Abstract
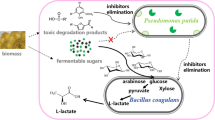
In this study, we constructed a coculture consortium comprising engineered Pseudomonas putida KT2440 and Escherichia coli MG1655. Provision of “related” carbon sources and synthesis of medium-chain-length polyhydroxyalkanoates (mcl-PHAs) were separately assigned to these strains via a modular construction strategy. To avoid growth competition, a preference for the use of a carbon source was constructed. Further, the main intermediate metabolite acetate played an important role in constructing the expected “nutrition supply–detoxification” relationship between these strains. The coculture consortium showed a remarkable increase in the mcl-PHA titer (0.541 g/L) with a glucose–xylose mixture (1:1). Subsequently, the titer of mcl-PHA produced by the coculture consortium when tested with actual lignocellulosic hydrolysate (0.434 g/L) was similar to that achieved with laboratory sugars’ mixture (0.469 g/L). These results indicate a competitive potential of the engineered E. coli–P. putida coculture consortium for mcl-PHA production with lignocellulosic hydrolysate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution caused by plastics and the consumption of fossil energy have accelerated the search for sustainable development materials to replace petroleum-based plastics. The bacterial polymers, polyhydroxyalkanoates (PHAs), are a class of high-molecular-weight polyesters comprising hydroxy fatty acids as monomers in microorganisms. PHAs have attracted extensive interest as a new green alternative to petroleum-based plastics because they are biodegradable and can be produced from renewable resources [4, 5, 39, 47]. PHAs include short-chain-length (scl, 3–5 carbon atoms) and medium-chain-length (mcl, 6–14 carbon atoms) hydroxyalkanoic acid monomers depending on the strain, carbon substrate, and culture conditions provided [10]. Mcl-PHAs are polyesters of hydroxyalkanoates primarily produced by fluorescent pseudomonads under unbalanced growth conditions [34, 43, 44]. Mcl-PHAs have lower crystallinity but are more elastic than scl-PHAs. Furthermore, mcl-PHAs are more diverse than scl-PHAs in structure; therefore, they can be used in a wide range of applications [33]. However, high production costs make large-scale application of mcl-PHAs difficult, unlike the application of low-cost petroleum-based plastics. Several factors lead to the relatively high production cost of mcl-PHAs, such as high substrate cost, low polymer concentration, as well as low productivity [22, 48]. According to statistics, substrate costs account for approximately 50% of the total production cost. Therefore, mcl-PHA synthesis using inexpensive, renewable carbon sources is an effective strategy to reduce production cost [1, 30].
Lignocellulose comprising cellulose, hemicellulose, and lignin is inexpensive and the most abundant non-grain biomass in nature [27]. Cellulose is a water-insoluble polymer comprising glucose, hemicellulose is a polymer comprising pentose and hexose linked by glycoside bonds, and lignin is a three-dimensional polymer based on phenylpropane [18]. Currently, lignocellulose is converted into fermentable sugars (glucose and xylose) by various methods, and these sugars have been used to synthesize PHAs. However, most studies have focused on polyhydroxybutyrates (PHBs), whereas studies on PHAs are limited [8, 10, 13, 28, 29, 32, 35, 52]. Davis et al. [10] used several different Pseudomonas strains to synthesize mcl-PHAs from a wild ryegrass hydrolysate treated by different methods. Although the yield of mcl-PHAs was similar to that of laboratory-grade sugars, the highest yield was 0.3 g/L, which is insufficient to meet the needs of industrial production. Based on previous reports, we speculated that the reason for this finding is that sugars are structurally “unrelated” carbon sources and unbeneficial for PHA biosynthesis [47]. Different carbon source species considerably affect PHA biosynthesis. In general, carbon sources can be classified as “related” carbon sources (structures associated with PHA monomers, e.g., fatty acids) and “unrelated” carbon sources (structures unrelated to PHA monomers, e.g., xylose and glucose) [11, 21].
Currently, synthetic microbial coculture strategy has become a novel strategy in metabolic engineering and synthetic biology and has been widely used to overcome the limitations of monoculture [16, 42, 53]. A major advantage of coculture consortia is that each expression system and pathway module can be constructed and optimized in parallel, which indicates substantially reduced product production time [54]. Moreover, coculture consortia can involve unique properties and functions of different species and minimize problems arising from feedback inhibition by means of spatial pathway module segregation [54]. Researchers have applied coculture consortia to the PHA production. Shalin et al. [36] achieved an increase in the PHB yield by constructing a coculture consortium comprising Bacillus firmus NII 0830 and Lactobacillus delbrueckii NII0925. Löwe et al. [25] used a coculture consortium comprising cyanobacteria and Pseudomonas to convert CO2 to mcl-PHAs, and they achieved a yield of 156 mg/L. However, to the best of our knowledge, the use of a coculture consortium system to synthesize mcl-PHA with a glucose–xylose mixture as a carbon source and lignocellulosic hydrolysate as a substrate has not been reported to date.
