Abstract
In contrast to top-down trophic cascades, few reviews have appeared of bottom-up trophic cascades. We review the recent development of research on bottom-up cascades in terrestrial food webs, focusing on tritrophic systems consisting of plants, herbivorous insects, and natural enemies, and attempt to integrate bottom-up cascade and material transfer among trophic levels. Bottom-up cascades are frequently reported in various tritrophic systems, and are important to determine community structure, population dynamics, and individual performance of higher trophic levels. In addition, we highlight several features of bottom-up cascades. Accumulation or dilution of plant nutritional and defensive materials by herbivorous insects provides a mechanistic base for several bottom-up cascades. Such a stoichiometric approach has the potential to improve our understanding of bottom-up cascading effects in terrestrial food webs. We suggest a future direction for research by integration of bottom-up cascades and material transfer among trophic levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trophic cascades, defined as reciprocal consumer-resource effects that alter the abundance, biomass or productivity of a population, community or trophic level across more than one link in a food web (Pace et al. 1999), generally represent indirect effects of a higher trophic level on nonadjacent lower trophic levels, i.e. top-down cascade. While the top-down cascade has been well documented in aquatic systems (Strong 1992), it is thought to occur rarely in terrestrial systems because of high diversity in food webs and strong antiherbivore defense of plants, under which trophic cascades are unlikely to occur (Strong 1992; Polis 1999; Halaj and Wise 2001). However, recent studies have revealed that the top-down cascade commonly occurs in a wide variety of terrestrial systems (Schmitz et al. 2000), and focus has shifted to a cross-ecosystem comparison of the strength of trophic cascades (Pace et al. 1999; Schmitz et al. 2000; Shurin et al. 2002; Borer et al. 2005).
While such a top-down concept in which a higher trophic level dominates populations and/or communities of lower trophic levels has been widely accepted, lower trophic levels can also propagate upward to nonadjacent higher trophic levels (Hunter and Price 1992; Price 2002); this is called “bottom-up cascade”, or “cascading upward” (Hunter and Price 1992). Hunter and Price (1992) proposed a model that synthesizes the top-down and bottom-up concepts for terrestrial food webs. They suggested that species at any trophic levels can dominate other trophic levels due to feedback loops of top-down and bottom-up cascading effects (Hunter and Price 1992). There is increasing evidence of bottom-up cascading effects in various terrestrial systems (e.g. Siemann 1998; Forkner and Hunter 2000; Teder and Tammaru 2002; Gratton and Denno 2003). Although the top-down cascade has been repeatedly reviewed (Pace et al. 1999; Schmitz et al. 2000; Haraj and Wise 2001; Shurin et al. 2002; Borer et al. 2005), few systematic reviews have appeared for the bottom-up cascade, although it has been partially documented by Andow (1991), Hare (2002), and Lövei and Arpaia (2004).
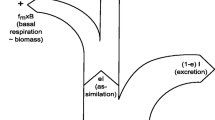
We review the recent development of research on the bottom-up cascades in terrestrial food webs, focusing on tritrophic systems consisting of plants, herbivorous insects, and natural enemies, and show how quantitative and qualitative traits of plants indirectly affect natural enemies via herbivorous insects at the community, population, and individual levels. We do not include another important bottom-up effect: the direct effect of plants on natural enemies via plant architecture or leaf volatile compounds (see Turlings et al. 2002; Langellotto and Denno 2004). Moreover, the relative importance of top-down and bottom-up forces in tritrophic systems is not considered here, because it has been repeatedly discussed by many researchers (e.g. Power 1992; Walker and Jones 2000; Dyer and Coley 2002; Stregbom et al. 2005). Instead, we attempt to integrate bottom-up cascading and material transfer in terrestrial food webs. Since nutrient or other materials, such as defensive chemicals, which are fixed or produced by plants are transmitted upward to higher trophic levels through food chains, the material flow is in the same direction as the effects of bottom-up cascades (Fig. 1). Such nutrient or defensive materials of plants would be important materials to determine survival and growth of predators as well as herbivores (Mattson 1980; Hagen 1987; White 1993). Hence, an integration of bottom-up cascades and material transfer among trophic levels may lead to a new aspect for mechanisms and consequences of the bottom-up cascades in terrestrial food webs.
