mukherjee, S. C., Saha, K. C., Pati, S., Quamruzzaman, Q., & Chakraborti, D. (2009). Groundwater arsenic contamination, its health effects and approach for mitigation in West Bengal, India and Bangladesh. Water Quality, Exposure and Health, 1(1), 5–21. https://doi.org/10.1007/s12403-008-0002-3
De Jesus, A., Zmozinski, A. V., Damin, I. C. F., Silva, M. M., & Vale, M. G. R. (2012). Determination of arsenic and cadmium in crude oil by direct sampling graphite furnace atomic absorption spectrometry. Spectrochimica Acta: Part B Atomic Spectroscopy, 71–72, 86–91. https://doi.org/10.1016/j.sab.2012.03.010
Demirbas, E., Kobya, M., Oncel, M. S., Şık, E., & Goren, A. Y. (2019). Arsenite removal from groundwater in a batch electrocoagulation process: Optimization through response surface methodology. Separation Science and Technology (philadelphia), 54(5), 775–785. https://doi.org/10.1080/01496395.2018.1521834
Eyvaz, M., Gürbulak, E., Kara, S., & Yüksel, E. (2014). Preventing of cathode passivation/deposition in electrochemical treatment methods—A case study on winery wastewater with electrocoagulation. Modern Electrochemical Methods in Nano, Surface and Corrosion Science. https://doi.org/10.5772/58580
Farmer, J. G., & Johnson, L. R. (1990). Assessment of occupational exposure to inorganic arsenic based on urinary concentrations and speciation of arsenic. British Journal of Industrial Medicine, 47(5), 342–348. https://doi.org/10.1136/oem.47.5.342
Fazal, M. A., Kawachi, T., & Ichion, E. (2001). Extent and severity of groundwater arsenic contamination in Bangladesh. Water International, 26(3), 370–379. https://doi.org/10.1080/02508060108686929
Ferreccio, C., González, C., Milosavjlevic, V., Marshall, G., Sancha, A. M., & Smith, A. H. (2000). Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology, 11(6), 673–679. https://doi.org/10.1097/00001648-200011000-00010
Figoli, A., Fuoco, I., Apollaro, C., Chabane, M., Mancuso, R., Gabriele, B., De Rosa, R., Vespasiano, G., Barca, D., & Criscuoli, A. (2020). Arsenic-contaminated groundwaters remediation by nanofiltration. Separation and Purification Technology. https://doi.org/10.1016/j.seppur.2019.116461
Garcia-Costa, A. L., Sarabia, A., Zazo, J. A., & Casas, J. A. (2021). UV-assisted catalytic wet peroxide oxidation and adsorption as efficient process for arsenic removal in groundwater. Catalysis Today, 361(March), 176–182. https://doi.org/10.1016/j.cattod.2020.03.054
Ghosh, S., Debsarkar, A., & Dutta, A. (2019). Technology alternatives for decontamination of arsenic-rich groundwater—A critical review. Environmental Technology and Innovation, 13, 277–303. https://doi.org/10.1016/j.eti.2018.12.003
Gong, Y., Qu, Y., Yang, S., Tao, S., Shi, T., Liu, Q., Chen, Y., Wu, Y., & Ma, J. (2020). Status of arsenic accumulation in agricultural soils across China (1985–2016). Environmental Research, 186, 109525. https://doi.org/10.1016/j.envres.2020.109525
Gonzalez, B., Heijman, S. G. J., Rietveld, L. C., & van Halem, D. (2019). Arsenic removal from geothermal influenced groundwater with low pressure NF pilot plant for drinking water production in Nicaraguan rural communities. Science of the Total Environment, 667, 297–305. https://doi.org/10.1016/j.scitotenv.2019.02.222
