Abstract
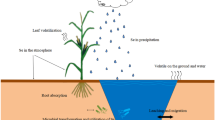
Selenium (Se) is an essential health element becoming rare in food as a result of intensive plant production. Indeed, several enzymes contain selenium in the form of the unusual selenocysteine amino acid. Selenium was found an essential nutrient in the late 1950s, when selenium was found to replace vitamin E in the diets of rats and chicks for the prevention of vascular, muscular, and hepatic lesions. At that time, selenium was considered solely as a toxic element in the northern Great Plains of the USA, because selenium was associated with the ‘alkali disease’ of grazing livestock. The major source of Se in soils is the weathering of Se-containing rocks. Secondary sources are volcanic activities, dusts such as in the vicinity of coal burning, Se-containing fertilizers, and some waters. Se cycles through the food system; Se is first removed from soils by plants and soil microorganisms, which can take up Se into their proteins and produce volatile forms such as dimethylselenide. Dimethylselenide enters the atmosphere to be brought down with precipitation and airborne particulates. Here, we review Se in agroecosystems. We focus on the production, biological effects, and use of nano-selenium particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), the essential poison, is a contradictory nutrient, because too much selenium in the diet can be toxic, whereas too little can result in chronic, and sometimes fatal, deficiency. Selenium is a key player in cellular metabolism, an essential component of enzymes that protect the body against oxidative damage and has important roles in metabolism of thyroid, human fertility, and many other vital functions (Reilly 2006). It is isolated and chemically characterized by Jöns Jakob Berzelius (1817). This scientist also discovered, or isolated for the first time, some new elements, including cerium (Ce), silicon (Si), thorium (Th), vanadium (V), and zirconium (Zr) (Hurd and Kipling 1964), as reviewed by Reilly (2006).
Selenium is playing an important role in human and animal health and is essential to all other organisms including bacteria and algae, whereas there is no evidence for selenium in higher plants (Novoselov et al. 2002; Zhang and Gladyshev 2010; El Mehdawi et al. 2011a). Although Se is not an essential nutrient for higher plants, it should be added to soil to ensure that both feed and food products contain the adequate amounts for the dietary needs. Therefore, it should be emphasized that the safety margin of Se concentrations is rather narrow (Kabata-Pendias 2011). Because of its similarity to sulfur (S), which leads to non-specific replacement of S by Se in proteins (El Mehdawi et al. 2011a), Se is toxic to most organisms at elevated concentrations (Stadtman 1990). In general, most plants contain rather low foliar Se (around 25 μg kg−1) and rarely exceed 100 μg kg−1 (Kabata-Pendias 2011). However, some plants have a great capability to accumulate Se (may be up to 100 mg kg−1 in seleniferous soils (Beath et al. 1939)). Some plants can grow in such seleniferous soils and hyperaccumulate Se levels up to 15,000 mg kg−1 dry weight (Galeas et al. 2007) as reviewed by El Mehdawi et al. (2011b).
Although Se concentration in most soils is low, it is particularly abundant in seleniferous soils, which typically contains from 1 to 10 mg Se kg−1 and may reach 100 mg Se kg−1 (Beath et al. 1939). The main bioavailable Se forms in different oxidizing and reducing environments are selenate (SeO4 2−) and selenite (SeO3 2−), respectively (White et al. 2007). Therefore, Se uptake by plants dependents on Se concentration and speciation, the concentration of competing anions (like SO4 2−), rhizosphere pH, and redox conditions (Mikkelsen et al. 1989). In general, roots take up selenate (SeO4 2−) faster than selenite (SeO3 2−) at the same concentration (de Souza et al. 1998; Zhao et al. 2005). It is well known that translocation of Se from the roots to the shoots is highly dependent on the supplied Se form, where SeO −24 is transported more readily than SeO −23 or organic Se compounds (de Filippis 2010). Furthermore, both selenate and selenite can be reduced to selenide (Se2−) and assimilated into amino acids including selenocysteine (SeCys) and selenomethionine (SeMet) via the S assimilation pathway (Terry et al. 2000). Both of these amino acids (SeCys and SeMet) can be incorporated into proteins (El Mehdawi and Pilon-Smits 2012).
In general, nanoparticles must traverse the cell wall before entering the intact plant cell protoplast, as reviewed by Dietz and Herth (2011). They also reported that the maximum pore size of plant cell walls is usually in the range of a few nanometers (nm): for example, 3.5–3.8 nm in case of root hairs and 4.5–5.2 nm in palisade parenchyma cells. Therefore, the specific objectives of this review are mainly to follow the distribution and occurence of Se in the different agricultural environments from soils, water, air, and plants to humans and animals. The nano-selenium, from agricultural nanotechnology and sustainable development, nanotechnology in agriculture and food, recent developments, risks and regulation of nanotechnology, and finally synthesis methods of nanoparticles will be also highlighted.
This article is an abridged version of the chapter by El-Ramady et al. (2014a) published in the book series Environmental Chemistry for a Sustainable Word.
Selenium characterization
Selenium is one of the rarest elements in the earth’s crust (0.05 mg kg−1), whereas it is 69th in abundance among the 88 elements (Shriver and Atkins 1999). Se is called the two-faced element (like the moon, where its name is originated); therefore, it has both a dark and a bright side (Table 1; Reilly 2006). It is also known as the “essential poison or double-edged sword element” for its dual toxic and beneficial character to health (Charlet et al. 2011). It is reported that the duality for Se came from how to reconcile its apparently contradictory properties and roles. Nevertheless, these gaps in our understanding of Se are rapidly being filled by great efforts of an extraordinary array of researchers, working in a range of disciplines, aided by powerful new research techniques and tools (Reilly 2006).
It is well known that Se has an atomic weight of 78.96 and its atomic number is 34. It is chemically related to other members of the chalcogen group (Group 16/VIA), which includes oxygen (O), sulfur (S), tellurium (Te), and polonium (Po). Therefore, it is classified as a half metal or metalloid, but elemental Se has several different allotropes (Chapman et al. 2010). This places Se in an important group of half metals or metalloids, elements that are neither fully metals nor non-metals, but share chemical and physical properties of both (Reilly 2006). This location accounts for many of its biological interactions with some important elements including sulfur, as well as with arsenic and its neighbor phosphorus (Frost 1972). About the outer electronic configuration of this element, it is 3d 104s 24p 4, with three completely filled inner shells.
Selenium has four valence states: −2 state, which predominates in organic Se compounds beside +2, +4, +6 states. There are around 50 Se minerals. The most important and relatively common ones include: clausthalite (PbSe), klockmanite (CuSe), tiemannite (HgSe), berzelianite (Cu2−x Se), crookesite (Cu, Tl, Ag)2Se, and ferroselenite (FeSe2) (Kabata-Pendias 2011). Therefore, the association of this element with host minerals, such as chalcopyrite, pyrite, and sphalerite, is relatively common. On the other hand, this element has a great affinity to different organic substances resulted in a large number of organic compounds that are analogous to those of S-organic compounds and are easily accumulated in some biolithes (Kabata-Pendias 2011). As mentioned before, the average content of Se in the Earth’s crust is estimated as 0.05 mg kg−1; however, a higher value (up to 0.5 mg kg−1) is also given. This element is slightly more concentrated in mafic rocks (rarely exceeds 0.1 mg kg−1), whereas Se is associated with clay fractions, and thus, its abundance in argillaceous sediments ranged from 0.3 to 0.6 mg kg−1 in sedimentary rocks. This concentration is higher than in sandstones and limestones (0.01–0.1 mg kg−1), as shown in Table 2 (Kabata-Pendias 2011).
On the other hand, Se is a quite unique trace element, because it is a component of some amino acids (SeCys and SeMet) and therefore involved in very specific biological roles. These main roles include protection against oxidative damages, defenses against infection, and modulation of growth and development. Therefore, the main exposure to Se occurs through food chain, and its distribution in natural environments has a marked effect on its content in soils, crops, and the human body (Marmiroli and Maestri 2008). In agriculture sector, Se is used mainly as sodium selenite (Na2SeO3) as an addition to fertilizers, insecticides, and foliar sprays. In small doses, Se is widely used in vitamins, other dietary supplements, and some livestock feeds (as a fortified element). Furthermore, it is a relatively common component of various medications and cosmetics, as a therapeutic agent (e.g., in cardiology as an antioxidant), as reported by Kabata-Pendias (2011).