Top-down and bottom-up cascades and material flow in the tritrophic system. Black, gray, and white arrows show the directions of direct effects, indirect effects, and material flow, respectively. A, B, C represent plants (lower trophic level), herbivores (mediating trophic level), and predators (higher trophic level), respectively
Bottom-up trophic cascades at various levels
While top-down cascades have been generally examined by experiments removing top predators in food webs (Schmitz et al. 2000), bottom-up cascades have been detected as indirect effects of heterogeneity of plants on third trophic levels through herbivorous insects. Plant heterogeneity represents differences in species richness, abundance, productivity, and quality (Table 1). Bottom-up cascades are categorized as community-, population-, and individual-level cascades, depending on the properties of the third trophic level (Table 1).
Community-level cascades
Effects of plant diversity on communities of higher trophic levels have been focused on agroecosystems as an issue in pest and natural enemy management, i.e. monoculture vs. polyculture (Andow 1991). Plant diversity may affect herbivore diversity, and subsequently predator diversity (Hunter and Price 1992). Siemann (1998) was the first to study experimentally this community-level cascade, demonstrating that plant diversity cascaded up to the natural enemy community through the herbivore community. Since then, several studies have detected community-level cascades which were initiated by manipulation of species richness or biomass of the plant community using fertilization or planting (Table 1). For example, Knops et al. (1999) showed that increasing plant species richness results in increased species richness of herbivores and predators. Similarly, Hawes et al. (2003) detected the same pattern in terms of abundance of each trophic level. Note that most of these studies showed that changes in species richness and abundance of herbivores and predators are in the same direction as changes in their resources, i.e. high diversity (abundance) of plants can support high diversity (abundance) of herbivores, which can support high diversity (abundance) of predators (Hunter and Price 1992). However, Koricheva et al. (2000) showed that the responses of each arthropod to plant diversity were different depending on taxa.
On the other hand, changes in plant diversity and abundance generally involve changes in vegetation structure and in abundance of alternative food, such as flower nectar. These changes may directly affect diversity and abundance of predators and parasitoids (Langellotto and Denno 2004). For example, Brose (2003) found that increasing plant diversity increased species richness in a carabid beetle community, and concluded that the increased diversity of the carabid beetles would result from direct effects of vegetation structure which depends on plant diversity. Therefore, the community-level cascades detected in several studies may include not only cascading effects through herbivores, but also direct effects of plant structure or alternative food on predators and parasitoids.
Population-level cascades
The population-level cascade (cf. species-level cascade, Polis 1999) is determined by changes in individual numbers of predators and/or parasitoids corresponding to changes in plant biomass, growth, and quality, through herbivorous insects (Table 1). Although there are a number of studies showing that host plants affect the attack or parasitism rates on herbivorous insects by natural enemies (e.g. Gross and Price 1988; Rank and Smiley 1994; Martinsen et al. 1998; Barbosa et al. 2001; Lill and Marquis 2001), the present review includes only studies that directly examined the density of natural enemies. Like the community-level cascades, changes in plant traits generally result in responses in the same direction of both herbivorous insects and their natural enemies in population-level cascades (e.g. Masters et al. 2001; Nakamura et al. 2005). In other words, when the direct effects of plants on herbivorous insects are positive, the indirect effects on natural enemies are also positive. Nakamura et al. (2005) showed that regrowth of riparian willows after damage due to flooding results in a higher density not only of a leaf beetle, but also of its natural enemies. In addition, population-level cascades are initiated by changes in plant quality induced by other herbivorous insects. Masters et al. (2001) found that root herbivory by coleopteran and dipteran larvae resulted in a greater number of both a seed predator and its parasitic wasps. This is because root herbivory was likely to increase resource allocation to reproductive organ of plants, which resulted in high quality of seeds. Then, such high quality seeds were attacked by greater number of seed predators, which attracted parasitic wasps (Masters et al. 2001). However, there is another possibility that changes in plant architecture or flower size may directly affect natural enemies.
While most studies have examined the cascading effects at a point in time, a few studies have detected temporal changes in bottom-up cascades (Gratton and Denno 2003; Bjökman et al. 2004). These studies have shown that the bottom-up cascade was maintained for several years after plant manipulation in the experiment (Gratton and Denno 2003), but the response each year differed between herbivorous insects and their natural enemies (Bjökman et al. 2004). For example, high quality in the resprouting willows after harvesting resulted in high densities of the leaf beetles and their natural enemies, but the leaf beetle density peaked 2 years after the harvesting, whereas the natural enemies consistently increased after 4 years (Bjökman et al. 2004). This result indicates that the leaf beetle density would be increased by high plant quality for the first 2 years after harvesting, but thereafter may be decreased by the top-down force of increased predators due to a delayed cascading effect of harvesting, as a feedback in the bottom-up cascade.