In this study, we have reported on the construction of a coculture consortium comprising Escherichia coli and Pseudomonas putida using division of labor, i.e., separation of tasks, between these bacteria to achieve efficient biosynthesis of mcl-PHAs with only “unrelated” carbon sources (Fig. 1a). E. coli secreted acetate and “related” carbon sources such as extracellular free fatty acids (FFAs). As an excellent mcl-PHA synthetic strain, P. putida undertook the task of efficient biosynthesis of mcl-PHAs. As shown in Fig. 1b, ptsG and manZ were knocked out in E. coli, thereby prompting E. coli to preferably utilize xylose and slowly utilize glucose. Consequently, this reduced growth competition between E. coli and P. putida. In addition, the ability of E. coli to synthesize acetate and extracellular FFAs was promoted, which can promote mcl-PHA biosynthesis, by knocking out atpFH and envR. The main intermediate metabolite acetate played an important role in constructing the expected “nutrition supply–detoxification” relationship between these two strains. It was consumed by engineered P. putida to synthesize mcl-PHAs and produced by engineered E. coli from xylose, which was toxic to the latter in the meantime. The ultimate goal was to achieve efficient production of mcl-PHAs using inexpensive “unrelated” carbon sources.
Materials and methods
Bacterial strains, plasmids, and reagents
The plasmids and bacterial strains used in this study are listed in Table 1. The parent strain P. putida KT2440 (American type culture collection; ATCC 47054) was obtained from ATCC (Manassas, VA, USA). The plasmid pBBR1MCS-2 was donated by Dr. Yingjin Yuan of Tianjin University, China. The plasmids pTKS/CS and pTKRED were donated by Dr. Tao Chen of Tianjin University. E. coli MG1655 was donated by Dr. Tao Chen of Tianjin University. Acetate [-performance liquid chromatography (HPLC) grade] was purchased from Concord Tech (China). Xylose (99% purity) was purchased from YuanYe (Shanghai, China). Glucose (AR) was purchased from Yuanli Chemical (Tianjin, China). DNA-manipulating agents, including restriction endonucleases and T4 DNA ligase, were purchased from Thermo Scientific (Beijing, China). Phanta Max Super-Fidelity DNA Polymerase and Taq for polymerase chain reaction (PCR) were purchased from Vazyme (Nanjing, China). PCR primers were synthesized by GENEWIZ (Suzhou, China) and are listed in Table 1.
Plasmid and strain construction
Plasmids and strains were constructed according to standard protocols [9]. P. putida KT2440 was employed in mcl-PHA synthesis studies; 1689-bp acs (Gene ID 1045574), 1203-bp ackA (Gene ID 946775), and 2145-bp pta (Gene ID 946778) were amplified using PCR and the primer pairs acs-F/acs-R, ackA-F/ackA-R, and pta-F/pta-R. These amplified genes were cloned into pBBR1MCS-2 [19], which resulted in pBBR1-acs, pBBR1-ackA, and pBBR1-ackA-pta, respectively. Expression vectors were confirmed using enzymatic digestion and DNA sequencing, and then transformed into P. putida KT2440, thereby generating engineered strains. Transformation of P. putida strains was accomplished via electroporation using a voltage of 2.5 V, 200 Ω, and 25 μF.
Escherichia coli MG1655 was used to synthesize “related” carbon sources using “unrelated” carbon sources and secrete them extracellularly. Gene knockout experiments based on λ-red and I-SceI described by Lin Zhenquan [23] were used to construct ptsG, manZ, envR, and atpFH knockout strains. ptsG knockout was performed as follows: using the genome of E. coli MG1655 as a template, the primers ptsG-Af/ptsG-Ar and ptsG-Bf/ptsG-Br were used to amplify the upstream and downstream homologous arm fragments A and B, respectively, of ptsG. Using the plasmid pTKS/CS as a template, the fragment tet1 and downstream fragment tet2 of the tetracycline resistance gene tet were amplified with the primers ptsG-tetf1/ptsG-tetr1 and ptsG-tetf2/ptsG-tetr2, respectively. Using the fragments A and tet1 as templates, the fragment A-tet1 was amplified by overlap extension PCR using the primer ptsG-Af/ptsG-tetr1. Similarly, using fragments tet2 and B as templates, the fragment tet2-B was amplified by overlap extension PCR using the primer ptsG-tetf2/ptsG-Br. Finally, overlap extension PCR was performed again using fragments A-tet1 and tet2-B as amplification templates and ptsG-Af/ptsG-Br as a primer to amplify the knockout fragment A-t-B. This knockout fragment A-t-B was transferred to E. coli MG1655 by electroporation. The ptsG knockout strain ECΔp was obtained after two screenings. manZ, envR, and atpFH were sequentially knocked out by the same method to obtain the strains ECΔp-m, ECΔp-m-a, ECΔp-m-a, and ECΔp-m-a-e. PCR primers were synthesized by GENEWIZ (Suzhou, China) and are listed in Table 1.
Culture media and conditions
Luria broth (LB) medium was used for strain maintenance and seed preparation. All batch fermentations, including pure culture and coculture, were performed in M9 medium comprising 12.8 g/L Na2HPO47H2O, 3 g/L KH2PO4, 1 g/L NH4Cl, and 0.5 g/L NaCl supplemented with the desired amounts of glucose and/or xylose. This basic solution was autoclaved and supplemented with 0.24 g/L MgSO4, and a trace element solution containing 6.0 mg/L FeSO47H2O, 2.7 mg/L CaCO3, 2.0 mg/LZnSO4H2O, 1.16 mg/L MnSO4H2O, 0.37 mg/L CoSO47H2O, 0.33 mg/L CuSO45H2O, and 0.08 mg/L H3BO3 (all filter sterilized). Appropriate amounts of antibiotics (50 μg/ml kanamycin and 100 μg/mL streptomycin) were added to the medium when needed. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to the medium at a final concentration of 2 mM when needed.