Production, sources, and uses of selenium
Globally, there are no mines that specifically extract Se; instead, it is a by-product of the production of other metals such as refining of Pb and Cu, or recovered from the sludge accumulated in H2SO4 factories (Johnson et al. 2010). The supply of Se is directly affected by the supply of the materials from which it is a by-product—Cu, and to a lesser extent, Ni. Estimated domestic Se production was slightly higher in 2012 compared with that of 2011 owing to a slight increase in Cu production (1,980 and 2,000 metric tons in 2011 and 2012, respectively; USGS 2013). Most of the world’s selenium is produced in the Japan, USA, and Canada, with smaller amounts coming from Finland, Australia, Peru, Zambia, Belgium, Russia, China, and other countries with a Cu-refining industry. Many compounds of Se are commercially available, including ferro- and nickel selenide, cadmium sulfoselenide, selenium dioxide, and selenium diethyldithiocarbamate, as well as sodium selenate and selenite, as reported by Reilly (2006).
At the global scale, Se is constantly recycled in the environment via the marine, atmospheric, and terrestrial systems. Estimates of Se flux indicate that anthropogenic activity (76,000–88,000 ton per year) is a major source of Se release in the cycle, whereas the marine system (38,250 ton per year) constitutes the main natural pathway (Haygarth 1994). Se cycling through the atmosphere (15,300 ton per year) is significant because of the rapidity of transport, but the terrestrial system (15,380 ton per year) is most important in terms of human and animal health because of the direct links with food chain and the agricultural activities. Although Se is derived from both natural and man-made sources, an understanding of the links between environmental geochemistry and health is particularly very important for Se as rocks are the primary source of this element in agroecosystems (Table 3; Fordyce 2013).
There are a number of important agricultural and horticultural applications for Se. These applications include the use of sodium selenate and selenite as additives and dietary supplements in animal feeds. It could be corrected soil deficiencies by adding Se compounds to fertilizers and top dressings. On the other hand, it could be noticed potassium ammonium sulfoselenide used as a pesticidal substrate and was one of the first systemic insecticides to be marketed in the 1930s. This compound is still in use, but is restricted to non-food crops because of its toxicity. In commercial greenhouses growing flowers for cutting, sodium selenate (Na2SeO4) has also been used for a similar purpose. This selenate (Na2SeO4) could be added to irrigation water, and the plant roots can take it up. Then, it is converted in the leaves into volatile selenide (Se2−), which is released by the plant to aphids, repel red spiders, and similar pests (Reilly 2006).
Occurrence of selenium in terrestrial environments
Naturally, Se occurrence is rarely recovered in a free state, and it can be existed in different oxidation states including +6 (VI), +4 (IV), 0 (Se0), and −2 (II). The oxidized water-soluble forms including selenate Se(VI) and selenite Se(IV) are recovered in both of natural water and in soil solution (Kabata-Pendias 2011). It is reported that the highly stable elemental selenium (Se0) is also recovered in soils, but not in water solution because it is insoluble (Di Gregorio 2008). This elemental selenium (Se0) could be existed in different allotropic forms including rhombohedral Se (containing Se6 molecules), three deep-red monoclinic forms, α-, β-, and γ-Se (containing Se8 molecules), trigonal gray Se (containing Se n helical chain polymers), amorphous red Se, and black vitreous Se. The gray (trigonal) Se is the most thermodynamically stable form, which contains countless helical chains of Se atoms, and it is the only allotropic form that conducts electricity (Di Gregorio 2008). The most likely amorphous allotropes forms that are occurring in soils include both of red and black. Furthermore, red amorphous Se can gradually revert to the black amorphous form at temperature greater than 30 °C. This black form can then slowly transform into the much more stable gray hexagonal allotrope or it will be re-oxidized depending on pH and redox conditions of soil (Di Gregorio 2008).
Selenium is distributed in different environments by several processes, such as volcanic activities and hot springs, combustion of fossil fuels, soils and rocks weathering, soil leaching, sea salt spray, forest wildfires, groundwater movements, chemical and biological redox reactions, and mineral formation, as well as incineration of municipal waste, Pb and Zn smelting, Cu/Ni production, Fe and steel production, crop growth and irrigation practices, and plant and animal uptake and release (Nriagu 1989; Di Gregorio 2008). As a general rule, Se concentration in soils or ground and fresh waters depends upon the parent material, topography, age of the soil, climate, and agricultural or industrial utilization. Elemental Se and selenides (Se2−) are the predominant species under acidic, reducing conditions in soils that may be waterlogged and rich in organic matter (McNeal and Balistrieri 1989; Di Gregorio 2008).
Selenium in soils
It is well known that Se content in soils is inherited from parent material and its distribution strongly reflects soil-forming processes and atmospheric deposition. Sandy soils, which developed under humid climate, particularly in podzols, have the lowest amounts of Se, whereas the highest contents occurring most often in organic and calcareous soils (Tables 2, 4; Kabata-Pendias 2011). In general, the concentrations of Se ranged from 0.05 to 1.5 mg kg−1 Se in worldwide soils, with a calculated average value 0.44 mg kg−1. It could be observed higher contents of Se in surface layer of volcanic soils, forest soils, organic rich soils, and calcareous soils. In general, the main factors controlling Se forms and behavior in soils are Eh or redox potential and pH; however, several other parameters such as organic ligands, clay content, and hydroxides also play very significant roles (Table 4; Kabata-Pendias 2011). It is reported that about different inorganic species of Se, which associated with defined soil parameters, reveal variable properties as follows: (1) selenates (mobile in inorganic forms in neutral and alkaline soils but not absorbed on hydrous sesquioxides in particular Fe2O3·H2O), (2) selenites (slightly mobile in neutral and acid soils of humid temperate regions and are easily absorbed on hydrous sesquioxides and organic matter), and (3) selenides (rather immobile in acid soils due to the formation of stable mineral and organic compounds) (Lakin and Dawidson 1967; Combs and Combs 1986; Frankenberger and Engberg 1998; Kabata-Pendias 2011).
The most important forms and concentrations of Se in soil solution are governed by various physicoal, chemical, and biological factors, and common inorganic anions include HSeO3 −, SeO3 2−, H2SeO4 −, SeO4 2−, and HSeO4 − (Kabata-Pendias and Sadurski 2004). Selenate anions (SeO4 2−) are the favored form under oxidizing conditions, whereas in mild reducing conditions, SeO3 2− is likely to dominate (Kabata-Pendias 2011). SeO3 2− is strongly sorbed on oxides and precipitates such as Fe(SeO3)3, whereas SeO4 2– anion is very weakly sorbed, especially at high pH. Therefore, mobile and easily phyto available (available to plants) Se occurs in well-aerated alkaline soils, which are common in arid and semi-arid regions. On the other hand, organic matter has a strong tendency to form organometallic complexes which remove Se from soil solution (Kabata-Pendias and Mukherjee 2007).
It is reported that the phyto-availability of different Se species in soils decreases in the following order: selenate > selenomethionine > selenocysteine > selenite > elemental selenium > selenide (Kabata-Pendias and Mukherjee 2007). It is also observed a close relationship between Se and organic carbon in most soils. Microbial processes play a crucial role in Se cycling in both the formation and mineralization of organic Se such as selenomethionine and selenocysteine and especially in its volatilization from Se-contaminated soils (Martens and Suarez 1998). These processes are important for the reduction of Se, principally through the reduction of selenate and selenite. Insoluble selenide compounds are likely to accumulate in case of poorly drained soils. Se may volatilize in the form of (CH3)2Se, as well as in forms of several other methane and sulfide Se compounds, due to methylation processes under anaerobic conditions (Kabata-Pendias and Mukherjee 2007). A number of microorganisms such as bacteria and fungi species are involved in volatilization processes of Se. It is reported that organic amendments may significantly increase the rate of Se volatilization from soils (Frankenberger and Karlson 1994; Kabata-Pendias and Mukherjee 2007).
Selenium in waters
In aquatic environments and under most pH and redox conditions, the two oxyanions (Se4+ and Se6+) are dominant with several forms of selenide (Se2−) also being present, as in soils (Cutter and Bruland 1984). A high biological activity and a locally oxidative environment may occur in contaminated aquifers. Therefore, these environmental conditions oxidize and then solubilize the reduced Se forms that enter into food web, and Se levels in biota can remain high for years after inputs have ceased (Lemly 1997; Di Gregorio 2008). Usually, natural waters contain Se < 1 μg l−1, whereas these concentrations in seawater commonly range between 0.1 and 0.35 μg l−1. The average global Se in river waters is about 0.07 μg l−1, with a range of 0.02–0.5 μg l−1 (Gaillardet et al. 2003). However, it is also reported by ATSDR (2002) in case of some rivers (e.g., Colorado River) contain Se in the range of 1–4 μg l−1, although much higher values, up to 400 μg l−1 (Kabata-Pendias and Mukherjee 2007).
