Abstract
Glycosylphosphatidylinositol (GPI)-anchored glycoproteins have diverse intrinsic functions in yeasts, and they also have different uses in vitro. The GPI-modified cell wall proteins GCW21, GCW51, and GCW61 of Pichia pastoris were chosen as anchoring proteins to construct co-expression strains in P. pastoris GS115. The hydrolytic activity and the amount of Candida antarctica lipase B (CALB) displayed on cell surface increased significantly following optimization of the fusion gene dosage and combination of the homogeneous or heterogeneous cell wall proteins. Maximum CALB hydrolytic activity was achieved at 4920 U/g dry cell weight in strain GS115/CALB-GCW (51 + 51 + 61 + 61) after 120 h of methanol induction. Changes in structural morphology and the properties of the cell surfaces caused by co-expression of fusion proteins were observed by transmission electron microscopy (TEM) and on plates containing cell-wall-destabilizing reagent. Our results suggested that both the outer and inner cell layers were significantly altered by overexpression of GPI-modified cell wall proteins. Interestingly, quantitative analysis of the inner layer components showed an increase in β-1,3-glucan, but no obvious changes in chitin in the strains overexpressing GPI-modified cell wall proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most eukaryotes harbor glycosylphosphatidylinositol (GPI) anchoring machinery, which is used to attach proteins to cell surface. A few GPI-modified proteins are located in the plasma membrane, while the majority are processed further to integrate into the cell wall via covalently linked to cell wall β-glucans [22]. Analysis of GPI-modified proteins in Saccharomyces cerevisiae showed that Man1 of the GPI core structure is glycosidically-linked to β-1,6 glucan, which in turn is attached to β-1,3 glucan and chitin [12, 25]. The classification of GPI proteins into cell wall proteins or plasma membrane proteins is regarded as relative rather than absolute, and most can be found in both locations. The position information of these proteins only indicates the predominant localization [36].
There are three important factors affecting the yeast cell-surface display system: the anchoring protein, the target protein, and the host strain. Each component affects the efficiency of the surface display of heterologous proteins [16]. GPI-modified proteins have been used for displaying heterologous proteins on the yeast cell wall via the covalent attachment, which has been developed to produce recombinant strains for use in the pharmacological and food industries. S. cerevisiae is considered one of the most suitable microorganisms for study the cell-surface expression systems, and these systems have been applied in a wide range of research areas [15]. The proteins displayed on the cell surface can be directly, conveniently, and rapidly analyzed using intact cells without the next concentration and purification. This method is convenient for high-throughput screening of protein libraries carrying comprehensive mutations and genetically modified mutant proteins. Enzymes displayed on the cell surface can be used as an immobilized bio-catalyst, enabling bioconversion of substrates with high- or low molecular mass. In addition, co-displaying multiple kinds of enzyme on the cell surface can achieve the multistep and synergistic reactions [15].
To further this method, additional cell wall anchor proteins are needed. In our previous study, 50 potential GPI-anchored proteins were predicted from the genome of P. pastoris based on universal characteristics of yeast GPI-anchored proteins. In total, 13 GPI cell wall proteins were confirmed by flow cytometry and western blot analysis [37], and were then used in the construction of P. pastoris cell-surface expression systems. The proteins were fused with the gene encoding Candida antarctica lipase B (CALB), which was applied in esterification, hydrolysis, and transesterification reactions, amongst others [7, 10].
To further develop P. pastoris cell-surface expression systems, in this study, we selected endogenous cell wall proteins from P. pastoris GS115 for use as anchor proteins to display CALB. By optimizing the gene dosage and combination of different anchoring proteins, we further improved the amount of protein displayed on the cell surface, as well as the enzyme activity.
Materials and methods
Strains, plasmids, and cultivation media
Escherichia coli Top 10 and P. pastoris strain GS115 cells were purchased from Invitrogen (Carlsbad, CA, USA). For cloning purposes, E. coli Top 10 cells were grown at 37 °C in Luria–Bertani low salt medium (0.5% w/v yeast extract, 1% w/v tryptone, and 0.5% w/v NaCl) supplemented with 25 µg/ml Zeocin. The vectors pKCALB-GCW21, pKCALB-GCW51, and pKCALB-GCW61, which contained the fusion genes encoding the mature CALB cDNA and gene fragments of GPI-modified cell wall proteins, have been described previously [37]. P. pastoris GS115 cells were grown at 30 °C in YPD consisting of 1% w/v yeast extract, 2% w/v tryptone, and 2% w/v dextrose for sub-cultivation, in BMGY and BMMY culture media, containing 1% w/v yeast extract, 2% w/v peptone, 1.34% w/v yeast nitrogen base, 100 mM potassium phosphate buffer pH 6.0, and 1% v/v glycerol (in the case of BMGY) or 1% v/v methanol (in the case of BMMY), for growth and recombinant protein production, respectively. For phenotypic analysis, the recombinants were spotted on several different agar plates: YPD + CR (YPD plates supplemented with 35 μg/ml Congo red), YPD + CW (YPD plates supplemented with 10 μg/ml calcofluor white), and YPD + SDS (YPD plates supplemented with 500 μg/ml sodium dodecyl sulfate).
