Abstract
Habitat structure in Oku-Nikko, Japan, has been substantially modified by the overabundance of sika deer (Cervus nippon). A deer-proof fence (15.0 km and 900 ha) was constructed in 2001 to conserve vegetation. Although the understory inside the fence is dominated by Sasa nipponica (hereafter, Sasa), an important forage plant for deer, that outside the fence is dominated by Aster ageratoides leiophyllus (hereafter, Aster), an unpalatable plant to deer, and, partly, by bare floor. In this study, we examined the effects of deer on ground-dwelling insects and earthworms, the primary food resources of raccoon dogs (Nyctereutes procyonoides), and, thus, the bottom-up cascading effects of the herbivore on the omnivorous carnivore. Between July and September 2008, we examined the abundance of insects and earthworms by pitfall trapping and hand-sorting methods, respectively, both inside and outside the fence. The abundance of earthworms and insects (Scarabaeidae and Rhaphidophoridae) was higher on forest floors with Aster and/or bare floors outside the fence than on those with Sasa inside the fence. These results indicate that the increasing deer population in this area probably increased the number of these invertebrates outside the fence by modifying understory vegetation and/or depositing dung. Furthermore, the sighting rates of raccoon dogs obtained by spotlight counts were greater outside than inside the fence, suggesting that deer probably exert bottom-up cascading effects on raccoon dogs, at least during May to November, when the invertebrates are predominantly fed on by the omnivorous carnivore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indirect effects occur when the effect of one species, the donor, is transmitted through a second species, the transmitter, to a third species, the receiver (Morin 1999). Trophic cascades, defined as reciprocal predator–prey effects that alter the abundance, biomass, or productivity of a population, community, or trophic level across more than one link in a food web, are indirect effects of one trophic level on lower levels (i.e., top-down cascade) or upper levels (i.e., bottom-up cascade; Hunter and Price 1992; Dyer and Letourneau 1999; Pace et al. 1999). Although trophic cascades are predominantly discussed in aquatic ecology studies, use of the term in terrestrial ecology studies has increased in recent years (Persson 1999; Schmitz et al. 2000). However, studies on bottom-up cascades still focus on invertebrates, and studies focusing on mammals on higher trophic levels are few (Strong 1992; Pace et al. 1999; Schmitz et al. 2000; Kagata and Ohgushi 2006).
In recent decades, deer have expanded their range and dramatically increased in abundance worldwide (Cote et al. 2004). The overabundance of deer has affected forest ecosystems in various ways; for example, the effects of deer on vegetation are particularly well known (Rooney and Waller 2003; Cote et al. 2004; Takatsuki 2009). Moreover, it has been shown that deer exert cascading effects on other animals both by directly competing for resources with other herbivores and by indirectly modifying the composition and physical structure of habitats; for example, browsing by deer affects the population and community composition of many invertebrates, birds, and small mammals (Flowerdew and Ellwood 2001; Rooney and Waller 2003; Cote et al. 2004; Shibata and Hino 2009). The effects of deer on other animals at lower trophic levels are expected to exert bottom-up cascading effects on predators feeding on the animals. For example, it has been demonstrated that in an African savanna, removal of large herbivorous mammals causes an increase in the density of small mammals, which probably contribute to a pronounced increase in the number of snakes belonging to the higher trophic levels (McCauley et al. 2006). Therefore, one would expect that deer also exert indirect effects on the mammals at higher trophic levels through such bottom-up cascades.
In this study, as a first step in investigating the interaction between deer and other mammals, we examined the bottom-up cascading effects of sika deer (Cervus nippon) on raccoon dogs (Nyctereutes procyonoides) in Oku-Nikko, Japan, for the following reasons. The raccoon dog is an omnivorous carnivore belonging to the higher trophic levels in Japan. Previous studies on the composition of the diet of raccoon dogs in the mountainous areas of Japan have shown that they feed on fruit, insects, earthworms, and small mammals (Sasaki and Kawabata 1994; Yamamoto 1994). In Oku-Nikko, raccoon dogs have been shown to feed primarily on ground-dwelling insects and earthworms from spring to autumn (Seki 2011). It has recently been demonstrated that deer overabundance affects the abundance and/or the numbers of ground-dwelling beetles and soil biota by modification of habitat structure (Suominen et al. 1999a, b, 2003; Wardle et al. 2001; Bardgett and Wardle 2003; Melis et al. 2006, 2007). Because habitat structure in Oku-Nikko has been substantially modified by deer overabundance (Koganezawa and Satake 1996; Hasegawa 2008), the abundance of ground-dwelling insects and earthworms could change, potentially exerting bottom-up cascading effects on raccoon dogs.