Although ground waters usually contain more Se than surface waters, in groundwater and surface water, Se concentrations range from 0.06 μg l−1 to about 400 μg l−1 in general (Lindberg 1968), whereas it may approach 6,000 μg l−1 in groundwater in some areas (Cannon 1964). Levels of selenium in tap water samples from public water supplies around the world are usually much less than 10 μg l−1 but may exceed 50 μg l−1 (Gore et al. 2010). It is reported that, in China and from high soil Se area content, drinking water ranged from 50 to 160 μg l−1 (WHO 2011).
Labile Se usually in most soils and the Se deposited atmospherically onto soils are rapidly leached into groundwater (Haygarth 1994). In Finland, it is reported that levels of Se in stream and river waters increased up to an average of 180 μg l−1 and in bottom sediments up to around 4 mg kg−1, after the Se-fertilizing program (Wang et al. 1994). WHO has been established the threshold value for Se in drinking water as 10 μg l−1, whereas in the USA, it ranges between 10 and 45 μg l−1. The maximum critical level value for the Se concentration in waters of all states of the USA is 50 μg l−1. The limit level for Se in water used for irrigation is 20 μg l−1 (ATSDR 2002). The maximum permissible Se concentration are as follows: for drinking water for humans 10 μg l−1, drinking water for livestock 50 μg l−1, and irrigation water 20 μg l−1 in most countries (Kabata-Pendias and Mukherjee 2007). There several physical and chemical factors govern concentrations and chemical forms of Se in soils or drainage water, including chemical and mineralogical composition, pH, adsorbing surface, and oxidation–reduction status (Dhillon and Dhillon 1999).
Selenium in air
It is reported that Se concentrations in the atmosphere are highly variable due to differentiated sources: (1) evaporation from ocean and sea surface, (2) volcanic eruption, and (3) industrial emissions (Kabata-Pendias and Mukherjee 2007). Concentration of Se in air above the South Pole is 0.06 ng m−3 with average value for worldwide air from remote regions is 0.2 ng m−3, whereas the median is 4.0 ng m−3 for polluted areas (Reimann and de Caritat 1998). It is found that, due to the impact of Se volatilization from the surface of seawaters, Se levels increased in mosses >1 mg kg−1 and in peat >2 mg kg−1 in the marine regions (Steinnes 2003). It is worth to mention that Se is released into the atmosphere as hydrogen selenide, produced metabolically by plants, and as selenates, selenites, and elemental selenium in particulate form (WHO 2011). Se level in most urban air ranges from 0.1 to 10 ng m−3, whereas higher levels may be found in certain areas like the vicinity of copper smelters (Zoller and Reamer 1976).
It is reported by the US Environmental Protection Agency that inhalation exposure limits for Se include 12,700 μg m−3 for hydrogen selenide, 400 μg m−3 for Se-hexafluoride, and 200 μg m−3 for other Se compounds (Fordyce 2005). Se concentration in air may vary from 160 to 1,000 μg m−3 (ATSDR 2002), whereas its recommended threshold limit is 200 μg m−3 in workplace in general, 50 μg m−3 in Germany, and 100 μg m−3 in Russia (Schrauzer 2004). During fossil fuel combustion, Se is released and its global emission is >6 kt year−1, in both small particles and volatile compounds which make around 40 % of the total aerial abundance (Kabata-Pendias and Mukherjee 2007).
Selenium in plants
In general, it could be increased Se content in plants in different ways including foliar applications, hydroponics, or aeroponic cultivation in a nutrient solution containing Se and soaking seeds in Se solution before sowing (Germ et al. 2007). Therefore, the Se uptake by plants (mainly as SeO4 2− or SeO3 2−, when it is present in soluble forms) depends on several factors related to soils and plants characterization, although differences between these plants species are very pronounced (Kabata-Pendias 2011). Rayman et al. (2008) reported that there is no bioavailability data for either Se-methyl-selenocysteine or γ-glutamyl-Se-methyl-selenocysteine. Although Se has not yet been classified as an essential element for higher plants, its role has been considered to be beneficial for plants that are capable of uptake and then accumulating in large amounts (Shanker 2006). There are several naturally occurring organic Se species including selenocysteine, methylselenocysteine, selenomethionine, selenotaurine, seleniobetaine, seleniocholine, dimethylselenide, dimethyldiselenide, and trimethylselenonium (Pyrzynska 1995). Although the essentiality of these selenoproteins in higher plants has not been documented, syntheses of them in some plants such as sugar beet have been reported by Terry et al. (2000). Furthermore, several selenoamino acids including selenomethionine (SeMet), selenocysteine (SeCys), and selenomethylocysteine (SeMC) in association with glutathione peroxidases have been found in both bacteria and higher plants (Kabata-Pendias 2011).
The uptake and metabolism of Se totally differ due to growth stage, plant species, and plant organs. More Se accumulates in shoot and leaf than in root tissues in several plants, but there are some exceptions (Zayed et al. 1998). It is observed that Se concentrations in the upper leaves, roots, stolons, and tubers of potato increased with increasing Se supplementation (Turakainen 2007). Furthermore, the Se concentration declined during the growing period in the aerial parts, roots, and stolons of potato plants, whereas an intensive accumulation took place in immature and mature tubers (Turakainen 2007). In seleniferous soils, a great variation in plants’ capability exists to uptake Se from these soils. It is worth to mention that most of the cultivated crop plants have a low tolerance to high Se levels and in general, they contain less than 25 μg Se g−1 DW and are considered to be non-accumulators like potato (White et al. 2004).
It is reported that the critical Se concentration in plant tissues, which decreased the yield in case of the following plants Indian mustard, maize, rice, and wheat (in μg g−1 DW), was 105, 77, 42, and 19, when Se rates (as selenite) were 5, 5, 4, and 10 μg Se g−1 soil, respectively (Rani et al. 2005). There are several physiological functions or roles of Se in higher plants (Tamaoki et al. 2008; Pilon-Smits and Colin 2010; Hasanuzzaman et al. 2010; Hajiboland 2012). Some of the beneficial effects of Se in plants subjected to stress conditions, which increase antioxidant activity. It is reported that treated plants with selenate induced higher increases in plant enzymes that detoxify H2O2, especially both of ascorbate peroxidase and glutathione peroxidase. It is indicated that selenate application at low rates can be used to promote the induction of plant antioxidant system, thereby improving stress resistance (Hasanuzzaman et al. 2010).
An excess of Se may decrease germination and growth rates of non-tolerant plant species and cause leaves chlorosis and black spots. It is found that the critical Se concentration in solid media for gain reed (Arundo donax L.) plant ranged from 20 to 50 mg kg−1 for the American and Hungarian ecotypes, respectively (El-Ramady et al. 2014b). In some plants, increased Se levels suppress their concentrations of N, S, and P, as well as several amino acids (Kabata-Pendias 2011). Inhibition uptake of some metals (mainly Cu, Cd, Mn, and Zn) may happen under high Se concentrations. Therefore, the application of N, S, and P is known to help in Se detoxifying, which may be a result either of depressing the Se uptake by roots or of establishing a beneficial ratio of Se to these previous elements (Kabata-Pendias 2011).
It is estimated Se range in cereals at the worldwide level to be 100–800 μg kg−1 FW (Fordyce 2005). This range of Se mean (in μg kg−1) varies from 142 to 970 and from 14 to 90 for countries with high and low Se levels in grains, respectively (Kabata-Pendias and Mukherjee 2007). Using soil application of 10 g Se ha−1 rate, it is found that Se contents in grains of barley and oats (in μg kg−1) ranged from 19 to 260 and from 32 to 440, respectively (Gupta and Gupta 2000), whereas using two foliar application rates of Se (10 and 20 g Se ha−1) increased Se contents of winter wheat grains from 0.094 to 0.192 mg kg−1 and the first Se rate was sufficient for reaching the required content in wheat grains (Duscay et al. 2006). A number of feed and forage samples from China were analyzed by Ge and Yang (1993). They found that these samples were from the Se-deficient regions, which contain the following Se levels (in μg kg−1): <20, 30–50, 60–90, and >100 for severe deficient, deficient, moderate deficiency, and adequate Se supply areas, respectively (Kabata-Pendias 2011). Thus, the agronomic biofortification with Se-supplemented fertilizers is a common practice in cereal crops to increase the Se content and nutritional quality of grains (Bañuelos et al. 2005). However, the transformation of Se by bacteria and the effect of these bacteria on the Se availability to plants still are poorly understood (Acuña et al. 2013).