Gu, Z., de Silva, S., & Reichman, S. M. (2020). Arsenic concentrations and dietary exposure in rice-based infant food in Australia. International Journal of Environmental Research and Public Health, 17(2), 415. https://doi.org/10.3390/ijerph17020415
Gude, J. C. J., Rietveld, L. C., & van Halem, D. (2018). Biological As(III) oxidation in rapid sand filters. Journal of Water Process Engineering, 21, 107–115. https://doi.org/10.1016/j.jwpe.2017.12.003
Guha Mazumder, D., & Dasgupta, U. B. (2011). Chronic arsenic toxicity: Studies in West Bengal, India. Kaohsiung Journal of Medical Sciences, 27(9), 360–370. https://doi.org/10.1016/j.kjms.2011.05.003
Gupta, A., Yunus, M., & Sankararamakrishnan, N. (2012). Zerovalent iron encapsulated chitosan nanospheres—A novel adsorbent for the removal of total inorganic arsenic from aqueous systems. Chemosphere, 86(2), 150–155. https://doi.org/10.1016/j.chemosphere.2011.10.003
Hanssen, B. L., Siraj, S., & Wong, D. K. Y. (2016). Recent strategies to minimise fouling in electrochemical detection systems. Reviews in Analytical Chemistry, 35(1), 1–28. https://doi.org/10.1515/revac-2015-0008
Hao, L., Liu, M., Wang, N., & Li, G. (2018). A critical review on arsenic removal from water using iron-based adsorbents. RSC Advances, 8(69), 39545–39560. https://doi.org/10.1039/c8ra08512a
Haque, I. U., Nabi, D., Baig, M. A., & Hayat, W. (2008). Groundwater arsenic contamination—A multi-directional emerging threat to water scarce areas of Pakistan. IAHS-AISH Publication, 324, 24–30.
Henke, K. R. (2009). Arsenic: Environmental chemistry, health threats and waste treatment. Wiley. https://doi.org/10.1002/9780470741122
Hong, Y. S., Song, K. H., & Chung, J. Y. (2014). Health effects of chronic arsenic exposure. Journal of Preventive Medicine and Public Health, 47(5), 245–252. https://doi.org/10.3961/jpmph.14.035
Hossain, M., Rahman, S. N., Bhattacharya, P., Jacks, G., Saha, R., & Rahman, M. (2015). Sustainability of arsenic mitigation interventions—An evaluation of different alternative safe drinking water options provided in Matlab, an arsenic hot spot in Bangladesh. Frontiers in Environmental Science, 3(MAY), 30. https://doi.org/10.3389/fenvs.2015.00030
Huang, Y., Liu, Z., Bo, A., Tang, X., Martens, W., Kou, L., Gu, Y., Carja, G., Zhu, H., & Sarina, S. (2022). High efficient arsenic removal by In-layer sulphur of layered double hydroxide. Journal of Colloid and Interface Science, 608, 2358–2366. https://doi.org/10.1016/j.jcis.2021.10.148
Issa, N. B., Rajaković-Ognjanović, V. N., Marinković, A. D., & Rajaković, L. V. (2011). Separation and determination of arsenic species in water by selective exchange and hybrid resins. Analytica Chimica Acta, 706(1), 191–198. https://doi.org/10.1016/j.aca.2011.08.015
Jalil, M. A., & Ahmed, F. (2001). Development of an activated alumina based household arsenic removal unit. Bangladesh University of Engineering & Technology (BUET).