In this paper, we discuss the effects of deer on ground-dwelling insects and earthworms, which are the primary food resources of raccoon dogs from spring to autumn, and thus, the bottom-up cascading effects of deer on raccoon dogs.
Materials and methods
Study area
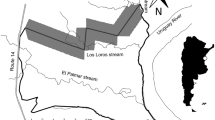
The study was conducted around Odashirohara and Senjugahara in Nikko National Park, Tochigi Prefecture, Central Honshu, Japan, which is located in the cool temperate zone (36°45′N, 139°25′E; 1,290–1,410 m elevation). In this area, a deer-proof fence was constructed by the Prefecture in 1998 (ca. 3.0 km and 50 ha) and another by the Ministry of the Environment in 2001 (ca. 15.0 km and 900 ha); we used the latter fence in this study. As the size of the home range of raccoon dogs in this area has been shown to range from 45 to 386 ha (Seki and Koganezawa 2011), the area enclosed by the fence is considered large enough for raccoon dogs. In addition, we observed that medium-sized carnivores such as raccoon dogs and red foxes (Vulpes vulpes) moved freely through several points of the fence. Therefore, we believe the fence is no barrier to movement of medium-sized carnivores. Spotlight counts of sika deer conducted from April to November 2008 in this area (Wildlife Management Laboratory of Utsunomiya University, unpublished results; details of the method are given by Koganezawa and Li 2002) gave mean numbers of deer observed per kilometer of 0.01 ± 0.05 (SD) inside the fence and 5.78 ± 4.11 (SD) outside the fence (n = 33). Thus, we consider the inside of the fence as the low-deer-density area and the outside of the fence as the high-deer-density area. The vegetative canopy of the study area is dominated by deciduous broad-leaved trees, for example Quercus crispula, Ulmus davidiana, and Betula platyphylla, and by the conifer Lalix kaempferi. The understory inside the fence is dominated by dwarf bamboo (Sasa nipponica), an important forage plant for sika deer (Takatsuki 1983, 1986; Yokoyama and Shibata 1998), and that outside the fence is dominated by plants unpalatable to deer, for example Aster ageratoides leiophyllus, and, partly, by bare floor (Koganezawa and Satake 1996; Hasegawa 2008). The Nikko Weather Station (1,292 m) reported that from 1971 to 2000 the mean annual temperature and the mean annual precipitation in this area were 6.7°C and 2,103 mm, respectively.
Sampling of insects and earthworms
Insect and earthworm sampling plots were established in sites of 6 forest types: forest floors with Sasa nipponica (hereafter, Sasa; inside the fence), forest floors with Aster ageratoides leiophyllus (hereafter, Aster; outside the fence) and bare floors (outside the fence) in the forest dominated by Quercus crispula (hereafter, Quercus) and Lalix kaempferi (hereafter, Lalix). The inclination of each plot was flat, and the percentage of canopy closure of each plot was ≥80%.
Insect sampling was conducted by pitfall trapping with transparent plastic cups (volume 224 ml, height 90 mm, top diameter 65 mm, and bottom diameter 45 mm) from July to September in 2008. Each trap was covered with a wooden board (10 × 10 cm, approximately 3 cm above the trap) to minimize capture of non-target species, provide cover from rain, and prevent predation from larger animals. We established a 10 × 10-m plot in each site and installed 16 traps at 2-m intervals, in a square pattern, in each plot. Trapping was conducted 6 times during the study period, each time for 3 days. The trapped insects were stored in vials containing 70% ethanol and classified according to family. The insects were dried for 72 h at 80°C, and their weight was measured. We focused our analysis on trapped insects belonging to Coleoptera and Orthoptera, which are predominantly fed on by raccoon dogs from spring to autumn in mountainous areas (Sasaki and Kawabata 1994; Seki 2011).
For earthworm sampling, we established a 5 × 5-m plot in each site and set up 16 quadrates of 25 × 25 cm at 1-m intervals, in a square pattern, in each plot. Sampling was conducted by the hand-sorting method, by digging the soil to a depth of 10 cm at each quadrate in September 2008. The wet weight of the collected earthworms was measured.