Selenium in food systems
There are several articles and books concerning with the relationship between Se and both of human health and plant foods (Combs 2005; Hartikainen 2005; Finley 2005; Reilly 2006; Rayman et al. 2008; Fairweather-Tait et al. 2010, 2011; WHO 2011; Hatfield et al. 2012; Bañuelos et al. 2014). In general, people can obtain virtually all of their Se requirements from eating foods, whereas Se is found mostly bound to proteins in both plant and animal tissues (WHO 2011). Therefore, meats, seafood, and cereals are considered to be the most important food sources of Se, because they have high-protein contents (for meats and seafood 0.3–0.5 mg Se kg−1), because of its consumption in large amounts (for cereals 0.1–10 mg Se kg−1). Vegetables and fruits (relatively low-protein-level foods) tend to have relatively low Se contents (<0.01 mg Se kg−1). In general, Se content in different food systems depends on and at the same time reflects the soil available Se to produce those food systems (WHO 2011).
It is reported that the global Se intakes (in μg day−1) vary significantly among different countries, whereas its average intakes were relatively low (10–20), moderate (40–90), and (85–150) in parts of China, Europe, and North America, respectively (FAO/WHO 1998; WHO 2011). In more details, it reported that dietary Se intake ranges from 7 to 4,990 μg per day, with mean values of 40 μg per day in Europe and 93 to 134 μg per day for women and men in the USA, respectively (Rayman 2012). Finally, it is recommended that the average Se intake is 60 and 53 μg per day for men and women, respectively (Rayman 2004). In UK, it is reported that the main food groups providing Se in the diet or contribution of each food group to total population dietary exposure include eggs (4 %), vegetables and fruits (7 %), fish (10 %), milk/dairy products (21 %), bread and cereals (26 %), and meat (26 %). On the other hand, some Brazil nuts are a rich source with Se concentrations ranging from ~0.03 to 512 mg kg−1 fresh weight (Rayman et al. 2008).
It is found that Se concentrations in heart, liver, and kidney of beef tissues were 0.55, 0.93, 4.5, and mg kg−1, respectively, whereas muscle tissue was in the region of 0.2 mg kg−1. Juniper et al. (2008) reported that supplementation of cattle with Se-enriched yeast increased muscle Se concentration up to ~0.6 mg kg−1, whereas the average Se content in chicken was ~0.2 mg kg−1 and beef ~0.25–0.3 mg kg−1 in the USA (Fairweather-Tait et al. 2011). Total Se content in fish ranged between 0.1 and ~5.0 mg kg−1 (Fairweather-Tait et al. 2010), where the Se content in some marine fish is considered relatively high for cod, shark, and canned tuna (~1.5, 2.0, and 5.6 mg kg−1, respectively; Reyes et al. 2009). It is worth to mention that the main Se species in fish include selenite/selenate (12–45 %) and selenomethionine (29–70 %) depending on both of fish species and the total Se content (Rayman et al. 2008; Fairweather-Tait et al. 2010).
Lipiec et al. (2010) reported that hens’ eggs contain from 3 to 25 mg Se per whole egg, whereas Se supplementation in hen’s diet may increase Se content of eggs up to 0.34–0.58 mg kg−1. Se-enriched eggs are widely produced around the world (Fisinin et al. 2009). The main Se species in eggs include selenomethionine, selenocysteine, and possibly selenite, where the predominant species (>50 %) involve selenomethionine and selenocysteine in egg white and egg yolk, respectively (Lipiec et al. 2010). The predominant Se species in cows’ milk include both of selenite and selenocysteine; furthermore, the supplementation program of dairy cows with Se-enriched yeast is already used, and after using this supplementation, the main species include selenite, selenocysteine, and selenomethionine (Muniz-Naveiro et al. 2007).
It is well known that both fruit and vegetables contain relatively small Se amounts. In case of un-enriched vegetables with low Se levels, the main species include selenate (4 %), Se-methyl-selenocysteine (12 %), γ-glutamyl-Se-methyl-selenocysteine (31 %), and selenomethionine (53 %) in garlic with natural Se content of <0.5 mg kg−1 (Kotrebai et al. 2000). However, certain vegetables such as broccoli, onions, and garlic when grown on Se-rich soil can accumulate Se, resulting in Se-enrichment from <0.5 up to 140–300 mg kg−1. The main Se species in Se-enriched food such as onions is γ-glutamyl-Se-methyl-selenocysteine (63 %), selenate (10 %), and selenomethionine (5 %) plus some other species (Hurst et al. 2010). It could be concluded that Se species profile in such vegetables, such as garlic, broccoli, and onions, is variable depending on the total Se level of enrichment, the forms of Se used for this enrichment, and the type of vegetable. The predominant species in Se-enriched vegetables include Se-methyl-selenocysteine or γ-glutamyl-Se-methyl-selenocysteine, whereas these forms of Se in plant foods have received attention due to purported protection against cancer in animal models when compared with other forms of this element (Fairweather-Tait et al. 2011).
Nano-selenium and the environment
It is reported that nano-materials exhibit novel properties, like a high surface activity, a great specific surface area, a lot of surface active centers, and a high catalytic efficiency (Gao and Hiroshi 2005). Due to both high surface reactivity and advantage of size effect, nanoparticle has been already used in pharmaceutical applications to increasing the bioavailability of drugs and targeting therapeutic agents to particular organs (Davda and Labhasetwar 2002). It has been reported that nanoparticles showed new characteristics of transport and uptake and exhibited higher absorption efficiencies (Zha et al. 2008; Liao et al. 2010). However, there is little data on intestinal absorption and Se retention of nano-Se (Hu et al. 2012).
Different Se forms including organic and some salts have been used in studying its biological effects several years ago, whereas recently, nanoparticles of elemental selenium (Se0) have gained the attention as a possible source of this beneficial element (Zhang et al. 2001). It is worth to mention that nanoparticles of Se0, which has a very low bioavailability (<5 %), could be formed from some bacterial strains within redox system of glutathione or ascorbate and selenite (Garbisu et al. 1996). Furthermore, red elemental Se, which formed from selenite reduction and glutathione or other reducing agents, could further aggregate into gray and black elemental Se (Fig. 1). Nano-Se, which is bright red, soluble, highly stable, and nano-defined size in the redox state of zero (Se0), has been manufactured for use in both of the nutritional supplements and developed for applications in medical therapy (Gao et al. 2002). It is well documented that black and gray elemental Se are biologically inert, which may be due to their insolubility, whereas the red elemental particulate Se has promising uses in the environmental protection from the pollution of the excessive Se (Garbisu et al. 1995). The size of this red elemental Se depends on the amount of protein in the redox system (Zhang et al. 2001). It is reported that nano-Se at 20–60 nm had a similar bioavailability to sodium selenite (Ball and Garwin 1992). Huang et al. (2003) suggested that the biological activities of nano-Se may come from the special properties of these nanoparticles.
Zhang et al. (2005) demonstrated that nano-Se has comparable efficacy to selenite in up-regulating seleno-enzymes and Se levels in tissue, but is less toxic. These results challenged to confirm that elemental Se has biological activities and stimulated us to further compare nano-Se with selenomethionine, which has excellent bioavailability and lower toxicity among various Se forms (Zhang et al. 2008). It has been reported that nano-Se has a higher efficiency in up-regulating seleno-enzymes and seem to be less toxicity comparing with selenite. In other words, comparing with selenomethionine, nano-Se has lower toxicity and possesses equal efficacy in increasing the activities of seleno-enzymes (Wang et al. 2007). These results indicated that nano-Se can serve as an antioxidant with reduced risk of Se toxicity (Zhang et al. 2008).
A variety of microorganisms including algae, yeast, fungi, and bacteria can adsorb and accumulate metals, but only a few groups can selectively reduce metal ions to produce nanoscale mineral phases (Oremland et al. 2004). These previous organisms can produce inorganic phases of constant chemical composition and size (Pearce et al. 2008). The majority of several studies on the biogenesis of nano-Se particles have focused on the anaerobic systems, which have certain limitations such as culture conditions and isolate characteristics that make optimization and scale up in bio-manufacturing processes challenging. However, Se-tolerant aerobic organisms provide the opportunity to overcome these limitations in the biosynthetic process. The isolate tolerates Se oxyanions and generates Se-nanoparticles, thus combining the detoxification of oxidized seleniferous environments with the biotechnological production of nano-materials (Prakash et al. 2010).
Elemental selenium (Se0)
As mentioned before, Se0 is usually formatted in natural environments through a biotic process involving the reduction of selenate (Zhang and Frankenberger 2005) or selenite (Di Gregorio et al. 2005) by bacteria, whereas possible transformations of Se0 include its oxidation to Se(IV) or Se(VI) or its incorporation into iron sulfides (FeS) or selenides (Belzile et al. 2000). Because of its existence between the higher chemical valence of Se(IV) and Se(VI) from one side, and the lower valence of Se(−II) from the other side, Se0 could be transformed to either direction depending on the environmental redox potential and/or the presence of biological activity (Chen et al. 2006). Some information of allotropic elemental Se is summarized in Table 5.