Regarding seed preparation, single colonies of E. coli and P. putida were inoculated into 5 mL LB medium in a 35-mL test tube and incubated overnight under aerobic conditions at 30 °C and 220 rpm. To produce mcl-PHAs, seed cultures of P. putida were inoculated into 500-mL baffled shake flasks comprising 50 mL defined M9 medium to an initial OD600 of 0.05 and placed on a rotary shaker (220 rpm) under aerobic conditions at 30 °C. E. coli sugar utilization and acid production experiments were conducted as follows. A certain amount of glucose or xylose mother liquor was added to a 250-mL Erlenmeyer flask comprising 50 mL M9 medium, and a certain amount of activated seed solution was added. Then, this mixture was cultured under aerobic conditions at 30 °C and 220 rpm.
Regarding a reactor study, cells were cultured at 30 °C in a 5-L bioreactor with a working volume of 2 L. Sterile air was delivered at a flow rate of 2 vvm, and the dissolved oxygen level was kept above 25% of air saturation by controlling the agitation speed. pH was maintained at 7.0 by automatic addition of 0.5 M H2SO4 and 1 M KOH.
Enzymatic hydrolysis of corn stover
Corn stalk pretreated with ammonia water (water content 7.57%, glucan content 65.34%, xylan content 17.26%, and insoluble lignin 4.75%) was used as a substrate for enzymatic hydrolysis. The powder was placed in a 500-mL conical flask containing a citric acid buffer solution (50 mM, pH = 4.8) and reacted at a concentration ratio of 10% (w/v). Cellulase and hemicellulase were added at a ratio of 2:1 at a reaction temperature of 50 °C and rotational speed of 200 rpm; enzymatic hydrolysis was for 72 h in total. At the end of enzymatic hydrolysis, the reaction solution was placed in a boiling water bath for 10 min to kill the enzyme and then subjected to suction filtration to obtain a hydrolyzate.
Extraction and characterization of mcl-PHAs
The cultivated cells were harvested by centrifugation (9000 rpm, 4 °C, 10 min) and lyophilized for 24 h at − 20 °C. The dried cells were placed in methanol (room temperature, treated at 160 rpm for 5 min, centrifuged at 9000 rpm for 10 min), and lyophilized again. The stem cells obtained in the previous step were placed in ten times (volume:mass) the acetone at 170 rpm and extracted at 22 °C for 5 h. The extracted PHAs were concentrated by rotary evaporation and precipitated with ten times the volume of ice-cold methanol [15]. The thermal properties of mcl-PHAs, including Tg, Tm, and ΔH, were analyzed by differential scanning calorimetry (DSC). The weight average molecular weight (Mw), number average molecular weight (Mn), and polydispersity index (PD, Mw/Mn) of mcl-PHA were analyzed by gel permeation chromatography (GPC) [6, 40].
Analysis
The growth of bacterial cells was determined by measuring optical density at a wavelength of 600 nm (OD600) using an UV-1200 spectrophotometer (Mapada, China). Based on the characteristics of tetracycline and chloramphenicol resistance in E. coli and P. putida, the culture solution was diluted to a certain multiple and then coated on a plate with tetracycline and chloramphenicol resistance. Colony counting was conducted to analyze the OD600 value of the two bacteria in the mixed culture process.
Acetate, glucose, and xylose were quantified in the culture supernatant using an Ultimate 3000 HPLC (Dionex, Sunnyvale, CA, USA) equipped with an Aminex HPX-87H ion-exchange column (Bio-Rad, USA) operating at 65 °C with a flow rate of 0.6 mL min of 5 mM H2SO4 using a differential refraction detector.
Extraction and detection of fatty acids were performed as described previously [37]. Fermentation supernatant was harvested by centrifugation at 12,000 rpm for 10 min. Subsequently, 200 μL acetate was added to 2 mL supernatant, 150 mg undecanoic acid was added as an internal standard, and then, it was extracted with 2 mL n-hexane:chloroform (4:1, v/v). After the organic layer was evaporated to dryness, it was dissolved in 1 mL chloroform methanolic sulfuric acid (10:8.5:1.5) and then subjected to methanolysis at 100 °C for 1 h. Gas chromatography (Agilent, USA) was used for detecting FFAs. The resulting 1 μL FFA methyl esters was injected for gas chromatography. The column temperature was initially maintained at 60 °C for 3 min, increased to 250 °C at the rate of 10 °C/min, and finally maintained at 250 °C for 10 min.