Individual-level cascades
Compared to the community- and population-level cascades, individual-level cascades have been studied more frequently (Table 1). Note that there are many studies which are not listed in Table 1 (e.g. see Groot and Dicke 2002; Lövei and Arpaia 2004). An important question arising from these studies is whether plant nutritional conditions and defensive chemicals affect performance of natural enemies via herbivorous insects. Hence, the variables measured in the individual-level cascades include various performance parameters, such as survivorship, developmental time, body size, and fecundity, while the heterogeneity of host plants almost represents plant quality (Table 1). Barbosa et al. (1991) examined the indirect effects of three defensive chemicals of plants, including nicotine, rutin, and hordenine, on performance of the parasitoid Cotesia congregata via tobacco hornworm Manduca sexta. Although the influences of these chemicals differed, in general they decreased the performance of both the hornworm and its parasitoid. Teder and Tammaru (2002) have demonstrated that plants with more vigorous growth are associated with a larger body size of the lepidopteran larvae, which subsequently leads to an increase in the body size of their parasitoids. Furthermore, effects of the plant quality can cascade up to the fourth trophic level, i.e. hyperparasitoid, via herbivorous insect and its primary parasitoid (Harvey et al. 2003). A more recent consideration is whether improving plant quality by increasing atmospheric CO2 or by using transgenic plants affects performance of natural enemies via herbivorous insects (Groot and Dicke 2002; Holton et al. 2003; Lövei and Arpaia 2004). For example, transgenic cotton expressing a toxin for herbivorous insects prolonged larval developmental time and shortened adult longevity of parasitoid, as a result of slow growth and reduced pupal size of the host lepidopteran larvae (Baur and Boethel 2003, but see Groot and Dicke 2002).
Thus, most studies of the individual-level cascades, as well as community- and population-level cascades, have shown that plant quality results in responses in the same direction of both herbivorous insects and their natural enemies (e.g. Barbosa et al 1991; Teder and Tammaru 2002; Zvereva and Rank 2003), i.e. high nutritional (defensive) plants increase (decrease) the performance of herbivorous insects, and subsequently increase (decrease) that of their natural enemies. In addition, Teder and Tammaru (2002) and Kagata et al. (2005) demonstrated that plant quality affects more strongly the performance of herbivorous insects than that of their natural enemies, while the responses of herbivorous insects and natural enemies to plant quality are in the same direction. For example, in high quality leaves of willows the developmental time of a leaf beetle was 14.7% shorter than in low quality leaves, but the developmental time of its predatory ladybird was only 6.3% shorter (Kagata et al. 2005). These studies indicate that effects of plant quality on insect performance may be weakened through trophic levels in the bottom-up cascades. On the other hand, a few studies have shown opposite responses of a herbivorous insect and its natural enemy to plant quality (Karowe and Schoonhoven 1992; Holton et al. 2003). Karowe and Schoonhoven (1992) examined the effects of four different plants, including Brussels sprouts, nasturtium, rape, and Swedish turnip, on the performance of the cabbage butterfly Pieris brassicae and its parasitoid Cotesia glomerata. They found that cabbage butterfly larvae on nasturtium showed the lowest performance (in terms of survivorship, growth rates, and body size), but the performance of the parasitoids developed in the host larvae on nasturtium was the highest (in terms of developmental time and longevity) among the four plants. Although the reason why there are different responses of the butterfly and its parasitoid to host quality remains unclear, the authors pointed out that a difference in sensitivity to plant chemicals between the herbivore and parasitoid could explain the different responses (Karowe and Schoonhoven 1992).
Absence of bottom-up cascades
Although many studies have investigated bottom-up cascades at various levels, several studies did not detect cascading effects from plants to natural enemies at the community, population, or individual level (Table 1). For example, Dyer and Letourneau (1999) found no evidence of effects of plant biomass on predator density through herbivores. The following reasons for the absence of bottom-up cascading effects have been suggested: strong top-down control (Dyer and Letourneau 1999; Karimzadeh et al. 2004), competition among herbivores (Karimzadeh et al. 2004), dilution of plant chemicals in the body of herbivores (Cowgill et al. 2004), and diet range of natural enemies (Koricheva et al. 2000).