Jameel, Y., Mozumder, M. R. H., Geen, A., & Harvey, C. F. (2021). Well-switching to reduce arsenic exposure in Bangladesh: Making the most of inaccurate field kit measurements. GeoHealth. https://doi.org/10.1029/2021GH000464
Jha, S. K., Mishra, V. K., Damodaran, T., Sharma, D. K., & Kumar, P. (2017). Arsenic in the groundwater: Occurrence, toxicological activities, and remedies. Journal of Environmental Science and Health Part C Environmental Carcinogenesis and Ecotoxicology Reviews, 35(2), 84–103. https://doi.org/10.1080/10590501.2017.1298359
Jiang, W., Gao, X., Xu, L., & Wang, J. (2016). Investigation of synchronous arsenic and salinity rejection via nanofiltration system and membrane cleaning. Desalination and Water Treatment, 57(14), 6554–6565. https://doi.org/10.1080/19443994.2015.1008577
Kar, S., Maity, J. P., Jean, J. S., Liu, C. C., Liu, C. W., Bundschuh, J., & Lu, H. Y. (2011). Health risks for human intake of aquacultural fish: Arsenic bioaccumulation and contamination. Journal of Environmental Science and Health Part A Toxic/hazardous Substances and Environmental Engineering, 46(11), 1266–1273. https://doi.org/10.1080/10934529.2011.598814
Karakurt, S., Pehlivan, E., & Karakurt, S. (2019). Removal of carcinogenic arsenic from drinking water by the application of ion exchange resins. Oncogen, 2(1), 1–8. https://doi.org/10.35702/onc.10005
Kazi, T. G., Arain, M. B., Baig, J. A., Jamali, M. K., Afridi, H. I., Jalbani, N., Sarfraz, R. A., Shah, A. Q., & Niaz, A. (2009). The correlation of arsenic levels in drinking water with the biological samples of skin disorders. Science of the Total Environment, 407(3), 1019–1026. https://doi.org/10.1016/j.scitotenv.2008.10.013
Khan, M., Chanda, B., & Ahmad, S. (2016). Diabetes mellitus among arsenic exposed and non-exposed young adults in Bangladesh. International Journal of Community Medicine and Public Health, 3(11), 3170–3178. https://doi.org/10.18203/2394-6040.ijcmph20163931
Khan, M. M. T., Yamamoto, K., & Ahmed, M. F. (2002). A low cost technique of arsenic removal from drinking water by coagulation using ferric chloride salt and alum. Water Science and Technology: Water Supply, 2(2), 281–288. https://doi.org/10.2166/ws.2002.0074
Khan, N. I., & Yang, H. (2014). Arsenic mitigation in Bangladesh: An analysis of institutional stakeholders’ opinions. Science of the Total Environment, 488–489(1), 493–504. https://doi.org/10.1016/j.scitotenv.2013.11.007
Kobya, M., Ozyonar, F., Demirbas, E., Sik, E., & Oncel, M. S. (2015). Arsenic removal from groundwater of Sivas-Şarkişla Plain, Turkey by electrocoagulation process: Comparing with iron plate and ball electrodes. Journal of Environmental Chemical Engineering, 3(2), 1096–1106. https://doi.org/10.1016/j.jece.2015.04.014
Kuivenhoven, M., & Mason, K. (2019). Arsenic (arsine) toxicity. StatPearls Publishing.
Kulik-Kupka, K., Koszowska, A., Brończyk-Puzoń, A., Nowak, J., Gwizdek, K., & Zubelewicz-Szkodzińska, B. (2016). Arsen—Trucizna czy lek? Medycyna Pracy, 67(1), 89–96. https://doi.org/10.13075/mp.5893.00322
Kumar, C. P. (2015). Status and mitigation of arsenic contamination in groundwater in India. The International Journal of Earth & Environmental Sciences, 1(1), 1–10.