Density of raccoon dogs
To compare the relative densities of raccoon dogs inside and outside the fence, we analyzed data from spotlight counts (n = 260) conducted from May to November during 2002–2010 by the Wildlife Management Laboratory of Utsunomiya University. Although the spotlight counts were conducted for monitoring of deer, other mammals were also recorded in the survey. The spotlight surveys were conducted by 1 driver and at least 2 observers from a vehicle driven at a speed of 10–14 km h−1 over a fixed 9.0 km route (3.4 km inside the fence and 5.6 km outside the fence). The observers scanned the fields with a 12 V 100 W hand-held spotlight from approximately 1.5 m above ground level. The car was stopped when shining eyes were seen and then placed perpendicular to the initial position of the animal to measure the distance. The perpendicular distance of each animal from the center of the road was then measured by use of a laser range finder (Yardage Pro Sport 450 Laser Rangefinder; Bushnell, USA).
Statistical analysis
In each plot, the abundance of insects was defined as the mean dry weight (g) from the 6 trapping periods; that of earthworms as the mean wet weight (g) from 16 quadrates. We used the Steel–Dwass test (Nagata and Yoshida 1997) to determine the significance of differences in the abundance of insects and earthworms among 3 forest floor types in both Quercus and Lalix sites. We computed sighting rates as the number of raccoon dogs observed per 10 km in each survey. We determined the significance of differences of the sighting rates between inside and outside the fence by use of the Wilcoxon signed-rank test. Because when animals are located >70 m from the census route it is difficult to accurately measure distance, because of the limited line of sight (Koganezawa and Li 2002), we only analyzed data for raccoon dogs observed ≤70 m from the road. Because the perpendicular distance for a raccoon dog observed outside the fence was not recorded, we excluded the data from the analysis. In addition, the probability of detection of raccoon dogs might be different inside and outside the fence because of the different height of Sasa and Aster. Therefore, to minimize the bias of oversight, we also examined the significant differences of the sighting rates of raccoon dogs observed ≤10 m between inside and outside the fence, by use of the Wilcoxon signed-rank test.
All statistical analysis was performed using R ver. 2.8.1 software (R Development Core Team 2009). Significance was set at P < 0.05 for all statistical analysis.
Results
The trapped insects belonged to Scarabaeidae, Carabidae and Silphidae (Coleoptera) and Rhaphidophoridae (Orthoptera). The abundance of these insects is shown in Fig. 1. Although the abundance of Carabidae and Silphidae was not significantly different between forest types (Steel–Dwass test, all, P > 0.05), that of Scarabaeidae was higher for bare floor than for Sasa in Quercus (Quercus, Sasa vs. bare floor, P = 0.020; others, P > 0.05), and that of Rhaphidophoridae was higher for Aster and bare floor than for Sasa in Lalix (Lalix, Sasa vs. Aster, P = 0.006; Sasa vs. bare floor, P = 0.006; others, P > 0.05).
Abundance of insects on forest floors with Sasa nipponica (Sasa; inside the deer-proof fence) and on those with Aster ageratoides leiophyllus (Aster; outside the fence) and bare floors (outside the fence) in the forest dominated by Quercus crispula and Lalix kaempferi in Oku-Nikko, Japan. Values are means (+SE) from 6 trapping periods in each forest type
The abundance of earthworms was higher for Aster than for Sasa in Quercus (Quercus, Sasa vs. Aster, P = 0.006; others, P > 0.05) (Fig. 2).
Abundance of earthworms on forest floors with Sasa nipponica (Sasa; inside the deer-proof fence) and on those with Aster ageratoides leiophyllus (Aster; outside the fence) and bare floors (outside the fence) in the forest dominated by Quercus crispula and Lalix kaempferi in Oku-Nikko, Japan. Values are means (+SE) for 16 quadrates of 25 × 25 cm in each forest type
We recorded observations of 3 and 64 raccoon dogs within the 0–70 m transect inside and outside the fence, respectively. Of these observations, 2 and 18 raccoon dogs were observed within the 0–10 m transect inside and outside the fence, respectively. The sighting rates of raccoon dogs were greater outside than inside the fence within both the 0–70 and 0–10 m transects (Wilcoxon signed-rank test, 0–70 m transect: V = 78, P < 0.001; 0–10 m transect: V = 23, P = 0.018) (Fig. 3).