Material transfer in bottom-up cascades
Previous studies on nutritional ecology (cf. Slansky and Rodrigues 1987) have focused on the fate of plant defensive chemicals and nutritional materials ingested by herbivorous insects (e.g. Mattson 1980; Lindroth 1991). They have provided an insight into the integration of material transfer among trophic levels and bottom-up trophic cascades in multitrophic systems.
Several herbivorous insects, especially specialists, can rapidly excrete or detoxify plant defensive or toxic chemicals (Tabashnik and Slansky 1987; Lindroth 1991; Glendinning 2002), which may weaken or interrupt the cascading effects of plant chemicals on natural enemies. Recently, this detoxification ability of herbivorous insects has been often examined in agroecosystems as a factor in the effects of plant chemicals produced by transgenic crops on multitrophic levels (Groot and Dicke 2002; Lövei and Arpaia 2004). For example, snowdrop lectin expressed by a transgenic potato that reduces aphid fecundity was absent or in negligible amounts within the aphids, and was not detected in their predatory ladybird (Down et al. 2000). Therefore, there are no cascading effects from transgenic plants on the performance of natural enemies (Down et al. 2000). However, oryzacystatin I, a protease inhibitor, in transgenic plants accumulates in aphid tissue, which decreases its parasitoid performance in terms of developmental time and body size (Azzouz et al. 2005). This negative effect may have resulted from the low ability of the aphid to excrete or invalidate the harmful transgenic products. On the other hand, several herbivorous insects can voluntarily accumulate plant defensive chemicals within their body to protect themselves from natural enemies (Pasteels et al. 1989; Martinsen et al 1998; Francis et al. 2001). Francis et al. (2001) demonstrated that the aphid Brevicoryne brassicae accumulates glucosinolates derived from host plants, which results in 100% mortality of the predatory ladybird feeding on these aphids. This indicates that the effects of plant defensive chemicals cascade up to a natural enemy through accumulation of the chemicals by herbivorous insects.
In addition to plant defensive chemicals, nutritional materials in host plants, such as nitrogen and phosphorus, also accumulate in insects through trophic levels (Fagan et al. 2002; Woods et al. 2002). The nitrogen content increases (or the C:N ratio decreases) from lower to higher trophic levels in tritrophic systems (i.e. Nplants<<Nherbivores<Npredators; Fagan et al. 2002; Matsumura et al. 2004). This difference in the nitrogen content among trophic levels indicates that consumer development would be limited by the amount of nitrogen in the resources (White 1993). Indeed, it is known that the amount of nitrogen in the resources is an important factor limiting survival and growth of several herbivores and predators (Mattson 1980; Denno and Fagan 2003). Therefore, the amount of nitrogen in host plants and its accumulation by herbivorous insects may influence the performance of natural enemies. One hypothesis for explaining individual-level cascades is that a higher nitrogen level in host plants leads to an increase in the nitrogen content of herbivorous insects, which in turn leads to an increase in predator performance (Mayntz and Toft 2001). H. Kagata and T. Ohgushi (unpublished) tested this hypothesis using a willow-leaf beetle-predatory ladybird system, but their findings did not support it. Although a higher nitrogen content of the willows was associated with better growth rates of both the leaf beetle and the predatory ladybird, the leaf beetle nitrogen content was not affected by the nitrogen content of the willow leaves, because of the nitrogen homeostasis in herbivorous insects (Slansky and Feeny 1977).
Conclusion and perspectives
Bottom-up trophic cascades in terrestrial food webs
This review clearly illustrated several features of bottom-up cascades in terrestrial systems. First, the responses of herbivores and natural enemies to plant heterogeneity are in the same direction, i.e. when the plant positively affects herbivores, the indirect effect on natural enemies is also positive. This contrasts to top-down cascading effects, in which the direction of the effects between adjacent trophic levels is generally opposite: an increase in the abundance of predators results in a decrease in herbivores and subsequently an increase in plant biomass (Pace et al. 1999). Second, a few studies have suggested that the impact of bottom-up cascades weakens through trophic levels, and that there is a time lag in the appearance of the bottom-up cascades between herbivores and natural enemies. These two features may make detecting feedback loops in the top-down and bottom-up cascading effects difficult (cf. Hunter and Price 1992; Bjökman et al. 2004). Third, several studies have shown that investigating bottom-up cascades is important to elucidate the effects of increasing CO2 and transgenic crops on the dynamics of multitrophic levels. In this context, we would like to emphasize that the concept of the bottom-up cascade can predict the ecosystem risk due to such environmental issues in the future. However, note that several studies of bottom-up cascades have shown direct effects of plant architecture and leaf volatiles on natural enemies, without mediation of herbivorous insects. Hence, the effects of plant heterogeneity due to experimental manipulation should be carefully interpreted, especially for community- and population-level cascades where it would be difficult to discriminate the direct effects of plants from the cascading effects on natural enemies.