Kumar, M., Rahman, M. M., Ramanathan, A. L., & Naidu, R. (2016). Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Science of the Total Environment, 539, 125–134. https://doi.org/10.1016/j.scitotenv.2015.08.039
Kundu, R., Bhattacharyya, K., Majumder, A., & Pal, S. (2013). Response of wheat cultivars to arsenic contamination in polluted soils of West Bengal, India. Cereal Research Communications, 41(1), 66–77. https://doi.org/10.1556/CRC.2012.0027
Lee, C. G., Alvarez, P. J. J., Nam, A., Park, S. J., Do, T., Choi, U. S., & Lee, S. H. (2017). Arsenic(V) removal using an amine-doped acrylic ion exchange fiber: Kinetic, equilibrium, and regeneration studies. Journal of Hazardous Materials, 325(V), 223–229. https://doi.org/10.1016/j.jhazmat.2016.12.003
Lee, K. J., Lee, Y., Yoon, J., Kamala-Kannan, S., Park, S. M., & Oh, B. T. (2009). Assessment of zero-valent iron as a permeable reactive barrier for long-term removal of arsenic compounds from synthetic water. Environmental Technology, 30(13), 1425–1434. https://doi.org/10.1080/09593330903186240
Li, J., Wu, Y. N., Li, Z., Zhu, M., & Li, F. (2014). Characteristics of arsenate removal from water by metal–organic frameworks (MOFs). Water Science and Technology, 70(8), 1391–1397. https://doi.org/10.2166/wst.2014.390
Li, Y., Wang, J., Luan, Z., & Liang, Z. (2010). Arsenic removal from aqueous solution using ferrous based red mud sludge. Journal of Hazardous Materials, 177(1–3), 131–137. https://doi.org/10.1016/j.jhazmat.2009.12.006
Luo, X., Liu, H., Huang, G., Li, Y., Zhao, Y., & Li, X. (2016). Remediation of arsenic-contaminated groundwater using media-injected permeable reactive barriers with a modified montmorillonite: Sand tank studies. Environmental Science and Pollution Research, 23(1), 870–877. https://doi.org/10.1007/s11356-015-5254-4
Luo, X., Wang, C., Wang, L., Deng, F., Luo, S., Tu, X., & Au, C. (2013). Nanocomposites of graphene oxide-hydrated zirconium oxide for simultaneous removal of As(III) and As(V) from water. Chemical Engineering Journal, 220, 98–106. https://doi.org/10.1016/j.cej.2013.01.017
Ma, S., Yang, F., Chen, X., Khor, C. M., Jung, B., Iddya, A., Sant, G., & Jassby, D. (2021). Removal of As(III) by electrically conducting ultrafiltration membranes. Water Research, 204(February), 117592. https://doi.org/10.1016/j.watres.2021.117592
Majumder, A., Bhattacharyya, K., Kole, S. C., & Ghosh, S. (2013). Efficacy of indigenous soil microbes in arsenic mitigation from contaminated alluvial soil of India. Environmental Science and Pollution Research, 20(8), 5645–5653. https://doi.org/10.1007/s11356-013-1560-x
Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: A review. Talanta, 58(1), 201–235. https://doi.org/10.1016/S0039-9140(02)00268-0
Martinez, V. D., Vucic, E. A., Becker-Santos, D. D., Gil, L., & Lam, W. L. (2011). Arsenic exposure and the induction of human cancers. Journal of Toxicology. https://doi.org/10.1155/2011/431287
Mazumder, D. G., Purkayastha, I., Ghose, A., Mistry, G., Saha, C., Nandy, A. K., Das, A., & Majumdar, K. K. (2012). Hypertension in chronic arsenic exposure: A case control study in West Bengal. Journal of Environmental Science and Health Part A Toxic/hazardous Substances and Environmental Engineering, 47(11), 1514–1520. https://doi.org/10.1080/10934529.2012.680329
Menon, M., Dong, W., Chen, X., Hufton, J., & Rhodes, E. J. (2021). Improved rice cooking approach to maximise arsenic removal while preserving nutrient elements. Science of the Total Environment, 755, 143341. https://doi.org/10.1016/j.scitotenv.2020.143341
Milton, A. H., Hore, S. K., Hossain, M. Z., & Rahman, M. (2012). Bangladesh arsenic mitigation programs: Lessons from the past. Emerging Health Threats Journal, 5(1), 7269. https://doi.org/10.3402/ehtj.v5i0.7269