Discussion
In Oku-Nikko, the population of sika deer has been increasing since 1984, and bamboo undergrowth outside the fence has been eliminated by heavy grazing of deer (Koganezawa and Satake 1996; Hasegawa 2008). The forest understory outside the fence, particularly around Senjugahara, is now almost dominated by Aster and, partly, by bare floor (Hasegawa 2008). Although the abundance of Carabidae and Silphidae was not different significantly between forest types, that of the earthworms and the other insects (Scarabaeidae and Rhaphidophoridae) was higher for Aster and/or bare floor outside the fence than for Sasa inside the fence (Figs. 1, 2). Because there was only 1 plot for each of the 3 types of vegetation in each forest in this study, it is difficult to use our results as a representative of each forest type in this area. Nevertheless, several possible, non-mutually exclusive, explanations can be proposed for the greater number of these invertebrates outside the fence.
It has been pointed out that changes in vegetation caused by deer contribute most to be the different invertebrate assemblage and/or abundance (Rambo and Faeth 1999; Suominen et al. 1999a, b; Stewart 2001; Melis et al. 2006, 2007). By modifying vegetation, deer may affect invertebrates in several ways:
-
by directly competing with herbivorous invertebrates for plant food;
-
by changing the abundance of the prey of omnivorous and carnivorous invertebrates; and
-
by altering elements of the microclimate, for example temperature and humidity (Stewart 2001; Suominen et al. 2003).
Seki and Koganezawa (2010) demonstrated that Aster but not Sasa is probably a good resource for earthworms, which is probably the reason for the greater abundance of earthworms outside the fence in this study area. Although changes in understory vegetation are also believed to contribute to the greater abundance of Rhaphidophoridae outside the fence in the Lalix forest, the exact factors remain unclear. Reasons for the different abundance of Rhaphidophoridae between the Quercus and Lalix forests outside the fence also remain unclear. To elucidate these factors, further studies on aspects such as food habits and microhabitat selection of Rhaphidophoridae are needed.
In addition to direct modification of vegetation by deer, the copious quantities of feces produced by deer will attract a diverse community of dung-associated invertebrates (Stewart 2001). In this study, the Scarabaeidae captured outside the fence consisted of dung beetles (mainly the genus Geotrupes), but these were not captured inside the fence (Fig. 1). Because it has been demonstrated that these dung beetles utilize the feces of sika deer (Koike et al. 2006), the amount of deer dung might contribute to the greater abundance of dung beetles outside the fence in the Quercus forest. The different abundances of the dung beetles in the Quercus and Lalix forests outside the fence could be because in Nikko deer preferentially barked the Quercus trees and avoided the Lalix trees (Kanzaki et al. 1998). This could affect the extent of utilization of Quercus and Lalix forests by deer, which might have contributed to the different abundances of the dung beetles in these forests.
Another possible factor which could cause the greater abundance of the invertebrate population outside the fence is lower predation pressure. Although it has been reported that these invertebrates are frequently eaten not only by raccoon dogs, but also by red foxes, Japanese martens (Martes melampus), and Japanese badgers (Meles anakuma) (Kondo 1980; Yamamoto 1991, 1994; Kaneko et al. 2006), the density of these predators tends to be higher outside the fence than inside (Seki 2011). This suggests that predation pressure is probably higher outside the fence than inside. Therefore, we infer that the major variable driving the greater abundance of invertebrates outside the fence is not the low predation pressure but, rather, modification of the understory vegetation by sika deer and the different abundance of deer dung. These results also indicate that the abundance of invertebrates outside the fence was probably underestimated, because we did not consider the higher predation pressure outside the fence. Therefore, the abundance of Carabidae could actually be higher outside the fence than inside, because raccoon dogs frequently eat Carabidae in this area (Seki 2011). The abundance of Carabidae has been shown to be increased by the grazing of deer in some areas (Suominen et al. 1999b, 2003; Melis et al. 2006, 2007). In Japan, however, it has been shown that the reduction of bamboo volume because of browsing by deer did not affect the diversity of ground-carabid-beetle assemblage but did affect its structure (Ueda et al. 2009). Thus, in further studies, we should extend our analysis to the species level to assess the effects of deer on Carabidae in more detail.