Construction of recombinant plasmids with different numbers of expression cassettes and expression in P. pastoris
pPICZαA was used to construct in vitro multimers by taking advantage of Bgl II and Bam HI restriction sites, which have four bases in common (GATC), at each end of the AOX1 promoter. The plasmids pKCALB-GCW21, pKCALB-GCW51, and pKCALB-GCW61 contained the FLAG tag, the mature CALB cDNA, and fragments of genes (without signal peptide sequences) encoding GPI-modified cell wall proteins (GCW21, GCW51, and GCW61), in addition to EcoR I and Not I restriction sites. The plasmids were digested using EcoR I and Not I and obtained fusion gene fragments CALB-GCW21, CALB-GCW51, and CALB-GCW61, respectively. The fusion gene fragments, and the plasmid pPICZaA digested with EcoR I and Not I, were ligated overnight. The resulting recombinant plasmids, named pZCALB-GCW21, pZCALB-GCW51, and pZCALB-GCW61, respectively, were transformed into E. coli Top 10 for propagation. The extracted plasmids were verified by restriction enzyme digestion and DNA sequencing. Single-copy plasmids were digested with Bgl II and Bam HI to release the Bgl II-Bam HI expression cassette. Single-copy plasmids were also linearized with BamHI, and a single copy of the Bgl II-Bam HI expression cassette was ligated into the linearized plasmid to generate plasmids containing two copies of the expression cassette. Three and four-copy plasmids were constructed in the same way. According to the combination of different fusion gene fragments, the multiple copy number plasmids pZCALB-GCW (21 + 21), pZCALB-GCW (51 + 51), pZCALB-GCW (61 + 61), pZCALB-GCW (21 + 51), pZCALB-GCW (51 + 61), pZCALB-GCW (61 × 3), pZCALB-GCW (61 × 4), and pZCALB-GCW (51 + 51 + 61 + 61) were constructed. The plasmids were transformed into P. pastoris GS115 by electroporation, according to the manufacturer’s instructions (Invitrogen), and the resulting transformants were selected by incubation at 30 °C for 72 h on YPDSZ plates.
Flow cytometry analysis
Flow cytometry analysis was performed using a modified version of Sun et al. [30]. After 120 h of methanol induction, the cells were harvested by centrifugation at 6000×g for 1 min and washed twice with 10 mM phosphate-buffered saline (PBS, pH 7.4). Finally cells were resuspended in PBS supplemented with 1% (m/v) bovine serum albumin (BSA) to block the cell surface. Subsequently, a monoclonal antibody against the FLAG tag (DYKDDDDK) was used as the primary antibody. A 200 μL cell suspension (OD600 = 5) was incubated with primary antibody (final concentration 10 ng/μL) on a rotator for 2 h at 37 °C. Then, cells were washed twice with PBS and resuspended in 200 μl of PBS (containing 1% m/v BSA) prior to being exposed to the secondary antibody, Alexa Fluor 488 goat anti-mouse IgG antibody (final concentration 10 ng/μL). Following incubation at 37 °C for 1 h, cells were washed three times with PBS, and then resuspended in 1.5 ml of PBS for flow cytometry analysis (Beckman Coulter, Fullerton, CA). A total of ten thousand cells of each sample were analyzed and the data were examined using the Exp032 software (Beckman Coulter).
Quantitative real-time PCR (qRT-PCR) assay to determine CALB copy number
qRT-PCR has been developed into an important and widely used analytical tool for determining gene copy number. qRT-PCR and the 2−ΔΔCT method were used to analyze CALB copy number in recombinants, as described previously [18]. The housekeeping gene GAPDH was selected as the internal control, and the genomic DNA of single-copy recombinant GS115/CALB-GCW21 was chosen as the calibrator. Genomic DNA of yeast recombinants was extracted using a yeast DNA extraction kit (Omega, USA), and diluted to obtain an approximately 1 ng/μl solution for the qRT-PCR assay. Assays were performed using a SYBR Premix Ex Taq II Kit (Takara, Japan) and Applied Biosystems 7500 fast real-time PCR instrument (Applied Biosystems Inc., Foster City, CA, USA). The mean CT values of CALB and GAPDH from the samples (CT-C sample, CT-G sample) and the calibrator (CT-C control, CT-G control) were entered into Eq. (1) to calculate the CALB copy number (ratio). Each sample was analyzed in triplicate:
Shake flask cultures and analysis of CALB activity
Transformants with a confirmed gene copy number were precultured in 250 mL flask with 25 ml BMGY medium. Cells were cultured at 30 °C to an optical density (OD600, 2–6) and then resuspended in BMMY medium. Pure methanol was added to make a final concentration of 1% (v/v) every 24 h to maintain induction of the expression of fusion proteins.