Mitra, A., Chatterjee, S., & Gupta, D. K. (2017). Uptake, transport, and remediation of arsenic by algae and higher plants. In D. K. Gupta & S. Chatterjee (Eds.), Arsenic contamination in the environment (pp. 145–169). Springer. https://doi.org/10.1007/978-3-319-54356-7_7
Molinari, R., & Argurio, P. (2017). Arsenic removal from water by coupling photocatalysis and complexation–ultrafiltration processes: A preliminary study. Water Research, 109, 327–336. https://doi.org/10.1016/j.watres.2016.11.054
Muthayya, S., Sugimoto, J., Montgomery, S., & Maberly, G. (2014). An overview of global rice production, supply, trade, and consumption. Annals of the New York Academy of Sciences. https://doi.org/10.1111/nyas.12540
Nath, K. J. (2018). Arsenic and fluoride contamination in groundwater: Mitigation strategies. Ground Water Development Issues and Sustainable Solutions. https://doi.org/10.1007/978-981-13-1771-2_15
Navas-Acien, A., Sharrett, A. R., Silbergeld, E. K., Schwartz, B. S., Nachman, K. E., Burke, T. A., & Guallar, E. (2005). Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. American Journal of Epidemiology, 162(11), 1037–1049. https://doi.org/10.1093/aje/kwi330
Nguyen, X. S., Pham, T. D., Tran, T. T. T., & Tung Vo, H. (2020). Photo-oxidation efficient As (III) under visible light using microporous Zn doped-Fe3O4 sphere. Materials Technology, 35(6), 335–348. https://doi.org/10.1080/10667857.2019.1682857
Nicomel, N. R., Leus, K., Folens, K., Van Der Voort, P., & Du Laing, G. (2015). Technologies for arsenic removal from water: Current status and future perspectives. International Journal of Environmental Research and Public Health, 13(1), 1–24. https://doi.org/10.3390/ijerph13010062
Nidheesh, P. V., & Singh, T. S. A. (2017). Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere, 181, 418–432. https://doi.org/10.1016/j.chemosphere.2017.04.082
Obiri-Nyarko, F., Grajales-Mesa, S. J., & Malina, G. (2014). An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere, 111, 243–259. https://doi.org/10.1016/j.chemosphere.2014.03.112
Oehmen, A., Valerio, R., Llanos, J., Fradinho, J., Serra, S., Reis, M. A. M., Crespo, J. G., & Velizarov, S. (2011). Arsenic removal from drinking water through a hybrid ion exchange membrane—Coagulation process. Separation and Purification Technology, 83, 137–143. https://doi.org/10.1016/j.seppur.2011.09.027
O’Farrell, C., Mahon, J. M., & Gill, L. W. (2016). Development of a continuous flow solar oxidation process for the removal of arsenic for sustainable rural water supply. Journal of Environmental Chemical Engineering, 4(1), 1181–1190. https://doi.org/10.1016/j.jece.2016.01.027
Omwene, P. I., Çelen, M., Öncel, M. S., & Kobya, M. (2019). Arsenic removal from naturally arsenic contaminated ground water by packed-bed electrocoagulator using Al and Fe scrap anodes. Process Safety and Environmental Protection, 121, 20–31. https://doi.org/10.1016/j.psep.2018.10.003
Oza, H., Anantha Singh, T. S., & Sasikumar Jampa, S. (2020). Removal of arsenic from aqueous solution using combined ultrasonic and electrocoagulation process. Materials Today: Proceedings, 47, 728–732. https://doi.org/10.1016/j.matpr.2021.01.569
Pal, P., Chakrabortty, S., & Linnanen, L. (2014). A nanofiltration–coagulation integrated system for separation and stabilization of arsenic from groundwater. Science of the Total Environment, 476–477, 601–610. https://doi.org/10.1016/j.scitotenv.2014.01.041
Pal, P., Sen, M., Manna, A., Pal, J., Pal, P., Roy, S., & Roy, P. (2009). Contamination of groundwater by arsenic: A review of occurrence, causes, impacts, remedies and membrane-based purification. Journal of Integrative Environmental Sciences, 6(4), 295–316. https://doi.org/10.1080/19438150903185077