Extraction and detection of PHAs were performed as described previously [30]. Bacterial cells were harvested by centrifugation at 10,000 rpm for 10 min, washed with distilled water, frozen at − 80 °C, and lyophilized in a freeze dryer. The obtained lyophilizate was subjected to methanolysis at 100 °C for 4 h in the presence of 15% (v/v) H2SO4, and benzoic acid was used as an internal standard. Gas chromatography (Agilent, USA) was used for this study. The resulting 1 μL PHA methyl esters was injected for gas chromatography. Column temperature was initially maintained at 80 °C for 1 min, then increased to 250 °C at a rate of 20 °C/min, and finally maintained at 250 °C for 1.5 min. To identify the mcl-PHA monomer composition, the sample was first analyzed by gas chromatography and mass spectrometry (GC–MS), the interface temperature of mass spectrometry was 250 °C, the ion source voltage was 70 eV, ion current was 40 μA, and the mass scanning range was from 50 to 650 m/z. The inlet temperature was set to 290 °C. And then the standards of different monomers were used as internal standards in gas chromatography, and the monomer composition of mcl-PHA was determined according to the different peak positions of different monomers.
Results and discussion
Engineering P. putida KT2440 and E. coli MG1655 to increase mcl-PHA production from “unrelated” carbon sources
Results of previous studies conducted at our laboratory have reported on the application of engineered P. putida KT2440 to synthesize mcl-PHAs using acetate by overexpressing acs and ackA-pta pathways; acs overexpression can increase mcl-PHA production. The final yield of KT2440-acs was 0.674 g/L, which was 92% higher than that of wild-type bacteria; further, the intracellular accumulation of mcl-PHAs increased by approximately 43%, and the yield of mcl-PHAs from acetate increased by approximately 50%. Real-time quantitative PCR has demonstrated that the expression level of acs of KT2440-acs was 12 times higher than that of wild-type bacteria [51].
To resolve the limitation of low mcl-PHA production of P. putida monocultures with glucose and xylose, we introduced E. coli into the culture system to provide relevant carbon sources for P. putida [10]. When glucose is used as a single carbon source, these two bacteria have a competitive relationship, resulting in instability and inconsistency in the system. Engineered and cocultivated strains using different carbon sources serve as an effective strategy to avoid competitive growth and enhance the coordination of the growth of two bacteria. However, in the presence of glucose, E. coli preferentially consumes glucose instead of xylose owing to carbon catabolite repression (CCR). To promote the ability of E. coli to prefer xylose and relieve the effect of glucose, Gosset et al. [12] demonstrated that ptsG inactivation abolishes the CCR of manZ involved in glucose uptake and secretion. Liu et al. [24] knocked out ptsG and manZ simultaneously in E. coli BMT 23 to determine its slow metabolism of glucose and preferential utilization of xylose.
We knocked out ptsG and manZ in E. coli MG1655. The engineered strain EC∆p-m was cultured, and the results are presented in Fig. 2a, b. When the strain was cultured with 20 g/L of glucose as the sole carbon source, its concentration decreased slowly, and only a small amount (3.06 g/L) of glucose was consumed after 48 h. However, OD600 increased to only 0.457, which indicated that the glucose metabolism ability of the engineered strain was greatly inhibited. When the strain was cultured with 20 g/L xylose as the sole carbon source, the strain could consume xylose rapidly (12.28 g/L xylose was consumed 48 h later), and OD600 increased rapidly to 1.946 at 48 h, which indicated that the strain had a strong xylose consumption capacity. When the strain was cultured with a mixture of 10 g/L glucose and 10 g/L xylose, xylose was rapidly consumed during cultivation, whereas glucose was almost unconsumed. Glucose was only slowly consumed when the xylose concentration was low. These results suggest that knocking out ptsG and manZ in E. coli MG1655 can promote its preferential use of xylose and slow down the metabolism of glucose in the presence of both glucose and xylose.
Sugar consumption (a) and biomass (b) of the strain EC∆p-m, (filled square) Glu: glucose was the single carbon source; (filled circle) Xyl: xylose was the single carbon source; (open triangle) Mix: carbon source was mixture of glucose and xylose; (open square) Mix-Glu: glucose concentration under mixed carbon source; (open circle) Mix-Xly: xylose concentration under mixed carbon source. Production of acetate and free fatty acids in E. coli (c), E. coli: wild-type E. coli and ECΔp-m-a-e: engineered strain ECΔp-m-a-e
As shown in Fig. 1a, glucose was preferentially used for growth and reproduction by P. putida, whereas acetate and FFAs secreted by E. coli were mainly used for mcl-PHA biosynthesis or other product biosynthesis. Therefore, increasing the production of acetate and FFAs in E. coli could promote mcl-PHA production. Causey et al. [7] used various metabolic modification strategies to improve the acetate production of E. coli W3110. Under aerobic conditions, E. coli converted approximately 50% of the substrate into biomass [41]. If carbon flux can be regulated to acetate synthesis, synthesis of acetate can be effectively increased, and oxidative phosphorylation is a key process related to this. This process can drive the main synthesis of ATP under aerobic conditions. Disruption of this pathway will force cells to synthesize ATP via the acetate synthesis pathway, thereby achieving the goal of metabolic flux regulation. Therefore, we attempted to promote acetate synthesis by knocking out the key gene atpFH in EC∆p-m oxidized phosphoric acid.
FFAs are synthesized by microorganisms using acyl-ACP as a substrate catalyzed by thioesterases. In the past decade, rational metabolic engineering was widely used to improve the synthesis of FFAs and their precursors [49, 50]. EnvR was known to inhibit the expression of the forward transporter AcrAB. AcrAB excretes intracellular antibiotics, surfactants, and organic complexes [14, 31]. AcrAB is the major transporter of fatty acids. Shin et al. [37] demonstrated that knocking out envR can eliminate AcrAB inhibition, thereby promoting the secretion of extracellular FFA by E. coli. Promoting the secretion of extracellular FFA by E. coli also contributed to mcl-PHA synthesis. Therefore, we knocked out envR in EC∆p-m-a.