Elemental Se is occurring mostly in sedimentary rocks, although it is rare in the nature (White et al. 2004). From three allotropes of elemental Se, the gray and the black one are biologically inert, which may due to their insolubility (Huang et al. 2003). The red allotrope has been produced by many kinds of bacteria from selenite, such as Hunter and Manter (2008), Prokisch et al. (2008), and Prokisch and Zommara (2008). It is found that the red elemental Se particles in nano-size scale have good free radical scavenging effects on different free radicals in vitro (Huang et al. 2003).
Recent developments, risks, and regulation of nano-Se
Recently, there is an increased interest in the potential use of nanotechnology applications in food sector and agriculture (Chaudhry et al. 2008). In general, nanotechnology-enabled products could be defined as material products derived or issued from materials less than 100 nm, although no unified definition has been approved internationally (Gruère et al. 2011). In food sector and agriculture, several applications of nanotechnology are being developed and commercialized with different targets, ranging from improved food safety, processing, and nutrition to reduced agricultural inputs and enhanced packaging (Yada 2009), and the global potential to promote sustainable agriculture and deliver better foods (Gruère et al. 2011).
Because of the limited information on the risks of handling nano-materials (like nano-Se), these materials have created an intense interest in their health risks under ultra-small scale. Therefore, the potential risks of nano-materials have surprised the public by taking a strong precautionary tone on safety and health risks (ETC 2005a, b). Furthermore, due to the information lack about the impacts of nanotechnology (like nano-Se) on food industry, public safety, and society, as well as the potential toxicity of nano-materials, until proven otherwise, it is probably wise to take a full precautionary to deliberate the possible regulatory control as a proactive approach (Chau et al. 2007). Further investigations should be done on the applications of nanotechnology in packaging, nanotoxicity, food processing, and risk/benefit analysis. These studies will include the following items: sustain the growth of nano-food industry, fill the knowledge gaps, and avoid any unpredictable health hazard (Chau et al. 2007).
In general, there are some regulation issued by the European Union to regulate nanotechnologies in the food industry include the following: EC Cosmetics Regulation (EC No 1223/2009), EC No. 1223/2009 (2009), EC No. 1272/2008 (2008), EC No. 1907/2006 (2006), EC No. 1935/2004 (2004), and EC No. 258/97 (1997). The regulation of nanotechnologies is within the scope of both horizontal and vertical legislation (Cushen et al. 2012). Due to using of nanoparticles, it is urgently required the following issues: risk assessment and management, as well as exposure assessment for existing products available on the world market. Furthermore, existing uncertainties for risk and exposure assessment of nano-materials arise, due to limited information on several aspects including behavior, bioaccumulation, and toxicity. These uncertainties also have implications for the effective regulation of the use of nano-materials (Cushen et al. 2012).
Synthesis methods of Se-nanoparticles
It is well documented that nanotechnology has different tools showing the capability of synthesizing nanoscale materials with specific opto-electronic, physical, and chemical properties. Furthermore, various physical and chemical methods have been designed for the synthesis of nanoparticles, whereas the different problems related to these methods enforced the researchers to search for alternative methods. It could be synthesized elemental nano-Se within the reduction of a Se-salt with a reducing agent, usually in the presence of a stabilizing agent to prevent the clusters of Se atoms from growing and to obtain stabilized nanoparticles in colloidal suspension (Zhang et al. 2004).
Concerning of the accurate determination of Se0, it is a key step in order to understand any process whether it is biological, geological, or environmental. However, finding an appropriate method for identification and measurement of Se0 in natural systems like sediments or soils is a difficult task due to its both matrix complexity and low concentration (Chen et al. 2006). It is known that the lack of appropriate standard reference materials (SRM) is one of the most difficult problems analysts who are facing in quantitative speciation of the environmental samples. Hence, it is particularly problematic for Se0, due to the exact mineralogical and chemical properties of this species in nature are unknown (Chen et al. 2006). It should be kept in mind that the original work on identification or synthesizing those forms of Se is very difficult to find and often lacks of details (Chen et al. 2006).
There are three different methods can be used for selenium synthesis including the chemical, physical, and biological methods, whereas the chemical and biological methods are common use.
Chemical methods
In general, the most often used method for the chemical synthesis of nanoparticles is the chemical reduction method, which deals with the reduction of metal particles to nanoparticles using chemical reducing agents such as sodium borohydride or sodium citrate (Cao and Hu 2009). Other chemical agents utilized for Se synthesis include N,N-dimethyl formamide (DMF) (Pastoriza-Santos and Liz-Marzan 2000), poly(N-vinyl pyrrolidine) (PVP), ethyl alcohol (Kim 2007), tetra-n-tetrafluoroborate (TFATFB), L-ascorbic acid, and hexadecyltrimethylammonium bromide (CTAB) (Hanauer et al. 2007). Electrochemical synthesis method induces chemical reactions in an electrolyte solution with the use of an applied voltage. A wide variety of nano-materials could be synthesized using this method (Sau and Rogach 2010).
In the chemical method, researchers do not use any living/organic material, but they usually start from inorganic selenite and add a reducer agent, like ascorbic acid. This method includes reduction of sodium selenite with glutathione at room temperature in aqueous solution. Therefore, glutathione, having a thiol group, reacts with sodium selenite to form selenodiglutathione, which decomposes to produce Se molecules and diglutahtione. Then, finally Se molecules aggregate together to form Se nano-spheres (Gao et al. 2003). It is reported by Ministry of Hygiene in China (1998) that nano-Se at dose of 180 µg Se daily was granted as health care food.
Biological methods
Researchers started recently to recognize the importance of the ability of certain microorganisms to produce nano-sized particles within their metabolism. In trace concentrations, several elements are essential for growth and reproduction of animals, plants, and microorganisms; however, these elements easily become toxic when their concentrations are higher than the physiological level. The complete understanding of the synthesis mechanism of nanoparticles using the biological agents has not been devised. In addition, the synthesis mechanism for both intra- and extracellular of nanoparticles is totally different in various biological agents, because these biological agents react differently with metal ions and also there are different biomolecules responsible for the synthesis of these nanoparticles (Rai et al. 2011).
Biological agents used for nanoparticles synthesis represent mainly microbes including bacteria, fungi, algae and yeast (Ingle et al. 2008; Birla et al. 2009), and plants (Song and Kim 2009). The biological methods used for nanoparticles synthesis include both extra- and intra-cellular methods (Rai et al. 2008; Shaligram et al. 2009). There several benefits makes microbes advantageous for nanoparticle production including their economic viability, large-scale secretion of extra-cellular enzymes, and ease in scale up in solid substrate fermentations (Gardea-Torresdey et al. 2002). As mentioned before, plants have also the ability to produce nanoparticles. It is reported that live plants can manage themself to fabric gold nanoparticles (Gardea-Torresdey et al. 2002). It is confirmed by using atomic resolution analysis that Au nanoparticles inside plants are in a crystalline state and pure gold (Eszenyi et al. 2011).
The nanoparticles synthesis using bacteria and actinomycetes usually involves the intra-cellular synthesis method, in which the bacterial cell filtrate is treated with metal salt solution and kept in a shaker in dark at ambient temperature and pressure conditions (Ahmad et al. 2003a, b). For the extra-cellular nanoparticles synthesis using bacteria, the bacterial culture is centrifuged at 8,000×g and the supernatant is challenged with metal salt solution (Ogi et al. 2010). In case of fungi, nanoparticles also are intra-cellularly synthesized by treating the fungal mycelium with metal salt solution and further incubation for 24 h (Mukherjee et al. 2001). In algal nanoparticles synthetism, washed culture of algae is treated with metal salt solution (without existence of any medium) and kept in dark with controlled pH and temperature conditions (Thakkar et al. 2010). The nanoparticles synthesis using yeast involves two steps including firstly nanoparticles synthesis and next recovery of the synthesized nanoparticles (Kowshik et al. 2003). Yeast culture is challenged with metal salt solution and incubated in dark for 24 h within the synthesis process (Fig. 2; Rai et al. 2011).