Pandey, V. C., Singh, J. S., Singh, R. P., Singh, N., & Yunus, M. (2011). Arsenic hazards in coal fly ash and its fate in Indian scenario. Resources, Conservation and Recycling, 55(9–10), 819–835. https://doi.org/10.1016/j.resconrec.2011.04.005
Park, J. B., Lee, S. H., Lee, J. W., & Lee, C. Y. (2002). Lab scale experiments for permeable reactive barriers against contaminated groundwater with ammonium and heavy metals using clinoptilolite (01–29B). Journal of Hazardous Materials, 95(1–2), 65–79. https://doi.org/10.1016/S0304-3894(02)00007-9
Pous, N., Casentini, B., Rossetti, S., Fazi, S., Puig, S., & Aulenta, F. (2015). Anaerobic arsenite oxidation with an electrode serving as the sole electron acceptor: A novel approach to the bioremediation of arsenic-polluted groundwater. Journal of Hazardous Materials, 283, 617–622. https://doi.org/10.1016/j.jhazmat.2014.10.014
Quansah, R., Armah, F. A., Essumang, D. K., Luginaah, I., Clarke, E., Marfoh, K., Cobbina, S. J., Edward, N.-A., Namujju, P. B., Obiri, S., & Dzodzomenyo, M. (2015). Association of arsenic with adverse pregnancy outcomes/infant mortality. Enviromental Health Perspectives, 123(5), 412–422.
Rahman, M. M., Mondal, D., Das, B., Sengupta, M. K., Ahamed, S., Hossain, M. A., Samal, A. C., Saha, K. C., Mukherjee, S. C., Dutta, R. N., & Chakraborti, D. (2014). Status of groundwater arsenic contamination in all 17 blocks of Nadia district in the state of West Bengal, India: A 23-year study report. Journal of Hydrology, 518(PC), 363–372. https://doi.org/10.1016/j.jhydrol.2013.10.037
Rai, P. K., Lee, S. S., Zhang, M., Tsang, Y. F., & Kim, K. H. (2019). Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environment International, 125, 365–385. https://doi.org/10.1016/j.envint.2019.01.067
Rao, P. R., Ngullie, N., Golder, A. K., & Ghosh, P. K. (2015). Arsenic removal from contaminated water by various physicochemical processes. International Journal of Environmental Science and Development, 6(4), 357–362. https://doi.org/10.7763/ijesd.2015.v6.618
Rasheed, H., Slack, R., & Kay, P. (2016). Human health risk assessment for arsenic: A critical review. Critical Reviews in Environmental Science and Technology, 46(19–20), 1529–1583. https://doi.org/10.1080/10643389.2016.1245551
Raza, M., Hussain, F., Lee, J. Y., Shakoor, M. B., & Kwon, K. D. (2017). Groundwater status in Pakistan: A review of contamination, health risks, and potential needs. Critical Reviews in Environmental Science and Technology, 47(18), 1713–1762. https://doi.org/10.1080/10643389.2017.1400852
Rezaei, F., & Vione, D. (2018). Effect of pH on zero valent iron performance in heterogeneous Fenton and Fenton-like processes: A review. Molecules, 23(12), 3127. https://doi.org/10.3390/molecules23123127
Robertson, W. D., Vogan, J. L., & Lombardo, P. S. (2008). Nitrate removal rates in a 15-year-old permeable reactive barrier treating septic system nitrate. Ground Water Monitoring and Remediation, 28(3), 65–72. https://doi.org/10.1111/j.1745-6592.2008.00205.x
Rossman, T. G., Uddin, A. N., & Burns, F. J. (2004). Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicology and Applied Pharmacology, 198(3), 394–404. https://doi.org/10.1016/j.taap.2003.10.016
Saeed, M., Masood Quraishi, U., & Naseem Malik, R. (2021). Arsenic uptake and toxicity in wheat (Triticum aestivum L.): An review of multi-omics approaches to identify tolerance mechanisms. Food Chemistry, 355(March), 129607. https://doi.org/10.1016/j.foodchem.2021.129607