The final engineered strain ECΔp-m-a-e, which was knocked out of ptsG, manZ, atpFH, and envR, was cultured and tested. The main components of extracellular FFAs were n-hexanoic acid, n-heptanoic acid, n-octanoic acid, and n-dodecanoic acid. These FFAs can be used by P. putida to synthetize mcl-PHAs. As shown in Fig. 2c, when 20 g/L xylose was a single carbon source, cultured for 60 h, wild-type E. coli MG1655 could synthesize 2.4 g/L acetate and 0.62 g/L FFA, whereas ECΔp-m-a-e could synthesize 5.5 g/L acetate and 0.91 g/L FFAs. Therefore, the ability of E. coli MG1655 to use xylose for synthesizing acetate and extracellular FFAs can be promoted by knocking out atpFH and envR.
Developing a coculture consortium for efficient production of mcl-PHA
Construction and optimization of culture conditions for the coculture consortium
Although E. coli and P. putida have been engineered and optimized, the question of how to coculture for PHA production remains unsolved. The relative inoculation time, inoculation ratio, and corresponding culture conditions (e.g., temperature and pH) have important effects on the coculture consortium. In addition, the nitrogen source also affects the production of mcl-PHA.
First, the relative inoculation time of the two bacteria mainly involves whether the double bacteria are simultaneously or sequentially inoculated to the medium. Using E. coli as a reference, P. putida was inoculated 12 h before E. coli inoculation, simultaneously with E. coli, 12 h after E. coli inoculation, and 24 h after E. coli inoculation. As shown in Fig. 3, the yield of mcl-PHAs by P. putida inoculated 12 h before E. coli inoculation and simultaneously with E. coli was 0.201 and 0.312 g/L, respectively. Then, 0.451 and 0.440 g/L of mcl-PHAs were synthesized when P. putida was inoculated 12 and 24 h after E. coli inoculation, respectively. The results showed that the latter two relative inoculation times were significantly better than the former two relative inoculation times for mcl-PHA production, and PHA production was the highest when P. putida was inoculated 12 h after E. coli inoculation. Consequently, the subsequent culture was inoculated with P. putida 12 h after E. coli inoculation.
Second, we researched three initial inoculation ratios (E. coli:P. putida, 1/2, 1/1, 2/1) of the two bacteria. The results are shown in Fig. 4a. Different inoculation ratios had no significant effect on the yield of mcl-PHAs. The initial inoculum can accelerate the entry of the strain into the log phase. Under the study conditions, both P. putida and E. coli grew rapidly. The change in inoculum had no significant effect on their growth rate. In addition, P. putida was inoculated after E. coli, further weakening the effect of inoculation on double bacteria exchange. Therefore, changing the inoculation ratio did not significantly affect mcl-PHA production. Next, we designed four groups of experiments on the concentration of the nitrogen source (1, 2, 3, and 4 g/L NH4Cl). As shown in Fig. 4b, we found that 2 g/L of H4Cl showed the highest yield of mcl-PHAs. Last, we explored the culture temperature and pH. The optimum culture pH of P. putida and E. coli was approximately 7.0; therefore, the culture pH was selected to be 7.0. However, there were differences between the two cultures in terms of optimum temperature (P. putida: 30 °C and E. coli: 37 °C). Therefore, we designed culture experiments at different temperatures for the two bacteria. The results presented in Fig. 4c show that the yield of mcl-PHAs was the highest at 30 °C, and the change in culture temperature did not significantly affect this yield.
Experiments related to coculture consortium were conducted under the abovementioned optimum conditions to produce mcl-PHAs (Fig. 4d). In M9 medium, with a total sugar concentration of 20 g/L (glucose:xylose, 1:1), the yield of mcl-PHA produced by the coculture consortium was 0.541 g/L, which was 3.9 times higher than that synthesized by P. putida monoculture.
“Nutrition supply–detoxification” relationship between E. coli and P. putida
As shown in Fig. 5, the sugar concentration changed and biomass were analyzed during culture. A comparison of the maximum OD600 revealed that the biomass yields of thecoculture consortium were higher than those of the respective monoculture (Fig. 5a). The increase in biomass yield of E. coli may be mainly owing to the release of acetate inhibition by P. putida through the metabolic consumption of acetate. As can be seen in Fig. 5b, acetate hardly accumulates in the coculture consortium compared with how it accumulates in monocultures. It can also be deduced that E. coli grows better based on the observation that xylose consumption was faster in the coculture consortium, and there was no residue (Fig. 5d). The increase in biomass yield of P. putida is due to the increase in available carbon sources in the coculture consortium. Regarding monocultures, the biomass yield of P. putida cannot continue to increase because the only carbon source glucose is consumed within 24 h of culture (Fig. 5c). In the coculture consortium, P. putida can also use acetate and FFAs secreted by E. coli to grow and synthesize mcl-PHAs (Fig. 5b).