The left one different steps of the biological synthesis of nano-Se using MRS broth. 1 The first step is the incubation at 37 °C for 48 h, the second is centrifuge, 2 then filter to separate the filtered bacteria. 3 Then, use concentrated hydrochloric acid for 3–4 days, 4 thus purifying nano-Se spheres, we get a solution or suspension called NanoSel, which is mainly for research. Photo 3 shows that nano-Se spheres consolidate to the bottom over time, but with a little shaking, we can get back the original state. The right one from photo 1–4 represents how we can prepare nano-Se using chemical method. Photo 5 represents the comparison between biological and chemical methods for nano-Se production. The right 100 measuring flask belongs to the chemical method, where it used vitamin C or ascorbic acid, and the other flasks belong to the biological method using MRS media and Lactobacillus casei as a bacteria strain (photo by El-Ramady, Nano Food Lab, Debrecen Uni., Hungary)
Conclusion
Selenium, the essential poison, is becoming more and more insufficient in food crops. It is biologically essential because it is an essential constituent of several enzymes. This essentiality of this nutrient was first recognized in the late 1950s. Since that time, Se has become a subject of several investigations in many parts all over the world. Furthermore, the main sources and behavior of Se in different terrestrial environments including soils, water, air, and plants are a very fertile field for investigation and research. The importance, production, and biological effects of this element and its use in the sustainable development were still an attractive issue. On the other hand, Se nanoparticles can be synthesized using different chemical, physical, and biological methods. The biological method is an environmental friendly method compared with other methods. There are certain open questions of research which need to be pointed out. These issues include different biological effects of Se and nano-Se on the terrestrial environments. A comparison between effects of both Se and nano-Se on soil microbial activities (including soils microbial enzymes and microbial counts) should be kept in mind.
References
Acuña JJ, Jorquera MA, Barra PJ, Crowley DE, Mora M (2013) Selenobacteria selected from the rhizosphere as a potential tool for Se biofortification of wheat crops. Biol Fertil Soils 49:175–185. doi:10.1007/s00374-012-0705-2
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003a) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28:313–318
Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Shrinivas V, Sastry M (2003b) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14:824–828
ATSDR (2002) Draft toxicological profile for several trace elements. U.S. Dept. Health & Human Services. Agency for Toxic Substances and Disease Registry, Atlanta
Ball P, Garwin L (1992) Science at the atomic scale. Nature 355:761–766
Bañuelos GS, Lin ZQ, Arroyo I, Terry N (2005) Selenium volatilization in vegetated agricultural drainage sediment from the San Luis Drain, Central California. Chemosphere 60:1203–1213
Bañuelos GS, Lin ZQ, Yin X (2014) Selenium in the environment and human health. Taylor & Francis, London
Beath OA, Gilbert CS, Eppson HF (1939) The use of indicator plants in locating seleniferous soils in the Western United States. I. General. Am J Bot 26:257–269
Belzile N, Chen YW, Xu R (2000) Early diagenetic behavior of selenium in lake sediments. Appl Geochem 15:1439–1454
Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK (2009) Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol 48:173–179
Cannon HG (1964) Geochemistry of rocks and related soils and vegetation in the Yellow Cat area, Grand County, Utah. Washington, DC, United States Geological Survey (Bulletin No. 1176)
Cao J, Hu X (2009) Synthesis of gold nanoparticles using halloysites. e-J Surf Sci Nanotechnol 7:813–815
Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (2010) Ecological assessment of selenium in the aquatic environment. Society of Environmental Toxicology and Chemistry (SETAC) Series. CRC Press, Taylor & Francis, London
Charlet L, Parsons C, Bardelli F, Fernandez-Martinez A, Rossetto L (2011) Effect of Flood-Induced Redox Oscillations on Selenium Mobility in Soils. In: Bañuelos GS, Lin Z-Q, Yin X, Duan N (eds) Selenium: global perspectives of impacts on humans, animals and the environment. University of Science and Technology of China Press, Hefei, ISBN 978-7-312-02929-5
Chau CF, Wu SH, Yen GC (2007) The development of regulations for food nanotechnology. Trends Food Sci Technol 18:269–280
Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Aitken R, Watkins R (2008) Applications and implications of nanotechnologies for the food sector. Food Addit Contam 25(3):241–258
Chen H, Weiss J, Shahidi F (2006) Nanotechnology in nutraceuticals and functional foods. Food Technol 3:30–36
Combs GF (2005) Global importance of selenium and its relation to human health. In: Welch RM, Ãakmak I (eds) Impacts of agriculture on human health and nutrition. Encyclopedia of life support systems (EOLSS), Developed under the auspices of the UNESCO. EOLSS Publishers, Oxford. http://www.eolss.net. Retrieved 25 Aug 2012
Combs GF, Combs SB (1986) The role of selenium in nutrition. Academic Press, Orlando
Cushen M, Kerry J, Morris M, Cruz-Romero M, Cummins E (2012) Nanotechnologies in the food industry—recent developments, risks and regulation. Trends Food Sci Technol 24:30–46
Cutter GA, Bruland KW (1984) The marine biogeochemistry of selenium: a reevaluation. Limnol Oceanogr 29:1179–1192
Davda J, Labhasetwar V (2002) Characterization of nanoparticle uptake by endothelial cells. Int J Pharm 233:51–59
De Filippis LF (2010) Biochemical and molecular aspects in phytoremediation of selenium. In: Ashraf M, Ozturk M, Ahmad MSA (eds) Plant adaptation and phytoremediation. Springer, Berlin, pp 193–226. doi:10.1007/978-90-481-9370-7_10
de Souza MP, Chu D, Zhao M, Zayed AM, Ruzin SE, Schichnes D, Terry N (1998) Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian mustard. Plant Physiol 119:565–574
Dhillon KS, Dhillon SK (1999) Adsorption–desorption reactions of selenium in some soils of India. Geoderma 93:19–31
Di Gregorio S (2008) Selenium: a versatile trace element in life and environment. In: Prasad AS (ed) Trace elements in human health and disease, vol 2. Academic Press, New York, pp 593–622
Di Gregorio S, Lampis S, Vallini G (2005) Selenite precipitation by a rhizospheric strain of Stenotrophomonas sp. isolated from the root system of Astragalus bisulcatus: a biotechnological perspective. Environ Int 31:233–241
Dietz KJ, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16:582–589. doi:10.1016/j.tplants.2011.08.003
Duscay L, Ložek O, Varga L, Lošák T (2006) Wheat supplementation with selenium. Chem Listy 100:519–521 (in Slovakien and cited from Kabata-Pendias 2011)
El Mehdawi AF, Pilon-Smits EAH (2012) Ecological aspects of plant selenium hyperaccumulation. Plant Biol 14:1–10. doi:10.1111/j.1438-8677.2011.00535.x
El Mehdawi AF, Quinn CF, Pilon-Smits EAH (2011a) Effects of selenium hyperaccumulation on plant–plant interactions: evidence for elemental allelopathy? New Phytol 191:120–131. doi:10.1111/j.1469-8137.2011.03670.x
El Mehdawi AF, Quinn CF, Pilon-Smits EAH (2011b) Selenium hyperaccumulators facilitate selenium-tolerant neighbors via phytoenrichment and reduced herbivory. Curr Biol 21:1440–1449. doi:10.1016/j.cub.2011.07.033
El-Ramady H, Domokos-Szabolcsy É, Shalaby TA, Sztrik A, Prokisch J, Fári M (2014a) Selenium in agriculture: water, air, soil, plants, food, animals and nanoselenium. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) Environmental chemistry for a sustainable world vol 5 “CO2 sequestration, biofuels and depollution”. Springer, Berlin
El-Ramady H, Alshaal T, Domokos-Szabolcsy É, Shalaby T, Bayoumi Y, Elhawat N, Sztrik A, Prokisch J, Fári M (2014b) Selenium and its role in higher plants. In: Lichtfouse E (ed) Environmental chemistry for a sustainable world, vol 6. Springer, Berlin
Enghag P (2004) Encyclopedia of the elements—technical data, history, processing and applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Eszenyi P, Sztrik A, Babka B, Prokisch J (2011) Elemental, nanosized (100–500 nm) selenium production by probiotic lactic acid bacteria. Int J Biosci Biochem Bioinform 1:148–152
ETC, Action Group on Erosion, Technology and Concentration (2005a) ETC group report: NanoGeoPolitics. ETC group surveys the political landscape. http://www.etcgroup.org/upload/publication/51/01/com89specialnanopoliticsjul05eng.pdf