CALB hydrolytic activity was measured using a modified version of a previously described assay [14, 32]. P-nitrophenyl butyrate (pNPB; Sigma, St. Louis, MO, USA) was the substrate, which was emulsified by sonication in ultrapure water containing 0.5% Triton X-100, resulting in a final concentration of 25 mM. The reaction solution containing 940 μl of 50 mM Tris–HCl buffer (pH 8.0), 50 μl of substrate solution, and 10 μl of the cell suspension at an appropriate dilution, was incubated at 45 °C for 5 min. After centrifuging at 6000×g for 1 min, the hydrolytic activity of CALB was determined by measuring the absorbance of the liberated p-nitrophenol (pNP) at 405 nm using a microplate reader. One unit of CALB hydrolytic activity was defined as the amount of enzyme required to release 1 μmol pNP per min under the assay conditions. Average values were generated from triplicates of each sample.
Analysis of cell wall components in recombinant P. pastoris cells
Recombinant strains were grown in liquid YPD at 30 °C overnight. About 5 × 107 cells from each recombinant strain were subjected to tenfold serial dilution (from 10−1 to 10−5 relative to the initial culture) in sterile water. Aliquots (5 μl) from each dilution were then spotted on the agar plates (YPD + CR, YPD + CW, and YPD + SDS) and cultured at 30 °C for 72 h.
The total cellular chitin content analysis was performed using the protocol of Bulawa et al., and modified by Imai et al. [2, 9]. At 120 h post-induction, the recombinant cells were harvested by centrifugation at 6000×g for 1 min, and the cells were washed three times with TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0). About 50 mg of cells (wet weight) were collected and resuspended in 500 μl of 6% KOH. After incubation at 80 °C for 90 min, the 50 μl of glacial acetic acid was subsequently added. The appeared precipitation was washed twice with water and then resuspended in 250 μl of 50 mM sodium phosphate buffer (pH 6.3) containing 2-mg aliquot of Streptomyces griseus chitinase (Sigma, St. Louis, MO, USA). The tubes were incubated at 25 °C for 2 h with gentle agitation. After centrifugation at 8000×g for 5 min, 250 μl of supernatant were incubated with 1 mg of Helix pomatia β-glucuronidase (Sigma, St. Louis, MO, USA) at 37 °C for 2 h with gentle agitation. An aliquot of the mixture for N-acetylglucosamine content was assayed according to Reissig et al. [24]. The cell wall chitin content was expressed as mg GlcNAc per 107 cells.
The β-1,3-glucan content was measured as described previously [27], with some modification. Induced cells expressing the recombinant proteins were harvested at 120-h post-induction and diluted to OD600 = 1 in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0). A 1-mL volume of cells was washed three times and then resuspended in 250 μl of TE buffer. NaOH was added to the suspension with a final concentration of 1 M and then incubated for 30 min at 80 °C, followed by the addition of 1.05 ml of aniline blue mix consisting of 0.03% aniline blue, 0.18 M HCl, and 0.49-M glycine–NaOH, pH 9.5. Tubes were then briefly vortexed (30 s) and incubated at 50 °C for 30 min. Fluorescence of β-1,3-glucan was measured with excitation wavelength at 386 nm and emission wavelength at 460 nm by an Infinite M200 microplate reader (Tecan, USA).
Results
Effect of anchoring protein on the display of CALB on the cell surface
To examine the effect of anchoring protein on enzyme activity and the amount of fusion protein expressed on the P. pastoris cell surface, CALB was used as the target protein. The GPI-modified cell wall proteins GCW21, GCW51, and GCW61 were selected as the anchoring based on previous findings that these proteins have an excellent effect on the display of heterologous proteins [37].
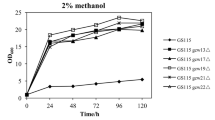
Based on our previous research, we constructed three recombinant strains with two copies of the fusion protein expression cassette. qRT-PCR assays were performed to precisely determine the fusion gene copy number in the genomes of the recombinant strains. Finally, six strains with either one or two copies of the fusion gene were analyzed, and the strains are presented in Table 1. As shown in Fig. 1, the hydrolytic activity of GS115/CALB-GCW61 was higher than that of GS115/CALB-GCW21 and GS115/CALB-GCW51 at 120 h. In addition, the CALB hydrolytic activity of strain GS115/CALB-GCW (61 + 61), containing two copies of the fusion protein expression cassette, was higher than that of the other four recombinant strains containing two copies of the gene at 120 h.