Acetate, the intermediate metabolite, was responsible for the symbiotic “nutrition supply–detoxification” relationship between two bacteria, i.e., P. putida used acetate and FFAs secreted by E. coli as carbon sources. Simultaneously, it relieved the inhibition of acetate on E. coli. Compared with the monoculture of P. putida, the coculture consortium can efficiently synthesize mcl-PHAs using a sugar-based carbon source.
Mcl-PHA production by the coculture consortium on cellulose hydrolysate
Currently, renewable resources such as lignocellulose are mainly used for scl-PHA production; for example, Narayanan [26] used Bacillus mycoides to synthesize 0.39 g/L P (3HB-co-3HV) with rice hull hydrolysate as a substrate. Johanna et al. [38] synthesized 0.23 g/L P (3HB-co-3HV) using acid-hydrolyzed wood chip hydrolysate. Kulkarni [20] synthesized 0.365 g/L P (3HB-co-3HV) using bagasse extract as a substrate along with Halomonas campisalis MCM B-1027 isolated from an extreme environment. However, there are a few reports on synthetic mcl-PHA studies. Davis [10] used wild ryegrass hydrolysate as a substrate to synthesize mcl-PHAs using several different Pseudomonas strains, producing a maximum yield of 0.3 g/L. Löwe et al. [25] constructed a coculture consortium comprising Synechococcus elongatus cscB and P. putida cscAB. The two bacteria synthesized mcl-PHAs using CO2 as a substrate through material exchange, but the production efficiency was only 23.8 mg/L/day. There remains a large gap between the mcl-PHAs produced by renewable resources and scl-PHAs in terms of yield and production efficiency.
We obtained the hydrolysate by enzymatic hydrolysis of corn straw (water content 7.57%, glucan content 65.34%, xylan content 17.26%, insoluble lignin 4.75%) pretreated with ammonia (181 °C, 30 min). HPLC was used to analyze the sugar composition in the hydrolysate. As a result, the main sugar components in the hydrolysate were glucose and xylose (ratio 4.3:1). Sugar solution obtained by enzymatic hydrolysis was used for fermentation. The total sugar concentration was approximately 20 g/L, and the glucose–xylose mixture (4.3:1) was used as a control. The result showed that the titer of mcl-PHAs produced by the coculture with the hydrolysate (0.434 g/L) was slightly lower than that of mcl-PHAs produced with a glucose–xylose mixture (0.469 g/L). This shows that the coculture consortium had the ability to utilize lignocellulosic resources. The mcl-PHA yield and production efficiency of the coculture consortium were improved compared with those previously reported (Table 2).
Property characterization of mcl-PHAs
Property characterization of mcl-PHAs showed that mcl-PHA monomer synthesized by the coculture consortium was composed of 3-hydroxyhexanoate, 3-hydroxyoctanoate, 3-hydroxydecanoate, 3-hydroxydodecanoate, and 3-hydroxytetradecanoate, with 3-hydroxydecanoate (accounting for 60% of the total mcl-PHA monomer) being the main component followed by 3-hydroxyoctanoate (accounting for 26.8% of the total mcl-PHA monomer). The monomer with the highest carbon atom number was hydroxytetradecanoate, whereas that with the lowest carbon atom number was hydroxyhexanoate.
As shown in Table 3, Tg of mcl-PHAs synthesized by the coculture consortium was − 46.78 °C, and Tm was 40.81 °C. It has typical thermal properties of mcl-PHAs mainly comprising a hydroxydecanoate monomer [2, 17]. The experimental data measured by GPC showed that Mw of mcl-PHA was 94146, Mn was 40222, and PD was 2.3. The molecular weight and thermal properties of mcl-PHAs synthesized by coculture are similar to those of mcl-PHAs synthesized by Davis et al. [10] using ryegrass hydrolysate.
Conclusions
In this study, we constructed a coculture consortium comprising engineered P. putida KT2440 and E. coli MG1655 for mcl-PHA production using “unrelated” carbon sources. E. coli used xylose to secrete acetate and “related” carbon sources such as extracellular FFAs, whereas P. putida undertook the task of efficient biosynthesis of mcl-PHAs. We knocked out ptsG, manZ, atpFH and envR in E. coli MG1655, and the yield of acetate and FFA in ECΔp-m-a-e was 2.3 and 1.4 times higher than the parent strain, respectively.
Consequently, E. coli used xylose to secrete acetate and extracellular FFAs, whereas P. putida used glucose as the main carbon source for growth. The main intermediate metabolite acetate played an important role in constructing the expected “nutrition supply–detoxification” relationship between these two strains. Next, the coculture consortium was applied to produce mcl-PHAs with renewable carbon resources; the glucose–xylose mixture first and then, the actual lignocellulosic hydrolysate. In M9 medium, with a total sugar concentration of 20 g/L (glucose:xylose, 1:1), the yield of mcl-PHAs produced by the coculture consortium was 0.541 g/L, which was 3.9 times higher than that observed for the engineered P. putida monoculture. The titer of mcl-PHA produced by the coculture consortium with lignocellulosic hydrolysate was 0.434 g/L, which was slightly lower than that of mcl-PHAs (0.469 g/L) produced with the glucose–xylose mixture (glucose:xylose, 4.3:1). These results indicate that the coculture consortium has the potential to apply lignocellulose resources. Simultaneously, it has a competitive advantage over other studies that use renewable resources to synthesize PHAs. Research on increasing FFA production is underway at our laboratory, which could be used to further improve the yield of mcl-PHAs.