ETC, Action Group on Erosion, Technology and Concentration (2005b) The potential impacts of nano-scale technologies on commodity markets: the implications for commodity dependent developing countries. http://www.etcgroup.org/upload/publication/45/01/southcentre.commodities.pdf
Fairweather-Tait SJ, Collings R, Hurst R (2010) Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr 91:1484S–1491S
Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R (2011) Selenium in human health and disease. Antioxid Redox Signal 14(7):1337–1383. doi:10.1089/ars.2010.3275
FAO/WHO (1998) Preparation and use of food-based dietary guidelines. Report of a joint FAO/WHO consultation. World Health Organization, Geneva (WHO technical report series, no. 880)
Finley JW (2005) Selenium accumulation in plant foods. Nutr Rev 63:196–202
Fisinin VI, Papazyan TT, Surai PF (2009) Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit Rev Biotechnol 29:18–28
Fordyce FM (2005) Selenium deficiency and toxicity in the environment. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P (eds) Essentials of medical geology. Elsevier, London, pp 373–415
Fordyce FM (2013) Selenium deficiency and toxicity in the environment. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P (eds) Essentials of medical geology. Elsevier, London, pp 373–415
Frankenberger WT, Engberg RA (1998) Environmental chemistry of selenium. Marcel Dekker, New York 736
Frankenberger WT, Karlson U (1994) Microbial volatilization of selenium from soils and sediments. In: Frankerberger WT, Benson S (eds) Selenium in the environment. Marcel Dekker, New York, pp 369–387
Frost DV (1972) Two faces of selenium—can selenophobia be cured? In: Hemphill D (ed) CRC critical reviews in toxicology. CRC Press, Boca Raton, pp 467–514
Gaillardet J, Viers J, Dupré B (2003) Trace elements in river waters. In: Drever JI (ed) Surface and ground water, weathering and soils. In: Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 5. Elsevier, Oxford, pp 225–227
Galeas ML, Zhang LH, Freeman JL, Wegner M, Pilon-Smits EAH (2007) Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators. New Phytol 173:517–525
Gao XY, Hiroshi M (2005) Peptide-based nanotubes and their applications in bionanotechnology. Adv Mater 17:2037–2050
Gao XY, Zhang JS, Zhang LD (2002) Hollow sphere selenium nanoparticles: their in vitro anti hydroxyl radical effect. Adv Mater 14:290–293
Gao X, Gao T, Zhang L, Mater J (2003) Solution-solid growth of α-monoclinic selenium nanowires at room temperature. Chemistry 13:6–8
Garbisu C, Gonzalez S, Yang WH (1995) Physiological mechanisms regulating the conversion of selenite to elemental selenium by Bacillus subtilis. BioFactors 5:29–37
Garbisu C, Ishii T, Leighton T, Buchanan BB (1996) Bacterial reduction of selenite to elemental selenium. Chem Geol 132:199–204
Gardea-Torresdey J, Parsons G, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nanoletters 2:397–401. doi:10.1021/nl015673
Ge K, Yang GQ (1993) The epidemiology of selenium deficiency of endemic disease in China. Am J Clin Nutr Supp 57:259–263
Germ M, Stibilj V, Kreft I (2007) Metabolic importance of selenium for plants. Eur J Plant Sci Biotechnol 1(1):91–97
Gore F, Fawell J, Bartram J (2010) Too much or too little? A review of the conundrum of selenium. J Water Health 8(3):405–416
Gruère G, Narrod C, Abbott L (2011) Agriculture, food, and water nanotechnologies for the poor: opportunities and constraints. http://www.ifpri.org/sites/default/files/publications/ifpridp01064.pdf/27.1.2013. International Food Policy Research Institute (IFPRI), NW, Washington
Gupta UC, Gupta SC (2000) Selenium in soils and crops, its deficiencies in livestock and humans: implications for management. Comm Soil Sci Plant Anal 31:1791–1807
Hajiboland R (2012) Effect of micronutrient deficiencies on plants stress responses. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, Berlin, pp 283–329. doi:10.1007/978-1-4614-0634-1_16
Hanauer M, Lotz A, Pierrat S, Sonnichsen C, Zins I (2007) Separation of nanoparticles by gel electrophoresis according to size and shape. Nano Lett 7(9):2881–2885
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol 18:309–318
Hasanuzzaman M, Hossain MA, Masayuki F (2010) Selenium in higher plants: physiological role, antioxidant metabolism and abiotic stress tolerance. J Plant Sci 5:354–375. doi:10.3923/jps.2010.354.375
Hatfield DL, Berry MJ, Gladyshev VN (2012) Selenium: its molecular biology and role in human health, 3rd edn. Springer, Berlin
Haygarth PM (1994) Global importance and cycling of selenium. In: Frankenberger WT, Benson S (eds) Selenium in the environment. Marcel-Dekker, New York, pp 1–28
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210
Huang B, Zhang J, Hou J, Chen C (2003) Free radical scavening efficiency of nano-Se in vitro. Free Rad Biol Med 35(7):805–813. doi:10.1016/S0891-5849(03)00428-3
Hunter WJ, Manter DK (2008) Bio-reduction of selenite to elemental red selenium by Tetrathiobacter kashmirensis. Curr Microbiol 57:83–88. doi:10.1007/s00284-008-9160-6
Hurd DL, Kipling JJ (1964) Origins and growth of physical science. Part 2. Penguin Books, Harmondsworth
Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, Broadley MR, Motley AK, Fairweather-Tait SJ (2010) Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 91:923–931
Ingle A, Gade A, Pierrat S, Sonnichsen C, Rai MK (2008) Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr Nanosci 4:141–144
Johnson CC, Fordyce FM, Rayman MP (2010) Symposium on “Geographical and geological influences on nutrition”: factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc Nutr Soc 69:119–132
Juniper DT, Phipps RH, Ramos-Morales E, Bertin G (2008) Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J Anim Sci 86:3100–3109
Kabata-Pendias E (2011) Trace elements in soils and plants, 4th edn. CRC Press, Taylor & Francis, Boca Raton
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer, Berlin
Kabata-Pendias A, Sadurski W (2004) Trace elements and compounds in soil. In: Merian E, Anke M, Ihnat M, Stoepppler M (eds) Elements and their compounds in the environment, 2nd edn. Wiley-VCH, Weinheim, pp 79–99
Kim JS (2007) Antibacterial activity of Ag+ ion-containing silver nanoparticles prepared using the alcohol reduction method. J Ind Eng Chem 13(4):718–722
Kotrebai M, Birringer M, Tyson JF, Block E, Uden PC (2000) Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ion-pairing agents. Analyst 125:71–78
Kowshik M, Ashataputre S, Kharrazi S, Kulkarni SK, Paknikari KM, Vogel W, Urban J (2003) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14:95–100
Lakin HW, Dawidson DF (1967) The relation of the geochemistry of selenium to its occurrence in soil. In: Proceedings of the selenium in biomedicine, Westport, CT, 27
Lemly AD (1997) Ecosystem recovery following selenium contamination in a freshwater reservoir. Ecotoxicol Environ Saf 36:275–281
Liao CD, Hung WL, Jan KC, Yeh AI, Ho CT, Hwang LS (2010) Nano/sub-microsized lignan glycosides from sesame meal exhibit higher transport and absorption efficiency in Caco-2 cell monolayer. Food Chem 119:896–902
Lindberg P (1968) Selenium determination in plant and animal material, and in water. A methodological study. Acta Vet Scand Suppl 23:1–48
Lipiec E, Siara G, Bierla K, Ouerdane L, Szpunar J (2010) Determination of selenomethionine, selenocysteine, and inorganic selenium in eggs by HPLC-inductively coupled plasma mass spectrometry. Anal Bioanal Chem 397:731–741
Marmiroli N, Maestri E (2008) Health implications of trace elements in the environment and the food chain. In: Prasad MNV (ed) Trace elements as contaminants and nutrients: consequences in ecosystems and human health. Wiley, Hoboken, pp 23–53
Martens DA, Suarez DL (1998) Sequential extracion of selenium oxidation states. In: Frankenberger WT, Engberg RA (eds) Environmental chemistry of selenium. Marcel Dekker, New York, pp 61–79
McNeal JM, Balistrieri LS (1989) Geochemistry and occurrence of selenium: An overview. In: Jacob LW (ed) Selenium in agriculture and the environment. Soil Science Society of America Special Publication no. 23. American Society of Agronomy and Soil Science Society of America, Madison, WI, pp 1–14
Mikkelsen RL, Page AL, Bingham FT (1989) Factors affecting selenium accumulation by agricultural crops. In: Selenium in agriculture and the environment. Soil Sci Soc Am J Spec Publ 23:65–94
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar PV, Alam M, Sastry M, Kumar R (2001) Bioreduction of AuC4 − ions by the fungus Verticillium sp and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed 40(19):3585–3588