Properties of recombinants with either one or two copies of the fusion protein expression cassettes. a CALB hydrolytic activity curves of single-copy number recombinants with different anchoring proteins. b CALB hydrolytic activity curves of two-copy recombinants with the same or different anchoring proteins. c Flow cytometry analysis of single-copy recombinants with different anchoring proteins. d Flow cytometry analysis of two-copy recombinants with the same or different anchoring proteins. Data were obtained from three independent experiments
The flow cytometry was used to analyze the amount of fusion protein on yeast cell surfaces by indirect immunofluorescence using the FLAG tag. The amount of fusion protein on the cell surface of double-copy strains was higher than in single-copy strains, but there were no significant differences among strains based on the type of GPI-modified cell wall protein, regardless of whether the strains were single copy or double copy. Therefore, GPI-modified cell wall proteins appear to have a greater influence on enzyme activity than on the amount of fusion protein expressed on the cell surface.
Effect of gene dosage on the display of CALB on the cell surface
To examine the effect of gene dosage on the amount of CALB displayed on the cell surface, we constructed recombinant strains with either homogeneous or heterogeneous anchor protein fusions. qRT-PCR assays were performed to precisely determine the fusion gene copy number in the genomes of the recombinant strains. An eight-copy GCW61 strain was confirmed with qRT-PCR when the plasmid pZCALB-GCW (61 × 4) was transformed into the host strain GS115. We compared seven recombinant strains, as shown in Table 2. As shown in Fig. 2a, b, the growth curve of the two-copy strain GS115/CALB-GCW (61 + 61) and GS115/CALB-GCW (51 + 61) shares a remarkable similarity to that of the strain GS115/CALB-GCW61. While the growth curves decreased when there were more than three copies of the fusion gene in the cells, regardless of whether the anchoring proteins were homogeneous or heterogeneous.
Growth characteristics and protein expression properties of recombinants with different gene dosages. a Growth curves of strains with different GCW61 gene dosages. b Growth curves of strains with different GCW51 and GCW61 gene dosages. c CALB hydrolytic activity curves of strains with different GCW61 gene dosages. d CALB hydrolytic activity curves of strains with different GCW51 and GCW61 gene dosages. e Flow cytometry analysis of strains with different GCW61 gene dosages. f Flow cytometry analysis of strains with different GCW51 and GCW61 gene dosages. Data were obtained from three independent experiments
The hydrolytic activity of the CALB displayed on the cell surface was measured in each of the recombinant strains. When GPI-modified cell wall protein GCW61 was used as the anchor protein, maximum CALB hydrolytic activity (4451 U/g dry cell weight) was observed at 120-h post-induction in strain GS115/CALB-GCW (61 + 61), which contained two copies of the fusion protein (Fig. 2c). The two-copy strain showed a 1.88-fold increase in hydrolytic activity compared with the single-copy strain GS115/CALB-GCW61, while three and four-copy GCW61 strains GS115/CALB-GCW (61 × 3) and GS115/CALB-GCW (61 × 4) showed 1.74 and 1.84-fold increases, respectively, in CALB activity compared with the single-copy strain at 120 h. However, the CALB hydrolytic activity decreased dramatically in the strain GS115/CALB-GCW (61 × 8).
When GPI-modified cell wall proteins GCW51 and GCW61 were used in conjunction, the highest CALB hydrolytic activity (4920 U/g dry cell weight) at 120 h was observed in strain GS115/CALB-GCW (51 + 51 + 61 + 61). In strains with fewer than four fusion gene copies, the CALB hydrolytic activity was positively correlated with the gene dosage. GS115/CALB-GCW (51 + 61) and GS115/CALB-GCW (51 + 51 + 61 + 61) showed increases of approximately 1.59- and 2.08-fold, respectively, compared with the single-copy strain GS115/CALB-GCW61 at 120 h post-induction (Fig. 2d). These results indicated that the hydrolytic activity of CALB could be improved by optimizing the gene dosage. However, the level of CALB activity of multi-copy strains varied depending on the anchor proteins used.
The amount of fusion protein on yeast cell surfaces was also analyzed by indirect immunofluorescence using the FLAG tag and flow cytometry. As shown in Fig. 2e, f, the amount of fusion protein on the cell surface increased with increasing fusion gene copy number. For recombinant strains with less than three fusion gene copies, the amount of fusion protein on the yeast cell surface was positively correlated with the gene dosage, regardless of whether the anchoring proteins were homogeneous or heterogeneous. The amount of fusion protein on the surface of GS115/CALB-GCW (61 × 4) and GS115/CALB-GCW (61 × 8) cells was very similar to that of GS115/CALB-GCW (61 × 3).