References
Agnew DE, Pfleger BF (2013) Synthetic biology strategies for synthesizing polyhydroxyalkanoates from unrelated carbon sources. Chem Eng Sci 103:58–67. https://doi.org/10.1016/j.ces.2012.12.023
Ashby RD, Solaiman DKY, Foglia TA, Liu CK (2001) Glucose/lipid mixed substrates as a means of controlling the properties of medium chain length poly(hydroxyalkanoates). Biomacromolecules 2:211–216. https://doi.org/10.1021/bm000098+
Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF (1997) The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Braunegg G, Bona R, Koller M (2004) Sustainable polymer production. J Macromol Sci Part D Rev Polym Process 43:1779–1793
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-β -hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6:29–37
Causey TB, Zhou S, Shanmugam KT, Ingram LO (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc Natl Acad Sci USA 100:825–832. https://doi.org/10.1073/pnas.0337684100
Cesario MT, Raposo RS, de Almeida M, van Keulen F, Ferreira BS, da Fonseca MMR (2014) Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol 31:104–113. https://doi.org/10.1016/j.nbt.2013.10.004
Sambrook J (2001) Molecular cloning: a laboratory manual. Anal Biochem 186(1):182–183
Davis R, Kataria R, Cerrone F, Woods T, Kenny S, O’Donovan A, Guzik M, Shaikh H, Duane G, Gupta VK (2013) Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Biores Technol 150:202–209
Escapa IF, del Cerro C, Garcia JL, Prieto MA (2013) The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol 15:93–110. https://doi.org/10.1111/j.1462-2920.2012.02790.x
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate: sugar phosphotransferase system. Microbial Cell Fact 4(1):14. https://doi.org/10.1186/1475-2859-4-14
Gowda V, Shivakumar S (2014) Agrowaste-based polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz Arch Biol Technol 57:55–61. https://doi.org/10.1590/s1516-89132014000100009
Hirakawa H, Takumi-Kobayashi A, Theisen U, Hirata T, Nishino K, Yamaguchi A (2008) AcrS/EnvR represses expression of the acrAB multidrug efflux genes in Escherichia coli. J Bacteriol 190:6276–6279
Jiang X, Ramsay JA, Ramsay BA (2006) Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J Microbiol Methods 67:212–219
Jones JA, Wang X (2018) Use of bacterial co-cultures for the efficient production of chemicals. Curr Opin Biotechnol 53:33–38
Kenny ST, Runic JN, Kaminsky W, Woods T, Babu RP, O’Connor KE (2012) Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl Microbiol Biotechnol 95:623–633. https://doi.org/10.1007/s00253-012-4058-4
Kobayashi H, Fukuoka A (2013) Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem 15:1740–1763. https://doi.org/10.1039/c3gc00060e
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. https://doi.org/10.1016/0378-1119(95)00584-1
Kulkarni SO, Kanekar PP, Jog JP, Sarnaik SS, Nilegaonkar SS (2015) Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int J Biol Macromol 72:784–789. https://doi.org/10.1016/j.ijbiomac.2014.09.028
Le Meur S, Zinn M, Egli T, Thony-Meyer L, Ren Q (2012) Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. Bmc Biotechnol 12(1):53. https://doi.org/10.1186/1472-6750-12-53
Lee GN, Na J (2013) Future of microbial polyesters. Microb Cell Fact 12:54
Lin ZQ, Xu ZB, Li YF, Wang ZW, Chen T, Zhao XM (2014) Metabolic engineering of Escherichia coli for the production of riboflavin. Microbial Cell Fact 13(1):104. https://doi.org/10.1186/s12934-014-0104-5
Liu X, Li XB, Jiang JL, Liu ZN, Qiao B, Li FF, Cheng JS, Sun XC, Yuan YJ, Qiao JJ, Zhao GR (2018) Convergent engineering of syntrophic Escherichia coli coculture for efficient production of glycosides. Metab Eng 47:243–253. https://doi.org/10.1016/j.ymben.2018.03.016
Lowe H, Hobmeier K, Moos M, Kremling A, Pfluger-Grau K (2017) Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB. Biotechnol Biofuels 10(1):190. https://doi.org/10.1186/s13068-017-0875-0
Narayanan A, Kumar VAS, Ramana KV (2014) Production and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus mycoides DFC1 using rice husk hydrolyzate. Waste Biomass Valoriz 5:109–118. https://doi.org/10.1007/s12649-013-9213-3
Nizami AS, Korres NE, Murphy JD (2009) Review of the integrated process for the production of grass biomethane. Environ Sci Technol 43:8496–8508. https://doi.org/10.1021/es901533j
Obruca S, Benesova P, Petrik S, Oborna J, Prikryl R, Marova I (2014) Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem 49:1409–1414. https://doi.org/10.1016/j.procbio.2014.05.013
Pan WY, Perrotta JA, Stipanovic AJ, Nomura CT, Nakas JP (2012) Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J Ind Microbiol Biotechnol 39:459–469. https://doi.org/10.1007/s10295-011-1040-6
Poblete-Castro I, Binger D, Rodrigues A, Becker J, dos Santos V, Wittmann C (2013) In-silico-driven metabolic engineering of Pseudomonas putida for enhanced production of poly-hydroxyalkanoates. Metab Eng 15:113–123. https://doi.org/10.1016/j.ymben.2012.10.004