Integrating bottom-up cascades and material transfer
This review also highlights the relationship between bottom-up cascades and material transfer in tritrophic systems, and argues that accumulation or dilution of materials that are fixed or produced by plants through trophic interactions provides a mechanistic base for individual-level cascades. Ecological stoichiometry links trophic interactions and changes in content of several materials between resources and consumers, and it is a useful idea to explore population and community dynamics in aquatic systems (Sterner and Elser 2002; Moe et al. 2005). However, in terrestrial systems most studies on stoichiometry have concerned soil–plant interactions, such as the relationship between the decomposition process and plant productivity (e.g. Vitousek 2004). This is probably because materials fixed or produced by plants almost flow into the decomposition system directly, but only small amounts into grazing food chains (Cebrian 1999; Polis 1999). However, many heterotrophs are supported by nutritional materials from plants through grazing food chains. Moreover, as we showed, the effects of plant materials cascade up to, and the materials themselves are transmitted to, higher trophic levels in several systems. Hence, the stoichiometric approach has the potential to improve our understanding of bottom-up cascading effects in terrestrial systems, as well as aquatic systems (Moe et al. 2005). In this context, although a few recent studies have shown a relationship between material transfer among trophic levels and individual-level cascades (Down et al. 2000), material transfer in population- and community-level cascades remains unclear, but this approach will provide a useful insight into the mechanisms or consequences of bottom-up cascades on population dynamics and community structure. For example, Schade et al. (2003) demonstrated that soil phosphorus availability links population dynamics of a herbivorous insect through changes in the carbon:phosphorus ratio (C:P ratio) of the host plants and in the phosphorus content of the herbivorous insect. Although their study was not expanded to multitrophic system, it indicates that a population-level cascade may occur with changes in the C:P ratio of each trophic level. Recent studies have revealed an important effect of phosphorus on insect growth (Frost and Elser 2002; Perkins et al. 2004) as well as on plant growth (Vitousek 2004). In addition, whether material transfer among trophic levels changes in community-level cascades which involve changes in species diversity is a challenging question. This could bridge the gap between species diversity of heterotrophs and its ecosystem functioning in terrestrial systems.
References
Andow DA (1991) Vegetational diversity and arthropod population response. Annu Rev Entomol 36:561–586
Azzouz H, Cherqui A, Campan EDM, Rahbé Y, Duport G, Jouanin L, Kaiser L, Giordanengo P (2005) Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae). J Insect Physiol 51:75–86
Barbosa P, Gross P, Kemper J (1991) Influence of plant allelochemicals on the tobacco hornworm and its parasitoid, Cotesia congregata. Ecology 72:1567–1575
Barbosa P, Segarra AE, Gross P, Caldas A, Ahlstrom K, Carlson RW, Ferguson DC, Grissell EE, Hodges RW, Marsh PM, Poole RW, Schauff ME, Shaw SR, Whitfield JB, Woodley NE (2001) Differential parasitism of macrolepidopteran herbivores on two deciduous tree species. Ecology 82:698–704
Baur ME, Boethel DJ (2003) Effects of Bt-cotton expressing CrylA(c) on the survival and fecundity of two hymenopteran parasitoids (Braconidae, Encyrtidae) in the laboratory. Biol Control 26:325–332
Bjökman C, Bommarco R, Eklund K, Höglund S (2004) Harvesting disrupts biological control of herbivores in a short-rotation coppice system. Ecol Appl 14:1624–1633
Borer ET, Seabloom EW, Shurin JB, Anderson KE, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2005) What determines the strength of a trophic cascade? Ecology 86:528–537
Bouchard É, Cloutier C, Michaud D (2003) Oryzacystatin I expressed in transgenic potato induces digestive compensation in an insect natural predator via its herbivorous prey feeding on the plant. Mol Ecol 12:2439–2446