Santacroce, L., Colella, M., & Charitos, I. A. (2021). The persistence and increase in sexually transmitted diseases (STDs) to pandemic levels. Venereology, 1(1), 2–8. https://doi.org/10.3390/venereology1010002
Schmidt, S. A., Gukelberger, E., Hermann, M., Fiedler, F., Großmann, B., Hoinkis, J., Ghosh, A., Chatterjee, D., & Bundschuh, J. (2016). Pilot study on arsenic removal from groundwater using a small-scale reverse osmosis system—Towards sustainable drinking water production. Journal of Hazardous Materials, 318, 671–678. https://doi.org/10.1016/j.jhazmat.2016.06.005
Senanayake, N., & Mukherji, A. (2014). Irrigating with arsenic contaminated groundwater in West Bengal and Bangladesh: A review of interventions for mitigating adverse health and crop outcomes. Agricultural Water Management, 135, 90–99. https://doi.org/10.1016/j.agwat.2013.12.015
Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., & Divya, B. V. (2021). Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geoscience Frontiers, 12(3), 101079. https://doi.org/10.1016/j.gsf.2020.08.015
Shankar, S., Shanker, U., & Shikha (2014). Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. The Scientific World Journal. https://doi.org/10.1155/2014/304524
Sharma, P. K., Mayank, M., Ojha, C. S. P., & Shukla, S. K. (2020). A review on groundwater contaminant transport and remediation. ISH Journal of Hydraulic Engineering, 26(1), 112–121. https://doi.org/10.1080/09715010.2018.1438213
Singh, R., Singh, S., Parihar, P., Singh, V. P., & Prasad, S. M. (2015). Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicology and Environmental Safety, 112, 247–270. https://doi.org/10.1016/j.ecoenv.2014.10.009
Smith, A. H., Marshall, G., Yuan, Y., Ferreccio, C., Liaw, J., von Ehrenstein, O., Steinmaus, C., Bates, M. N., & Selvin, S. (2006). Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environmental Health Perspectives, 114(8), 1293–1296. https://doi.org/10.1289/ehp.8832
Song, P., Yang, Z., Xu, H., Huang, J., Yang, X., Yue, F., & Wang, L. (2016). Arsenic removal from contaminated drinking water by electrocoagulation using hybrid Fe–Al electrodes: Response surface methodology and mechanism study. Desalination and Water Treatment, 57(10), 4548–4556. https://doi.org/10.1080/19443994.2014.992973
Song, S., Lopez-Valdivieso, A., Hernandez-Campos, D. J., Peng, C., Monroy-Fernandez, M. G., & Razo-Soto, I. (2006). Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite. Water Research, 40(2), 364–372. https://doi.org/10.1016/j.watres.2005.09.046
Sorlini, S., Gialdini, F., & Stefan, M. (2014). UV/H2O2 oxidation of arsenic and terbuthylazine in drinking water. Environmental Monitoring and Assessment, 186(2), 1311–1316. https://doi.org/10.1007/s10661-013-3481-z
Su, J., Lyu, T., Cooper, M., Mortimer, R. J. G., & Pan, G. (2021). Efficient arsenic removal by a bifunctional heterogeneous catalyst through simultaneous hydrogen peroxide (H2O2) catalytic oxidation and adsorption. Journal of Cleaner Production, 325(May), 129329. https://doi.org/10.1016/j.jclepro.2021.129329
Sumathi, T., & Alagumuthu, G. (2014). Adsorption studies for arsenic removal using activated Moringa oleifera. International Journal of Chemical Engineering. https://doi.org/10.1155/2014/430417
Sun, Y., Zhou, G., Xiong, X., Guan, X., Li, L., & Bao, H. (2013). Enhanced arsenite removal from water by Ti(SO4)2 coagulation. Water Research, 47(13), 4340–4348. https://doi.org/10.1016/j.watres.2013.05.028
Syam Babu, D., & Nidheesh, P. V. (2022). Treatment of arsenite contaminated water by electrochemically activated persulfate oxidation process. Separation and Purification Technology, 282(PA), 119999. https://doi.org/10.1016/j.seppur.2021.119999