Analysis of cell wall components of recombinant strains
To further characterize changes in the cell wall introduced by anchoring proteins, the co-expression recombinants with homogeneous or heterogeneous GPI-modified cell wall protein were observed on plates containing cell-wall-destabilizing reagents CW (calcofluor white), CR (congo red), and SDS (sodium dodecyl sulfate). Disruption or overexpression of a gene related to chitin synthesis makes the cell more resistant to CW [1, 21], whereas chitin is the primary binding target for CR in yeast [9]. As shown in Fig. 3, growth of the recombinant strains was unchanged on YPD + CW and YPD + CR plates, confirming that the chitin content of the inner layer of the cell wall showed no obvious change compared with P. pastoris GS115. Interestingly, recombinant strains were more resistant to SDS than wild-type cells, indicating that the outer layer of the cell wall in these strains, which is composed predominantly of mannoproteins, was strengthened.
The chitin and β-1,3-glucan content of each recombinant strain were also measured. As shown in Fig. 4a, b, compared with P. pastoris GS115, there were no significant differences in the chitin content of the cell wall of multi-copy strains, regardless of whether homogeneous or heterogeneous anchoring proteins were used. However, the β-1,3-glucan content in the cell wall of strains with more than two copies of the fusion gene was higher than that of control strain GS115, single-copy strains, and two-copy strains, irrespective of whether homogeneous or heterogeneous anchoring proteins were used (Fig. 4c, d).
Discussion
Yeast cell-surface display technology has been used to produce enzymes by attaching the proteins to the outer side of the yeast cell wall [13]. The enzymes displayed on the cell surface are synchronously produced and immobilized in a single step [5, 35]. Yeast cell-surface display can also be used to construct multiple enzymes on a single cell and to catalyze a series of bio-reactions. For these applications, many cell wall proteins have been used as anchors, including α-agglutinin and Flo1 from S. cerevisiae [13]. The covalent binding of the anchor protein to the cell wall is strong [31], and exogenous anchoring proteins from yeast have been used to improve the efficiency of enzyme display [29]. In this study, endogenous cell wall proteins from P. pastoris were used to display the lipase CALB. Compared with the enzyme activity of strains using different GPI-modified cell wall proteins, we found that GPI-modified cell wall protein GCW61 produced the best results for displaying CALB on the surface of P. pastoris. This anchoring protein also exhibited high efficiency in the display of other heterologous proteins on the surface of P. pastoris, including hydrophobins [34], phytase [17], and β-glucosidase [6].
However, the performance of bio-catalyst can be affected by many factors, such as promoter strength, signal peptide source, type and size of the anchor, host secretion system, size and conformation of enzyme, availability of the active site, and so on. As such, it is not easy to find a balance between these aspects [8]. Previous studies have suggested that the conformation of the enzyme and its interaction with the anchor on the cell surface play an important role on the activity of the bio-catalyst [26]. As shown by Sun et al., the catalytic efficiency can also be affected by the interactions with the active site of the protein [30]. Based on our previous work, we investigated whether a combination of different anchor proteins could be used to display CALB on the cell surface. Strain GS115/CALB-GCW (51 + 51 + 61 + 61) contained the CALB fusion protein with two GCW51 anchor proteins and two GCW61 anchor proteins. The maximum hydrolytic activity (4920 U/g) of CALB from GS115/CALB-GCW (51 + 51 + 61 + 61) was 1.13 times that of strain GS115/CALB-GCW (61 × 4) (4338 U/g), even though the two strains both contained four copies of the fusion gene construct. To compare the efficiency of other combinations of anchor proteins, we constructed another strain GS115/CALB-GCW (12 + 51 + 61), containing the CALB fusion protein with anchor proteins GCW12, GCW51, and GCW61. The hydrolytic activity of GS115/CALB-GCW (12 + 51 + 61) was similar to that of the strains containing four copies of the fusion gene construct and 1.08 times that of strain GS115/CALB-GCW (61 × 3). However, the two-copy strains GS115/CALB-GCW (21 + 51) and GS115/CALB-GCW (51 + 61) did not show an increase in hydrolytic activity compared with GS115/CALB-GCW (61 + 61). We speculated that using different anchoring proteins to display enzymes on the cell surface could promote proper enzyme conformation and increase the availability of the active sites, usually in high levels of protein expression.
For the growth and protein expression of multi-copy strains, we found that the cell growth was decreased when there were more than three copies of the fusion gene in the cells, regardless of whether the proteins were homogeneous or heterogeneous. Cos et al. [4] and Sha et al. [28] also reported that increasing gene copy number decreased growth of recombinant strains. From the analysis of flow cytometry, the amount of fusion protein expressed on the yeast cell surface could be improved by optimization of the gene dosage. However, the amount of fusion protein on the cell surface was reduced when the fusion gene copy number reached a certain limit. This phenomenon might be caused by limitations in the number of binding sites available for anchoring proteins on the cell wall. When the copy number reached eight, cell growth and heterogeneous protein production may be inhibited because of overloading.