In Oku-Nikko, insects (particularly Carabidae, Scarabaeidae, and Rhaphidophoridae) and earthworms are the primary food resources of raccoon dogs from May to November: the mean relative frequency of occurrence (RFO) of the invertebrates in fecal samples from raccoon dogs in this period was 61% (SE 5, range 44–78%) (Seki 2011). Therefore, we infer that deer overabundance increased the abundance of the primary food resources of raccoon dogs from May to November outside the fence in the area studied. However, in October–November, raccoon dogs also frequently feed on fruit (particularly, Actinidia arguta, Vitis coignetiae, and Malus toringo; RFO = 32–37%) in this area (Seki 2011). Sika deer could negatively affect the consumption of fruit by raccoon dogs by causing tree death by bark stripping and by competing for fallen fruit. However, the effects of sika deer on fruit consumed by raccoon dogs are believed to be low, for two reasons. First, of the tree species of which raccoon dogs consume fruits, Malus toringo was confirmed to be barked by sika deer in Oku-Nikko, but the tree has been shown not to be one of their preferred foods (Kanzaki et al. 1998). Second, many studies have shown that sika deer feed primarily on graminoids, forbs, browses, barks, and twigs, but rarely on fruit, even in autumn (Takatsuki 1983, 1986; Asada and Ochiai 1996; Yokoyama et al. 1996, 2000; Jayasekara and Takatsuki 2000; Takahashi and Kaji 2001; Campos-Arceiz and Takatsuki 2005). Furthermore, even on the few occasions when these deer feed on fruit, its composition is dominated by acorns (Asada and Ochiai 1996; Jayasekara and Takatsuki 2000; Takahashi and Kaji 2001). The effects of the sika deer on animals that feed on acorns (e.g., Asiatic black bears (Ursus thibetanus); Koike et al. 2008) might be significant when there are only a few acorns in the area. However, because acorns are not a food resource for raccoon dogs in mountainous areas, including Oku-Nikko (Sasaki and Kawabata 1994; Koike et al. 2008; Seki 2011), we believe the effects of the competition between the sika deer and raccoon dogs for fallen fruit are not significant. In conclusion, the overall effect of sika deer on the primary food resources of raccoon dogs during the period between May and November is positive in Oku-Nikko.
Our results also indicate that the greater abundance of the primary food resources of raccoon dogs outside the fence caused by deer probably contributed to the greater density of raccoon dogs. Several possible explanations for this, which are not mutually exclusive, can be proposed. First, the sighting rates of raccoon dogs by spotlight counts were greater outside than inside the fence (Fig. 3). Second, in spotlight counts (n = 18), conducted along the same route as that in this study, from July to October 1979, when the density of deer was still low (Koganezawa and Satake 1996), no raccoon dogs was observed (M. Koganezawa, unpublished results). This suggests that the density of raccoon dogs was probably low before the deer population increased. Koganezawa (1983) and Koganezawa and Kurokawa (1983) indicated that only a few raccoon dogs inhabited this area in 1979–1980. Because only 3 raccoon dogs were observed inside the fence in this study, their current density is regarded as low, which is the same as their density before the deer density increased. However, the greater sighting rates of raccoon dogs outside the fence (Fig. 3) and the fact that the probability of detection of latrines created by raccoon dogs outside the fence was 11 times greater than that inside the fence (Seki 2011) indicate that the population of raccoon dogs probably increased after the density of deer increased. We conclude that sika deer exert bottom-up cascading effects on raccoon dogs, at least during the period between May and November, in Oku-Nikko.
In this study, we have demonstrated the positive effects of sika deer on the populations of ground-dwelling insects and earthworms, the primary food resources of raccoon dogs, particularly from May to November, exerting bottom-up cascading effects on raccoon dogs, at least during this period. Although Stewart (2001) has drawn attention to possible effects on birds and bats at higher trophic levels, as a result of the increase in their prey brought about by deer, there is little evidence of cascading effects on vertebrates, particularly on mammals at higher trophic levels. Future research on the effects of deer should, therefore, focus on whether changes in the abundance of such prey because of the presence of deer alter the population of vertebrates at higher trophic levels.