Brose U (2003) Bottom-up control of carabid beetle communities in early successional wetlands: mediated by vegetation structure or plant diversity? Oecologia 135:407–413
Bultman TL, McNeil MR, Goldson SL (2003) Isolate-dependent impacts of fungal endophytes in a multitrophic interaction. Oikos 102:491–496
Cebrian J (1999) Patterns in the fate of production in plant communities. Am Nat 154:449–468
Cowgill SE, Danks C, Atkinson HJ (2004) Multitrophic interactions involving genetically modified potatoes, nontarget aphids, natural enemies and hyperparasitoids. Mol Ecol 13:639–647
Denno RF, Fagan WF (2003) Might nitrogen limitation promote omnivory among carnivorous arthropods. Ecology 84:2522–2531
Down RE, Ford L, Woodhouse SD, Raemaekers RJM, Leitch B, Gatehouse JA, Gatehouse AMR (2000) Snowdrop lectin (GNA) has no acute toxic effects on a beneficial insect predator, the 2-spot ladybird (Adalia bipunctata L.). J Insect Physiol 46:379–391
Dyer LA, Coley PD (2002) Tritrophic interactions in tropical versus temperate communities. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 67–88
Dyer LA, Letourneau DK (1999) Relative strengths of top-down and bottom-up forces in a tropical forest community. Oecologia 119:265–274
Dyer LA, Stireman JO III (2003) Community-wide trophic cascades and other indirect interactions in an agricultural community. Basic Appl Ecol 4:423–432
Fagan WF, Siemann E, Mitter C, Denno RF, Huberty AF, Woods HA, Elser JJ (2002) Nitrogen in insects: implications for trophic complexity and species diversification. Am Nat 160:784–802
Fonseca CR, Prado PI, Almeida-Neto M, Kubota U, Lewinsohon TM (2005) Flower-heads, herbivores and their parasitoids: food web structure along a fertility gradient. Ecol Entomol 30:36–46
Forkner PE, Hunter MD (2000) What goes up must come down? Nutrient addition and predation pressure on oak herbivores. Ecology 81:1588–1600
Francis F, Lognay G, Wathelet J, Haubruge E (2001) Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bipunctata. J Chem Ecol 27:243–256
Frost PC, Elser JJ (2002) Growth responses of littoral mayflies to the phosphorus content of their food. Ecol Lett 5:232–240
Fuentes-Contreras E, Pell JK, Niemeyer HM (1998) Influence of plant resistance at the third trophic level: interactions between parasitoids and entomopathogenic fungi of cereal aphids. Oecologia 117:426–432
Glendinning JI (2002) How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomol Exp Appl 104:15–25
Gratton C, Denno RF (2003) Inter-year carryover effects of a nutrient pulse on Spartina plants, herbivores, and natural enemies. Ecology 84:2692–2707
Groot A, Dicke M (2002) Insect-resistant transgenic plants in a multi-trophic context. Plant J 31:387–406
Gross P, Price PW (1988) Plant influences on parasitism of leafminers: a test of enemy free space. Ecology 69:1506–1516
Gruner DS (2004) Attenuation of top-down and bottom-up forces in a complex terrestrial community. Ecology 85:3010–3022
Haddad NM, Haarstad J, Tilman D (2000) The effects of long-term nitrogen loading on grassland insect communities. Oecologia 124:73–84
Hagen KS (1987) Nutritional ecology of terrestrial insect predator. In: Slansky F, Rodriguez JR (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York, pp 533–578
Halaj J, Wise DH (2001) Terrestrial trophic cascades: how much do they trickle? Am Nat 157:262–281
Hare JD (2002) Plant genetic variation in tritrophic interactions. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 8–43
Harvey JA, Van Dam NM, Gols R (2003) Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid. J Anim Ecol 72:520–531
Havill NP, Raffa KF (2000) Compound effects of induced plant responses on insect herbivores and parasitoids: implications for tritrophic interactions. Ecol Entomol 25:171–179
Hawes C, Haughton AJ, Osborne JL, Roy DB, Clark SJ, Perry JN, Rothery P, Bohan DA, Brooks DR, Champion GT, Dewar AM, Heard MS, Woiwod IP, Daniels RE, Young MW, Parish AM, Scott RJ, Firbank LG, Squire GR (2003) Responses of plants and invertebrate trophic groups to contrasting herbicide regimes in the farm scale evaluations of genetically modified herbicide-tolerant crops. Phil Trans R Soc Lond B 358:1899–1913