Pos KM (2009) Drug transport mechanism of the AcrB efflux pump. Biochim Biophys Acta Proteins Proteom 1794:782–793
Prabu CS, Murugesan AG (2010) Effective utilization and management of coir industrial waste for the production of poly-β-hydroxybutyrate (PHB) using the bacterium Azotobacter beijerinickii. Int J Environ Res 4:519–524
Rai R, Keshavarz T, Roether JA, Boccaccini AR, Roy I (2011) Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng R-Rep 72:29–47. https://doi.org/10.1016/j.mser.2010.11.002
Sang YL (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Sawant SS, Salunke BK, Kim BS (2015) Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp LL1. Biores Technol 194:247–255. https://doi.org/10.1016/j.biortech.2015.07.019
Shalin T, Sindhu R, Pandey A, Faraco V, Binod P (2016) Production of poly-3-hydroxybutyrate from mixed culture. Biologia 71(7):736–742
Shin KS, Lee SK (2017) Increasing extracellular free fatty acid production in Escherichia coli by disrupting membrane transport systems. J Agric Food Chem 65(51):11243–11250
Silva JA, Tobella LM, Becerra J, Godoy F, Martínez MA (2007) Biosynthesis of poly-β-hydroxyalkanoate by Brevundimonas vesicularis LMG P-23615 and Sphingopyxis macrogoltabida LMG 17324 using acid-hydrolyzed sawdust as carbon source. J Biosci Bioeng 103:542–546
Silva LF, Taciro MK, Michelin Ramos ME, Carter JM, Pradella JGC, Gomes JGC (2004) Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J Ind Microbiol Biotechnol 31:245–254
Sim SJ, Snell KD, Hogan SA, Stubbe J, Rha C, Sinskey AJ (1997) PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat Biotechnol 15:63–67. https://doi.org/10.1038/nbt0197-63
Griffiths E (1991) Prokaryotic physiology in perspective. Physiology of the Bacterial Cell: a molecular approach by F. C. Neidhart, J. L. Ingraham and M. Schaechter, Sinauer Associates, 1990. £34.95 (xii + 506 pages) ISBN 0 87893 608 4. Trends Biotechnol 9(1):442–443
Song H, Ding MZ, Jia XQ, Ma Q, Yuan YJ (2014) Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev 43:6954–6981. https://doi.org/10.1039/c4cs00114a
Steinbüchel A, Füchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Valappil SP, Rai R, Bucke C, Roy I (2008) Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J Appl Microbiol 104:1624–1635
Wang BQ, Sharma-Shivappa RR, Olson JW, Khan SA (2013) Production of polyhydroxybutyrate (PHB) by Alcaligenes latus using sugarbeet juice. Ind Crops Prod 43:802–811. https://doi.org/10.1016/j.indcrop.2012.08.011
Wang Q, Tappel RC, Zhu CJ, Nomura CT (2012) Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks. Appl Environ Microbiol 78:519–527. https://doi.org/10.1128/aem.07020-11
Wang Q, Zhuang QQ, Liang QF, Qi QS (2013) Polyhydroxyalkanoic acids from structurally-unrelated carbon sources in Escherichia coli. Appl Microbiol Biotechnol 97:3301–3307. https://doi.org/10.1007/s00253-013-4809-x
Wang Y, Yin J, Chen G-Q (2014) Polyhydroxyalkanoates, challenges and opportunities. Curr Opin Biotechnol 30:59–65
Xu P, Li L, Zhang F, Stephanopoulos G, Koffas M (2014) Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci USA 111:11299–11304
Xu P, Qiao K, Ahn WS, Stephanopoulos G (2016) Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc Natl Acad Sci 113:10848–10853
Yang SY, Li SH, Jia XQ (2019) Production of medium chain length polyhydroxyalkanoate from acetate by engineered Pseudomonas putida KT2440. J Ind Microbiol Biotechnol 46:793–800. https://doi.org/10.1007/s10295-019-02159-5
Yu J, Stahl H (2008) Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Biores Technol 99:8042–8048. https://doi.org/10.1016/j.biortech.2008.03.071
Zhang HR, Wang XN (2016) Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng 37:114–121. https://doi.org/10.1016/j.ymben.2016.05.007
Zhou K, Qiao KJ, Edgar S, Stephanopoulos G (2015) Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33(4):377–383
Acknowledgements
The authors are grateful for the kind donation of Escherichia coli MG1655 from Dr. Tao Chen and the plasmid pBBR1MCS-2 from Dr. Yingjin Yuan at Tianjin University. The authors wish to acknowledge the financial support provided by the National Key Research and Development Program of China (Project no. 2018YFA0902100), National Natural Science Foundation of China (No. 21576197), and Tianjin Research Program of Application Foundation and Advanced Technology (No. 18JCYBJC23500).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Yang, S. & Jia, X. Construction of a “nutrition supply–detoxification” coculture consortium for medium-chain-length polyhydroxyalkanoate production with a glucose–xylose mixture. J Ind Microbiol Biotechnol 47, 343–354 (2020). https://doi.org/10.1007/s10295-020-02267-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02267-7