References
Asada M, Ochiai K (1996) Food habits of sika deer on the Boso Peninsula, central Japan. Ecol Res 11:89–95
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Campos-Arceiz A, Takatsuki S (2005) Food habits of sika deer in the Shiranuka Hills, eastern Hokkaido: a northern example from the north-south variations in food habits in sika deer. Ecol Res 20:129–133
Cote SD, Rooney TP, Tremblay JP, Dussault C, Waller DM (2004) Ecological impacts of deer overabundance. Annu Rev Ecol Evol Syst 35:113–147
Dyer LA, Letourneau DK (1999) Relative strengths of top-down and bottom-up forces in a tropical forest community. Oecologia 119:265–274
Flowerdew JR, Ellwood SA (2001) Impacts of woodland deer on small mammal ecology. Forestry 74:277–287
Hasegawa J (2008) Changes in nature of Tochigi Prefecture. Self publication, Tochigi (in Japanese)
Hunter MD, Price PW (1992) Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73:724–732
Jayasekara P, Takatsuki S (2000) Seasonal food habits of sika deer population in the warm temperature forests of the westernmost part of Honshu, Japan. Ecol Res 15:153–157
Kagata H, Ohgushi T (2006) Bottom-up trophic cascades and material transfer in terrestrial food webs. Ecol Res 21:26–34
Kaneko Y, Maruyama N, Macdonald DW (2006) Food habits and habitat selection of suburban badgers (Meles meles) in Japan. J Zool 20:78–89
Kanzaki N, Maruyama N, Koganezawa M, Taniguchi M (1998) Tree barking by sika deer in Nikko, Tochigi prefecture. Wildl Conserv Jpn 3:107–117 (in Japanese with English summary)
Koganezawa M (1983) Altitudinal distribution of middle-sized mammals in Nikko area, Tochigi Prefecture (I). Mem Tochigi Pref Mus 1:39–66 (in Japanese with English summary)
Koganezawa M, Kurokawa M (1983) Altitudinal distribution of middle-sized mammals in Nikko area, Tochigi Prefecture (II)—focused on the winter distribution and the food habits of the red fox (Vulpes vulpes). Mem Tochigi Pref Mus 1:67–82 (in Japanese with English summary)
Koganezawa M, Li Y (2002) Sika deer response to spotlight counts: implications for distance sampling of population density. Mammal Study 27:95–99
Koganezawa M, Satake C (1996) Effects of grazing by sika-deer on the vegetation of Oku-Nikko and their management. Trans Nat Found Proj 5:57–66 (in Japanese with English summary)
Koike S, Kato M, Morimoto H, Furubayashi K (2006) Study of dung beetles in feces of sika deer, Cervus nippon, in Tanzawa Mountains, central Japan. Wildl Conserv Jpn 10:45–60 (in Japanese with English summary)
Koike S, Morimoto H, Goto Y, Kozakai C, Yamazaki K (2008) Frugivory of carnivores and seed dispersal of fleshy fruits in cool-temperate deciduous forests. J For Res 13:215–222
Kondo T (1980) Food habits of the red fox (Vulpes vulpes japonica) and the Japanese marten (Martes melampus melampus). Bull Osaka Kyoiku Univ 29:19–23 (in Japanese with English summary)
McCauley DJ, Keesing F, Young TP, Allan BF, Pringle RM (2006) Indirect effects of large herbivores on snakes in an African Savanna. Ecology 87:2657–2663
Melis C, Buset A, Aarrestad PA, Hanssen O, Meisingset EL, Andersen R, Moksnes A, Roskaft E (2006) Impact of red deer Cervus elaphus grazing on bilberry Vaccinium myrtillus and composition of ground beetle (Coleoptera, Carabidae) assemblage. Biodivers Conserv 15:2049–2059
Melis C, Sundby M, Andersen R, Moksnes A, Pedersen B, Roskaft E (2007) The role of moose Alces alces L. in boreal forest: the effect on ground beetles (Coleoptera, Carabidae) abundance and diversity. Biodivers Conserv 16:1321–1335
Morin PJ (1999) Community ecology. Blackwell, Oxford
Nagata Y, Yoshida M (1997) The basis of multiple comparison procedure. Scientist, Tokyo (in Japanese)
Pace ML, Cole JJ, Carpenter SR, Kitchell JF (1999) Trophic cascades revealed in diverse ecosystems. Trends Ecol Evol 14:483–488
Persson L (1999) Trophic cascades: abiding heterogeneity and the trophic level concept at the end of the road. Oikos 85:385–397
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rambo JL, Faeth SH (1999) Effects of vertebrate grazing on plant and insect community structure. Conserv Biol 13:1047–1054
Rooney TP, Waller DM (2003) Direct and indirect effects of white-tailed deer in forest ecosystems. For Ecol Manag 181:165–176