Holton MK, Lindroth RL, Nordheim EV (2003) Foliar quality influences tree-herbivore–parasitoid interactions: effects of elevated CO2, O3, and plant genotype. Oecologia 137:233–244
Hunter MD, Price PW (1992) Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural community. Ecology 73:724–732
Kagata H, Nakamura M, Ohgushi T (2005) Bottom-up cascade in a tri-trophic system: different impacts of host-plant regeneration on performance of a willow leaf beetle and its natural enemy. Ecol Entomol 30:58–62
Karimzadeh J, Bonsall MB, Wright DJ (2004) Bottom-up and top-down effects in a tritrophic system: the population dynamics of Plutella xylostella (L.)–Cotesia plutellae (Kurdjumov) on different host plants. Ecol Entomol 29:285–293
Karowe DN, Schoonhoven LM (1992) Interactions among three trophic levels: the influence of host plant on performance of Pieris brassicae and its parasitoid, Cotesia glomerata. Entomol Exp Appl 61:241–251
Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, Ritchie ME, Howe KM, Reich PB, Siemann E, Groth J (1999) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett 2:286–293
Koricheva J, Mulder CPH, Schmid B, Huss-Danell JJK (2000) Numerical responses of different trophic groups of invertebrates to manipulations of plant diversity in grasslands. Oecologia 125:271–282
Langellotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139:1–10
Le Rü B, Mitsipa A (2000) Influence of the host plant of the cassava mealybug Phenacoccus manihoti on life-history parameters on the predator Exochomus flaviventris. Entomol Exp Appl 95:209–212
Lill JT, Marquis RJ (2001) The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126:418–428
Lindroth RL (1991) Differential toxicity of plant allelochemicals to insects: roles of enzymatic detoxication systems. In: Bernays E (ed) Insect–plant interactions. CRC Press, Boca Raton, pp 1–33
Lövei GL, Arpaia S (2004) The impact of transgenic plants on natural enemies: a critical review of laboratory studies. Entomol Exp Appl 114:1–14
Martinsen GD, Driebe EM, Whitham TG (1998) Indirect interactions mediated by changing plant chemistry: beaver browsing benefits beetles. Ecology 79:192–200
Masters GJ, Jones TH, Rogers M (2001) Host-plant mediated effects of root herbivory on insect seed predators and their parasitoids. Oecologia 127:246–250
Matsumura M, Trafelet-Smith GM, Gratton C, Finke DL, Fagan WE, Denno RF (2004) Does intraguild predation enhance predator performance? A stoichiometric perspective. Ecology 85:2601–2615
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Mayntz D, Toft S (2001) Nutrient composition of the prey’s diet affects growth and survivorship of a generalist predator. Oecologia 127:207–213
Moe SJ, Stelzer RS, Forman MR, Harpole WS, Daufresne T, Yoshida T (2005) Recent advances in ecological stoichiometry: insights for population and community ecology. Oikos 109:29–39
Nakamura M, Utsumi S, Miki T, Ohgushi T (2005) Flood initiates bottom-up cascades in a tritrophic system: host plant regrowth increases densities of a leaf beetle and its predators. J Anim Ecol (in press)
Ode PJ, Berenbaum MR, Zangerl AR, Hardy ICW (2004) Host plant, host plant chemistry and the polyembryonic parasitoid Copidosoma sosares: indirect effects in a tritrophic interaction. Oikos 104:388–400
Pace ML, Cole JJ, Carpenter SR, Kitchell JF (1999) Trophic cascades revealed in diverse ecosystems. Trends Ecol Evol 14:483–488
Pasteels JM, Rowell-Rahier M, Raupp MJ (1989) Plant-derived defense in chrysomelid beetles. In: Barbosa P, Letourneau D (eds) Novel aspects of insect–plant interactions. Wiley, New York, pp 235–272
Perkins MC, Woods HA, Harrison JF, Elser JJ (2004) Dietary phosphorus affects the growth of larval Manduca sexta. Arch Insect Biochem Physiol 55:153–168
Perner J, Voigt W, Bährmann R, Heinrich W, Marstaller R, Fabian B, Gregor K, Lichter D, Sander FW, Jones TH (2003) Responses of arthropods to plant diversity: changes after pollution cessation. Ecography 26:788–800
Polis GA (1999) Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos 86:3–15
Power ME (1992) Top-down and bottom-up forces in food webs: do plants have primacy ? Ecology 73:733–746