Syam Babu, D., Vijay, K., Nidheesh, P. V., & Suresh Kumar, M. (2021). Performance of continuous aerated iron electrocoagulation process for arsenite removal from simulated groundwater and management of arsenic-iron sludge. Sustainable Energy Technologies and Assessments, 47(June), 101476. https://doi.org/10.1016/j.seta.2021.101476
Tolkou, A. K., & Zouboulis, A. I. (2020). Application of composite pre-polymerized coagulants for the treatment of high-strength industrial wastewaters. Water, 12(5), 1258. https://doi.org/10.3390/w12051258
Upadhyay, M. K., Shukla, A., Yadav, P., & Srivastava, S. (2019). A review of arsenic in crops, vegetables, animals and food products. Food Chemistry, 276, 608–618. https://doi.org/10.1016/j.foodchem.2018.10.069
van Genuchten, C. M., Bandaru, S. R. S., Surorova, E., Amrose, S. E., Gadgil, A. J., & Peña, J. (2016). Formation of macroscopic surface layers on Fe(0) electrocoagulation electrodes during an extended field trial of arsenic treatment. Chemosphere, 153, 270–279. https://doi.org/10.1016/j.chemosphere.2016.03.027
Wang, C. H., Hsiao, C. K., Chen, C. L., Hsu, L. I., Chiou, H. Y., Chen, S. Y., Hsueh, Y. M., Wu, M. M., & Chen, C. J. (2007). A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicology and Applied Pharmacology, 222(3), 315–326. https://doi.org/10.1016/j.taap.2006.12.022
Wang, M., & Liu, X. (2021). Applications of red mud as an environmental remediation material: A review. Journal of Hazardous Materials, 408, 124420. https://doi.org/10.1016/j.jhazmat.2020.124420
Xie, X., Lu, C., Xu, R., Yang, X., Yan, L., & Su, C. (2022). Arsenic removal by manganese-doped mesoporous iron oxides from groundwater: Performance and mechanism. Science of the Total Environment, 806, 150615. https://doi.org/10.1016/j.scitotenv.2021.150615
Yu, Y., Zhao, C., Wang, Y., Fan, W., & Luan, Z. (2013). Effects of ion concentration and natural organic matter on arsenic(V) removal by nanofiltration under different transmembrane pressures. Journal of Environmental Sciences (china), 25(2), 302–307. https://doi.org/10.1016/S1001-0742(12)60044-8
Yudovich, Y. E., & Ketris, M. P. (2005). Arsenic in coal: A review. International Journal of Coal Geology, 61(3–4), 141–196. https://doi.org/10.1016/j.coal.2004.09.003
Zanotto, F. M., López Teijelo, M., & Dassie, S. A. (2019). Hanging meniscus rotating disk electrode: A theoretical perspective. Electrochimica Acta, 327, 135032. https://doi.org/10.1016/j.electacta.2019.135032
Zaved Kaiser Khan, M. (2012). Mesures d’atténuation de l’arsenic au Bangladesh. Revue Des Sciences De L’eau, 25(1), 49–67. https://doi.org/10.7202/1008535ar
Zhao, F. J., McGrath, S. P., & Meharg, A. A. (2010). Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annual Review of Plant Biology, 61, 535–559. https://doi.org/10.1146/annurev-arplant-042809-112152
Acknowledgements
The authors are grateful to Department of Civil Engineering and Department of Chemical Engineering, School of Technology, Pandit Deendayal Energy University for the permission to publish this research.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contribution to this manuscript. MS conceptualized the research goal and methodology. MS, BP, RG, BD, MS, and JS drafted the original manuscript. DK and AK carried out review and editing. All the authors discussed the results and implication of the manuscript at every stage.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, B., Gundaliya, R., Desai, B. et al. Groundwater arsenic contamination: impacts on human health and agriculture, ex situ treatment techniques and alleviation. Environ Geochem Health 45, 1331–1358 (2023). https://doi.org/10.1007/s10653-022-01334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01334-5