Of the many fungal GPI-modified proteins, most are integrated into the cell wall by covalent attachment to cell wall glucans via a remnant of their GPI anchor. The GPI of a membrane-anchored protein is thought to be released from the plasma membrane within its glycan, and an intermediate form appears, whose GPI remnant is then transferred to cell wall β-1,6-glucan. The glucan portion is in turn covalently cross-linked to β-1,3-glucan [11, 19, 20, 33]. When fusion proteins containing GPI-modified proteins were overexpressed in P. pastoris, the β-1,3-glucan portion was needed to support more linkage sites to display fusion proteins, which might lead to an increase in β-1,3-glucan. Meanwhile, if increasing numbers of fusion proteins are displayed on the cell surface, the outer layer of the cell wall could become more compact. It has been reported that if the chitin (or glucan) content of the cell wall is reduced, the cell wall mutant strain synthesizes excessive glucan (or chitin). The result may be explained by a compensatory response to ensure cell integrity [3, 23]. However, in our study, the overexpression of fusion proteins with GPI-modified cell wall proteins led to an increase in β-1,3-glucan in the cell wall compared with P. pastoris GS115, while the chitin content remained unchanged. The β-1,3-glucan level in the cell wall was increased when GPI-modified cell wall proteins were overexpressed, regardless of whether homogeneous or heterogeneous anchor proteins were used to display CALB. Similar results were obtained by TEM study for the GCW51 displaying, as shown in Fig. 5. The outer layer of the cell wall was compact and dense in recombinant strains GS115/CALB-GCW51 and GS115/CALB-GCW (51 × 4) compared with P. pastoris GS115, and the thickness of the cell wall inner layer was greater in the GS115/CALB-GCW (51 × 4) recombinant strain. These findings were in accordance with the estimated β-1,3-glucan content in the strains with high levels of cell wall protein expression, and indicated that the high level expression of GPI-modified cell wall fusion proteins increased the amount of β-1,3-glucan and altered the thickness of the inner layer of the cell wall.
Electron microscopy of the three recombinant yeast strains. The structural preservation was very good, as demonstrated in the whole-cell micrographs of a GS115, b GS115/CALB-GCW51, and c GS115/CALB-GCW (51 × 4). Images were further magnified respectively in d GS115, e GS115/CALB-GCW51, and f GS115/CALB-GCW (51 × 4)
In summary, endogenous GPI-modified proteins from P. pastoris could be used as anchors to construct a cell-surface display system, and increase the expression and activity of lipase in a multi-copy gene strategy. The conformation and activity of lipase were better when heterogeneous GPI-modified proteins were used as anchors than when homogeneous anchors were used, most likely because the heterogeneous GPI-modified proteins reduced the steric hindrance and the competition with each other on the cell surface. This co-expression system is the starting point for further research that may have great value for the development of high efficiency whole-cell biocatalysts, multi-enzyme complexes, and vaccines.
References
Bulawa CE (1992) CSD2, CSD3, and CSD4, genes required for chitin synthesis in Saccharomyces cerevisiae: the CSD2 gene product is related to chitin synthases and to developmentally regulated proteins in Rhizobium species and Xenopus laevis. Mol Cell Biol 12(4):1764–1776. doi:10.1128/MCB.12.4.1764
Bulawa CE, Slater M, Cabib E, Au-Young J, Sburlati A, Adair WL Jr, Robbins PW (1986) The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell 46(2):213–225. doi:10.1016/0092-8674(86)90738-5
Carotti C, Ferrario L, Roncero C, Valdivieso MH, Duran A, Popolo L (2002) Maintenance of cell integrity in the gas1 mutant of Saccharomyces cerevisiae requires the Chs3p-targeting and activation pathway and involves an unusual Chs3p localization. Yeast 19(13):1113–1124. doi:10.1002/yea.905
Cos O, Serrano A, Montesinos JL, Ferrer P, Cregg JM, Valero F (2005) Combined effect of the methanol utilization (Mut) phenotype and gene dosage on recombinant protein production in Pichia pastoris fed-batch cultures. J Biotechnol 116(4):321–335. doi:10.1016/j.jbiotec.2004.12.010
Fukuda H, Hama S, Tamalampudi S, Noda H (2008) Whole-cell biocatalysts for biodiesel fuel production. Trends Biotechnol 26(12):668–673. doi:10.1016/j.tibtech.2008.08.001