Muniz-Naveiro O, Dominguez-Gonzalez R, Bermejo-Barrera A, Bermejo-Barrera P, Cocho JA, Fraga JM (2007) Selenium speciation in cow milk obtained after supplementation with different selenium forms to the cow feed using liquid chromatography coupled with hydride generation atomic fluorescence spectrometry. Talanta 71:1587–1593
Novoselov SV, Rao M, Onoshko NV, Zhi H, Kryukov GV, Xiang Y, Weeks DP, Hatfield DL, Gladyshev VN (2002) Selenoproteins and selenocysteine insertion system in the model plant system, Chlamydomonas reinhardtii. EMBO J 21:3681–3693
Nriagu JO (1989) Global cycling of selenium. In: Ihnat Milan (ed) Occurrence and distribution of selenium. CRC Press, Boca Raton, pp 327–340
Ogi T, Saitoh N, Nomura T, Konishi Y (2010) Room-temperature synthesis of gold nanoparticles and nanoplates using Shewanella algae cell extract. J Nanopart Res. doi:10.1007/s11051-009-9822-8
Oremland RS, Herbal MJ, Blum JS, Langely S, Beveridge TJ, Ajayan PM, Sutto T, Ellis AV (2004) Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl Environ Microbiol 70:52–60
Pastoriza-Santos I, Liz-Marzan LM (2000) Reduction of silver nanoparticles in DMF formation of monolayers and stable colloids. Pure Appl Chem 72(1–2):83–90
Pauling L (1932) The nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J Am Chem Soc 54(9):3570–3582. doi:10.1021/ja01348a011
Pearce CI, Coker VS, Charnock JM, Pattrick RAD, Mosselmans JFW, Law N, Beveridge TJ, Lloyd JR (2008) Microbial manufacture of calcogenide-based nanoparticles via reduction of selenite using Veillonella atypica: an in situ EXAFS study. Nanotechnology. doi:10.1088/0957-4484/19/15/155603
Pilon-Smits EAH, Colin FQ (2010) Selenium metabolism in plants. In: Hell R, Mendel R-R (eds) Cell biology of metals and nutrients. Springer, Berlin. Plant Cell Monogr 17:225–241. doi:10.1007/978-3-642-10613-2_10
Prakash NT, Sharma N, Prakash R, Raina K, Fellowes J, Pearce C, Lloyd J, Pattrick R (2010) Aerobic microbial manufacture of nanoscale selenium: exploiting nature’s bio-nanomineralization potential. Biotechnol Lett 31:1857–1862
Prokisch J, Zommara M (2008) Process for producing elemental selenium nanospheres PCT/IB2008/052838 date of receipt: 15 July 2008 receiving office: International Bureau of the World intellectual property organization your reference: P104315
Prokisch J, Széles E, Kovács B, Darόczy L, Zommara M (2008) Formation of metal selenium nanospheres in bacteria: is it a possible detoxification mechanism? Cer Res Comm 36:947–995
Pyrzynska K (1995) Solid phase extraction for preconcentration and separation of selenium. Solvent Extr Ion Exch 13:369–389
Rai M, Yadav A, Gade A (2008) Current trends in phytosynthesis of metal nanoparticles. Crit Rev Biotechnol 28(4):277–284
Rai M, Gade A, Yadav A (2011) Biogenic nanoparticles: an introduction to what they are, how they are synthesized and their applications. In: Rai M, Duran N (eds) Metal nanoparticles in microbiology. Springer, Berlin, pp 1–14. doi:10.1007/978-3-642-18312-6-1
Rani N, Dhillon KS, Dhillon SK (2005) Critical levels of selenium in different crops grown in an alkaline silty loam soil treated with selenite-Se. Plant Soil 277:367–374
Rayman MP (2004) The use of high-selenium yeast to raise selenium status: how does it measure up? Br J Nutr 92:557–573
Rayman MP (2012) Selenium and human health. The Lancet, 379(9822):1256–1268. doi:10.1016/S0140-6736(11)61452-9
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100:238–253
Reilly C (2006) Selenium in food and health, 2nd edn. Springer, Berlin
Reimann C, de Caritat P (1998) Chemical elements in the environment. Springer, Berlin
Reyes LH, Mar JL, Rahman GM, Seybert B, Fahrenholz T, Kingston HM (2009) Simultaneous determination of arsenic and selenium species in fish tissues using microwave assisted enzymatic extraction and ion chromatography inductively coupled plasma mass spectrometry. Talanta 78:983–990
Sau TK, Rogach AL (2010) Nonspherical noble metal nanoparticles: colloid-chemical synthesis and morphology control. Adv Mater 22(16):1781–1804
Schrauzer GN (2004) Selenium. In: Merian E, Anke M, Ihnat M, Stoeppler M (eds) Elements and their compounds in the environment, 2nd edn. Wiley-VCH, Weinheim, pp 1365–1406
Shaligram NS, Bule M, Bhambure RM, Singhal RS, Singh SK, Szakacs G, Pandey A (2009) Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem 44:939–948
Shanker AK (2006) Countering UV-B stress in plants: does selenium have a role? Plant Soil 282:21–26
Shehata SA, El-Ramady H (2012) Micronutrients: uptake and its roles in plants. Print house Fares Abd El-Hameed for Printing and Publishing. ISBN-No 978-977-403-505-0 (in Arabic)
Shriver DF, Atkins PW (1999) Inorganic chemistry, 3rd edn. Oxford University Press, Oxford
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 44:1133–1138. doi:10.1007/s00449-008-0224-6
Stadtman TC (1990) Selenium biochemistry. Annu Rev Biochem 59:111–127
Steinnes E (2003) Biogeochemiacl cycling of iodine and selenium and potential geomedicval relevance. In: Skinner HC, Berger AR (eds) Geology and health: closing gap. Oxford University Press, Oxford, pp 57–60
Tamaoki M, Freeman JL, Pilon-Smits EAH (2008) Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis [W][OA]. Plant Physiol 146:1219–1230
Terry N, Zayed AM, de Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomedicine 6(2):257–262
Turakainen M (2007) Selenium and its effects on growth, yield and tuber quality in potato. University of Helsinki, Helsinki. ISBN 9521034661
U.S. Geological Survey (USGS) (2013) Mineral commodity summaries 2013. http://minerals.usgs.gov/minerals/pubs/mcs/2013/mcs2013.pdf. 26.1.2013
Wang D, Alfthan G, Aro A (1994) The impact of selenium fertilization on the distribution of selenium in rivers in Finland. Agric Ecosyt Environ 50:133–149
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42:1524–1533
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937. doi:10.1093/jxb/erh192
White PJ, Bowen HC, Marshall B, Broadley MR (2007) Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann Bot 100:111–118
World Health Organization (WHO) (2011) Selenium in drinking-water—background document for development of WHO guidelines for drinking-water quality. WHO/HSE/WSH/10.01/14
Yada R (2009) Nanotechnology: a new frontier in foods, food packaging, and nutrient delivery. In: Pray L, Yaktine A (eds) Nanotechnology in food products. National Academies Press, Washington DC
Zayed AM, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–289
Zha LY, Xu ZR, Wang MQ, Gu LY (2008) Chromium nanoparticle exhibits higher absorption efficiency than chromium picolinate and chromium chloride in Caco-2 cell monolayers. J Anim Physiol Anim Nutr 92:131–140
Zhang YQ, Frankenberger WT Jr (2005) Removal of selenium from river water by a microbial community enhanced with Enterobacter taylorae in organic carbon coated sand columns. Sci Total Environ 346:280–285
Zhang Y, Gladyshev VN (2010) General trends in trace element utilization revealed by comparative genomic analyses of Co, Cu, Mo, Ni and Se. J Biol Chem 285:3393–3405
Zhang JS, Gao XY, Zhang LD, Bao YP (2001) Biological effects of a nano red elemental selenium. BioFactors 15:27–38
Zhang J, Wang H, Bao Y, Zhang L (2004) Nano red elemental selenium has no size effect in the induction of seleno-enzymes in both cultured cells and mice. Life Sci 75:237–244. doi:10.1016/j.lfs.2004.02.004
Zhang J, Wang H, Yan X, Zhang L (2005) Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci 76:1099–1109
Zhang J, Wang X, Xu T (2008) Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice. Toxicol Sci 101(1):22–31. doi:10.1093/toxsci/kfm221
Zhao C, Ren J, Xue C, Lin E (2005) Study on the relationship between soil selenium and plant selenium uptake. Plant Soil 277:197–206
Zoller WH, Reamer DC (1976) Selenium in the atmosphere. In: Proceedings of the symposium on selenium-tellurium in the environment. Industrial Health Foundation, Pittsburgh, PA, pp 54–66
Acknowledgments
El-Ramady and Abd Alla acknowledge the Hungarian Ministry of Education and Culture (Hungarian Scholarship Board, HSB and the Balassi Institute) for funding and supporting this work. He also thanks Prof. Eric Lichtfouse for his support and revising this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Ramady, H.R., Domokos-Szabolcsy, É., Abdalla, N.A. et al. Selenium and nano-selenium in agroecosystems. Environ Chem Lett 12, 495–510 (2014). https://doi.org/10.1007/s10311-014-0476-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-014-0476-0