Sasaki H, Kawabata M (1994) Food habits of the raccoon dog Nyctereutes procyonoides viverrinus in a mountainous area of Japan. J Mammal Soc Jpn 19:1–8
Schmitz OJ, Hamback PA, Beckrman AP (2000) Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am Nat 155:141–153
Seki Y (2011) Trophic interaction between the sika deer and the raccoon dog in Oku-Nikko, Japan. PhD thesis, Tokyo University of Agriculture and Technology (in Japanese)
Seki Y, Koganezawa M (2010) Factors influencing the increase in earthworms outside deer-proof fences in Oku-Nikko, central Japan: the influence of the modification of understory vegetation by sika deer. J Jpn For Soc 92:241–246 (in Japanese with English summary)
Seki Y, Koganezawa M (2011) Factors influencing winter home ranges and activity patterns of raccoon dogs Nyctereutes procyonoides in a high-altitude area of Japan. Acta Theriol 56:171–177
Shibata E, Hino T (2009) Ecology of sika deer and forest ecosystem of Mt. Ohdaigahara. Tokai University Press, Kanagawa (in Japanese)
Stewart AJA (2001) The impact of deer on lowland woodland invertebrates: a review of the evidence and priorities for future research. Forestry 74:259–270
Strong DR (1992) Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73:747–754
Suominen O, Danell K, Bergstrom R (1999a) Moose, trees, and ground-living invertebrates: indirect interactions in Swedish pine forests. Oikos 84:215–226
Suominen O, Danell K, Bryant JP (1999b) Indirect effects of mammalian browsers on vegetation and ground-dwelling insects in an Alaskan floodplain. Ecoscience 6:505–510
Suominen O, Niemela J, Martikainen P, Niemela P, Kojola I (2003) Impact of reindeer grazing on ground-dwelling Carabidae and Curculionidae assemblages in Lapland. Ecography 26:503–513
Takahashi H, Kaji K (2001) Fallen leaves and unpalatable plants as alternative foods for sika deer under food limitation. Ecol Res 16:257–262
Takatsuki S (1983) The importance of Sasa nipponica as a forage for sika deer (Cervus nippon) in Omote-Nikko. Jpn J Ecol 33:17–25
Takatsuki S (1986) Food habits of sika deer on Mt. Goyo, northern Honshu. Ecol Res 1:119–128
Takatsuki S (2009) Effects of sika deer on vegetation in Japan: a review. Biol Conserv 142:1922–1929
Ueda A, Hino T, Ito H (2009) Relationships between browsing on dwarf bamboo (Sasa nipponica) by sika deer and the structure of ground beetle (Coleoptera: Carabidae) assemblage. J Jpn For Soc 91:111–119 (in Japanese with English summary)
Wardle DA, Barker GM, Yeates GW, Bonner KI, Ghani A (2001) Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Yamamoto Y (1991) Food habits of Meles meles anakuma in Mt. Nyugasa, Nagano Pref., Japan. Nat Environ Sci Res 4:73–83 (in Japanese with English summary)
Yamamoto Y (1994) Comparative analyses on food habits of Japanese marten, red fox, badger and raccoon dog in Mt. Nyugasa, Nagano Prefecture, Japan. Nat Environ Sci Res 7:45–52 (in Japanese with English summary)
Yokoyama S, Shibata E (1998) Characteristics of Sasa nipponica grassland as a summer forage resource for sika deer on Mt. Ohdaigahara, central Japan. Ecol Res 13:193–198
Yokoyama S, Koizumi T, Shibata E (1996) Food habits of sika deer as assessed by fecal analysis in Mt. Ohdaigahara, central Japan. J For Res 1:161–164
Yokoyama M, Kaji K, Suzuki M (2000) Food habits of sika deer and nutritional value of sika deer diets in eastern Hokkaido, Japan. Ecol Res 15:345–355
Acknowledgments
We are grateful to Dr Tatsuhiro Okubo, Utsunomiya University, Dr Koichi Kaji and Dr Yayoi Kaneko, Tokyo University of Agriculture and Technology, Dr Takeshi Yasue, Ibaraki University, Ms Reiko Horie, Utsunomiya University, and two anonymous referees for their valuable advice and constructive criticism of the manuscript. In this study we used the data on spotlight counts conducted from 2002 to 2010 by the students of the Wildlife Management Laboratory of Utsunomiya University. We are grateful to them for the use of the data.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Seki, Y., Koganezawa, M. Does sika deer overabundance exert cascading effects on the raccoon dog population?. J For Res 18, 121–127 (2013). https://doi.org/10.1007/s10310-011-0332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10310-011-0332-z