Guo D, Xu Y, Kang Y, Han S, Zheng S (2016) Synthesis of octyl-β-d-glucopyranoside catalyzed by Thai rosewood β-glucosidase-displaying Pichia pastoris in an aqueous/organic two-phase system. Enzyme Microb Tech 85:90–97. doi:10.1016/j.enzmictec.2015.07.006
Guo DH, Zi J, Xu YS, Ping W, Ying L, Han SY, Zheng SP (2015) Scaling-up the synthesis of myristate glucose ester catalyzed by a CALB-displaying Pichia pastoris whole-cell biocatalyst. Enzyme Microb Tech 75–76:30–36. doi:10.1016/j.enzmictec.2015.04.002
He X, Liu N, Li W, Zhang Z, Zhang B, Ma Y (2008) Inducible and constitutive expression of a novel thermostable alkaline β-mannanase from alkaliphilic Bacillus sp. N16-5 in Pichia pastoris and characterization of the recombinant enzyme. Enzyme Microb Tech 43 (1):13–18. doi:10.1016/j.enzmictec.2008.03.011
Imai K, Noda Y, Adachi H, Yoda K (2005) A novel endoplasmic reticulum membrane protein Rcr1 regulates chitin deposition in the cell wall of Saccharomyces cerevisiae. J Biol Chem 280(9):8275–8284. doi:10.1074/jbc.M409428200
Jin Z, Han SY, Zhang L, Zheng SP, Wang Y, Lin Y (2013) Combined utilization of lipase-displaying Pichia pastoris whole-cell biocatalysts to improve biodiesel production in co-solvent media. Bioresour Technol 130(2):102–109. doi:10.1016/j.biortech.2012.12.020
Kapteyn JC, Montijn RC, Vink E, de la Cruz J, Llobell A, Douwes JE, Shimoi H, Lipke PN, Klis FM (1996) Retention of Saccharomyces cerevisiae cell wall proteins through a phosphodiester-linked beta-1,3-/beta-1,6-glucan heteropolymer. Glycobiology 6(3):337–345. doi:10.1093/glycob/6.3.337
Kollar RR, Reinhold BB, Petráková EE, Yeh HJC, Ashwell GG, Drgonová JJ, Kapteijn JC, Klis FM, Cabib EE (1997) Architecture of the yeast cell wall. b (1-6)-glucan interconnects mannoprotein, b (1-3)-glucan, and chitin. J Biol Chem 272(28):17762–17775. doi:10.1074/jbc.272.28.17762
Kondo A, Ueda M (2004) Yeast cell-surface display-applications of molecular display. Appl Microbiol Biot 64(1):28–40. doi:10.1007/s00253-003-1492-3
Kordel M, Hofmann B, Schomburg D, Schmid RD (1991) Extracellular lipase of Pseudomonas sp. strain ATCC 21808: purification, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol 173(15):4836–4841
Kuroda K, Ueda M (2013) Arming technology in yeast-novel strategy for whole-cell biocatalyst and protein engineering. Biomolecules 3(3):632–650. doi:10.3390/biom3030632
Lee SY, Choi J, Xu Z (2003) Microbial cell-surface display. Trends Biotechnol 21:45–52. doi:10.1016/S0167-7799(02)00006-9
Li C, Lin Y, Huang Y, Liu X, Liang S (2014) Citrobacter amalonaticus phytase on the cell surface of Pichia pastoris exhibits high pH stability as a promising potential feed supplement. PLoS One 9(12):e114728. doi:10.1371/journal.pone.0114728
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)). Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Lu CF, Kurjan J, Lipke PN (1994) A pathway for cell wall anchorage of Saccharomyces cerevisiae alpha-agglutinin. Mol Cell Biol 14(7):4825–4833. doi:10.1128/MCB.14.7.4825
Lu CF, Montijn RC, Brown JL, Klis F, Kurjan J, Bussey H, Lipke PN (1995) Glycosyl phosphatidylinositol-dependent cross-linking of alpha-agglutinin and beta 1,6-glucan in the Saccharomyces cerevisiae cell wall. J Cell Biol 128(3):333–340
Martin H, Dagkessamanskaia A, Satchanska G, Dallies N, Francois J (1999) KNR4, a suppressor of Saccharomyces cerevisiae cwh mutants, is involved in the transcriptional control of chitin synthase genes. Microbiology 145 (Pt1) (1):249–258. doi:10.1099/13500872-145-1-249
Pittet M, Conzelmann A (2007) Biosynthesis and function of GPI proteins in the yeast Saccharomyces cerevisiae. Biochim Et Biophys Acta 1771(1771):405–420. doi:10.1016/j.bbalip.2006.05.015
Ram AF, Kapteyn JC, Montijn RC, Caro LH, Douwes JE, Baginsky W, Mazur P, van den Ende H, Klis FM (1998) Loss of the plasma membrane-bound protein Gas1p in Saccharomyces cerevisiae results in the release of beta1,3-glucan into the medium and induces a compensation mechanism to ensure cell wall integrity. J Bacteriol 180(6):1418–1424
Reissig JL, Storminger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217(2):959–966
Roman K, Reinhold BB, Gilbert A (1997) Architecture of the yeast cell wall. J Biol Chem 272(3):17762–17775
Sato N, Matsumoto T, Ueda M, Tanaka A, Fukuda H, Kondo A (2002) Long anchor using Flo1 protein enhances reactivity of cell surface-displayed glucoamylase to polymer substrates. Appl Microbiol Biot 60(4):469–474. doi:10.1007/s00253-002-1121-6