Price PW (2002) Resource-driven terrestrial interaction webs. Ecol Res 17:241–247
Rank NE, Smiley JT (1994) Host-plant effects on Parasyrphus melanderi (Diptera: Syrphidae) feeding on a willow leaf beetle Chrysomela aeneicollis (Coleoptera: Chrysomelidae). Ecol Entomol 19:31–38
Roth S, Knorr C, Lindroth RL (1997) Dietary phenolics affects performance of the gypsy moth (Lepidoptera: Lymantriidae) and its parasitoid Cotesia melanoscela (Hymenoptera: Braconidae). Environ Entomol 26:668–671
Salvo A, Valladares GR (2002) Plant-related intraspecific size variation in parasitoids (Hymenoptera: Parasitica) of a polyphagous leafminer (Diptera: Agromyzidae). Environ Entomol 31:874–879
Schade JD, Kyle M, Hobbie SE, Fagan WF, Elser JJ (2003) Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol Lett 6:96–101
Schmitz OJ, Hambäck PA, Beckerman AP (2000) Trophic cascades in terrestrial systems: a review of the effects of carnivore removals on plants. Am Nat 155:141–153
Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2002) A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:758–791
Siemann E (1998) Experimental tests of effects of plant productivity and diversity on grassland arthropod diversity. Ecology 79:2057–2070
Siemann E, Tilman D, Haarstad J, Ritchie ME (1998) Experimental tests of the dependence of arthropod diversity on plant diversity. Am Nat 152:738–750
Slansky F, Feeny P (1977) Stabilization of the rate of nitrogen accumulation by larvae of the cabbage butterfly on wild and cultivated food plants. Ecol Monogr 47:209–228
Slansky F, Rodrigues D (eds) (1987) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York
Stamp NE, Yang Y, Osier TL (1997) Responses of an insect predator to prey fed multiple allelochemicals under representative thermal regimes. Ecology 78:203–214
Sterner RW, Elser JJ (2002) Ecological stoichiometry. Princeton University Press, Princeton
Stregbom J, Witzell J, Nordin A, Ericson L (2005) Do multitrophic interactions override N fertilization effects on Operophtera larvae? Oecologia 143:241–250
Strong DR (1992) Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73:747–754
Sznajder B, Harvey JA (2003) Second and third trophic level effects of differences in plant species reflect dietary specialisation of herbivores and their endoparasitoids. Entomol Exp Appl 109:73–82
Tabashnik BE, Slansky F (1987) Nutritional ecology of forb foliage-chewing insects. In: Slansky F, Rodrigues JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York, pp 71–104
Teder T, Tammaru T (2002) Cascading effects of variation in plant vigour on the relative performance of insect herbivores and their parasitoids. Ecol Entomol 27:94–104
Traugott MS, Stamp NE (1997) Effects of chlorogenic acid- and tomatine-fed caterpillars on performance of an insect predator. Oecologia 109:265–272
Turlings TC, Gouinguené S, Degen T, Fritzche-Hoballah ME (2002) The chemical ecology of plant–caterpillar–parasitoid interactions. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 148–173
Vitousek P (2004) Nutrient cycling and limitation. Princeton University Press, Princeton
Walde SJ (1995) How quality of host plant affects a predator–prey interaction in biological control. Ecology 76:1206–1219
Walker M, Jones TH (2000) Relative roles of top-down and bottom-up forces in terrestrial tritrophic plant–insect herbivore–natural enemy systems. Oikos 93:177–187
White TCR (1993) The inadequate environment. Springer, Berlin Heidelberg New York
Woods HA, Perkins MC, Elser JJ, Harrison JF (2002) Absorption and storage of phosphorus by larval Manduca sexta. J Insect Physiol 48:555–564
Zvereva EL, Rank NE (2003) Host plant effects on parasitoid attack on the leaf beetle Chrysomela lapponica. Oecologia 135:258–267
Acknowledgments
We thank T. Hayashi for valuable comments on this manuscript. This research was supported by the 21st Century COE Program to Kyoto University (A14).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kagata, H., Ohgushi, T. Bottom-up trophic cascades and material transfer in terrestrial food webs. Ecol Res 21, 26–34 (2006). https://doi.org/10.1007/s11284-005-0124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-005-0124-z