Sekiya-Kawasaki M, Abe M, Saka A, Watanabe D, Kono K, Minemura-Asakawa M, Ishihara S, Watanabe T, Ohya Y (2002) Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, Saccharomyces cerevisiae. Genetics 162(2):663–676. doi:10.3103/S0096392510040279
Sha C, Yu XW, Li F, Xu Y (2013) Impact of gene dosage on the production of lipase from Rhizopus chinensis CCTCC M201021 in Pichia pastoris. Appl Biochem Biotechnol 169(4):1160–1172. doi:10.1007/s12010-012-0050-9
Su GD, Huang DF, Han SY, Zheng SP, Lin Y (2010) Display of Candida antarctica lipase B on Pichia pastoris and its application to flavor ester synthesis. Appl Microbiol Biot 86(5):1493–1501. doi:10.1007/s00253-009-2382-0
Sun YF, Lin Y, Zhang JH, Zheng SP, Ye YR, Liang XX, Han SY (2012) Double Candida antarctica lipase B co-display on Pichia pastoris cell surface based on a self-processing foot-and-mouth disease virus 2A peptide. Appl Microbiol Biot 96(6):1539–1550. doi:10.1007/s00253-012-4264-0
Fukuda TT, Kondo HA (2006) Construction of a Pichia pastoris cell-surface display system using Flo1p anchor system. Biotechnol Prog 22(4):989–993. doi:10.1021/bp060133
Tanino T, Ohno T, Aoki T, Fukuda H, Kondo A (2007) Development of yeast cells displaying Candida antarctica lipase B and their application to ester synthesis reaction. Appl Microbiol Biotechnol 75(6):1319–1325. doi:10.1007/s00253-007-0959-z
Van der Vaart JM, te Biesebeke R, Chapman JW, Klis FM, Verrips CT (1996) The beta-1, 6-glucan containing side-chain of cell wall proteins of Saccharomyces cerevisiae is bound to the glycan core of the GPI moiety. FEMS Microbiol Lett 145(3):401–407. doi:10.1016/S0378-1097(96)00440-5
Wang P, He J, Sun Y, Reynolds M, Zhang L, Han S, Liang S, Sui H, Lin Y (2016) Display of fungal hydrophobin on the Pichia pastoris cell surface and its influence on Candida antarctica lipase B. Appl Microbiol Biotechnol 100(13):1–13. doi:10.1007/s00253-016-7431-x
Yan J, Zheng X, Li S (2014) A novel and robust recombinant Pichia pastoris yeast whole cell biocatalyst with intracellular overexpression of a Thermomyces lanuginosus lipase: preparation, characterization and application in biodiesel production. Bioresour Technol 151(1):43–48. doi:10.1016/j.biortech.2013.10.037
Yin QY, de Groot PW, Dekker HL, De JL, Klis FM, de Koster CG (2005) Comprehensive proteomic analysis of Saccharomyces cerevisiae cell walls: identification of proteins covalently attached via glycosylphosphatidylinositol remnants or mild alkali-sensitive linkages. J Biol Chem 280(21):20894–20901. doi:10.1074/jbc.M500334200
Zhang L, Liang SL, Zhou XY, Jin Z, Jiang FC, Han SY, Zheng SP, Lin Y (2013) Screening for glycosylphosphatidylinositol-modified cell wall proteins in Pichia pastoris and their recombinant expression on the cell surface. Appl Environ Microb 79(18):5519–5526. doi:10.1128/AEM.00824-13
Acknowledgements
The authors thank Jie He, Shufeng Sun, and Matthew Reynolds at the Wadsworth Center for their assistance in yeast cell growth, specimen preparation, and electron microscopy. This work was supported in whole by the grants to Ying Lin from, the China National High Technology Research and Development Program (863-2012AA022205), High Technology Research and Development of Guangdong Province (2012A080800013), and The Recruitment Program of Leading Talents in Innovation and Entrepreneurship of Guangzhou (LCY201322), and in part by the National Institute of Health (NIH) Grants GM097010 and GM101026 to Haixin Sui.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported in whole by the grants to Ying Lin from, the China National High Technology Research and Development Program (863-2012AA022205), High Technology Research and Development of Guangdong Province (2012A080800013), and The Recruitment Program of Leading Talents in Innovation and Entrepreneurship of Guangzhou (LCY201322), and in part by the National Institute of Health (NIH) Grants GM097010 and GM101026 to Haixin Sui.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Rights and permissions
About this article
Cite this article
Wang, P., Zhang, L., Fisher, R. et al. Accurate analysis of fusion expression of Pichia pastoris glycosylphosphatidylinositol-modified cell wall proteins. J Ind Microbiol Biotechnol 44, 1355–1365 (2017). https://doi.org/10.1007/s10295-017-1962-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1962-8