Abstract
The non-conventional yeast Kluyveromyces marxianus is an emerging industrial producer for many biotechnological processes. Here, we show the application of a biomass-linked stoichiometric model of central metabolism that is experimentally validated, and mass and charge balanced for assessing the carbon conversion efficiency of wild type and modified K. marxianus. Pairs of substrates (lactose, glucose, inulin, xylose) and products (ethanol, acetate, lactate, glycerol, ethyl acetate, succinate, glutamate, phenylethanol and phenylalanine) are examined by various modelling and optimisation methods. Our model reveals the organism’s potential for industrial application and metabolic engineering. Modelling results imply that the aeration regime can be used as a tool to optimise product yield and flux distribution in K. marxianus. Also rebalancing NADH and NADPH utilisation can be used to improve the efficiency of substrate conversion. Xylose is identified as a biotechnologically promising substrate for K. marxianus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kluyveromyces marxianus is an ascomycoteus yeast with enormous biotechnological potential for multiple industrial applications. There are a number of characteristics of K. marxianus that are industrially useful, including fast growth, broad substrate spectrum, thermotolerance, limited fermentation at sugar excess, and secretion of extracellular glycolytic enzymes. In addition, K. marxianus enjoys GRAS (Generally Regarded as Safe) status and therefore is useful in food- or pharma-related applications [30, 46].
Kluyveromyces marxianus can grow on glucose, fructose, xylose, galactose, lactose and inulin as the sole carbon sources [24]. Many of these carbon sources are of particular interest since they are waste products of forestry (xylose) or dairy (lactose) industries. Xylose is a pentose and the main sugar of plant hemicellulose; its content in hard wood wastes can be up to 30% [50]. K. marxianus has been engineered for xylitol production from xylose [42]. Cheese whey is a lactose-rich byproduct of the dairy industry produced in an approximate 10–1 (v/w) ratio to cheese. Currently, whey is considered as a potential substrate for future microbial fermentations [25, 65]. Inulin is one of the widely available plant polysaccharides common in many taxonomic groups (Asteraceae family, wheat, onion, banana, etc.). Some of those (e.g. Jerusalem artischoke, chicory) accumulate inulin in their underground tubers in vast amounts [11, 18]. These plants might serve as “niche” substrates for fermentations by yeasts including K. marxianus, [11] if not deprecated on account of competition with food use.
Kluyveromyces marxianus is a prospective producer for a range of important food additives and chemicals: phenylethanol, phenylalanine [60], hexanoic acid [10], xylitol [107, 108] and ethylacetate [52]. Due to its protein excretion, K. marxianus is suitable for extracellular protein production (galactosidase, inulinase, etc.) [26, 98].
Stoichiometric models and reconstructions significantly facilitate analysis of metabolic effects and limitations of microorganism metabolism, as well as predicting the phenotype of recombinant strains [39, 40]. Modelling attempts on K. marxianus to date have been concentrated on particular problems: e.g. kinetic models of ethanol batch fermentation [77], and of growth on cheese whey [51]. The first attempt at a genome-scale metabolic reconstruction [47] is patented in unreadable form and cannot be used for metabolic flux calculations. A genome-scale metabolic model for the related species K. lactis has been published [16]. Analysing medium-scale stoichiometric models of central metabolism, where the most significant metabolic fluxes are, has been successful for biotechnological applications. Examples include assessment and selection of productive routes in Escherichia coli [88–90] and Zymomonas mobilis [68]. Medium-scale modelling also proved to be a successful strategy for describing the uncharacterised central metabolism of the non-conventional yeast Pichia pastoris on the basis of limited wet experimentation [87]. Recent extensive attempts at K. marxianus metabolic engineering [33, 42, 43, 105, 109] underline the immediate need for modelling of limits of its metabolic potential.
The aim of this study was to assess the biotechnological potential of K. marxianus by a constraint-based stoichiometric [79] modelling approach. A biomass-coupled model of central metabolism was developed to be a basis for design of metabolic engineering and to assess in silico the production of ethanol, acetate, lactate, glycerol, ethylacetate, succinate, glutamate phenylethanol, phenylalanine. As well as being useful products in their own right, they are also representatives of other products that could be derived from the same precursor metabolites.
Materials and methods
Modelling methodology and software
Two major strands of stoichiometric modelling are the constraint-based flux balance analysis (FBA) [63, 94] and elementary modes analysis [80]. A constraint-based model of central metabolism including biomass production of K. marxianus was created adapting and combining the high-quality genome-scale metabolic reconstructions protocol [79] and structural modelling approach for development of medium-scale reconstruction and models [39].
Our medium-scale K. marxianus central carbon metabolism model is based on the general mass balance equation:
With respect to intermediate metabolite accumulation, a cell’s metabolism is in pseudosteady state and can be described by the following equation [85]:
We also assume the following:
-
the specific growth rate (μ, h−1) during the exponential growth phase is constant,
-
the cells are at pseudosteady state: substrate uptake, metabolite and product fluxes are constant when μ is constant.
For constraint-based and structural analysis, the ScrumPy modelling package [71] was used. Flux balance analysis (FBA) was carried out by setting a constant rate of substrate uptake to 10 mM g−1 DW h−1, and searching for the maximum yield of one of the following products: ethanol, acetate, lactate, glycerol, ethylacetate, succinate, glutamate, phenylethanol or phenylalanine. Solutions were further examined using flux variability analysis (FVA) [55] to determine the ranges of internal fluxes that are consistent with the maximum if there were multiple equivalent FBA solutions. Inconsistencies in the model formulation were additionally detected through null space analysis [21] combined with determination of inconsistent enzyme subsets [69] using ScrumPy. The essentiality of genes and reactions was analysed using FBA to check whether biomass production was feasible after deleting the relevant reaction(s) from the model. The gene essentiality test took into account the gene–protein-reaction (GPR) associations [86] that were determined for the model (next subsection). FVA was also used to calculate the potential range in product production taking into account minimal and maximal oxygen respiration levels at a fixed substrate uptake value.
Reactions
The K. lactis genome-scale reconstruction [16] was used as a starting point given the high degree of similarity between its metabolic networks and that of K. marxianus. The amino acid sequences of K. lactis genes from the NIH genetic sequence database GenBank [3] were compared against fungal species using NCBI BLAST [38]. The corresponding K. marxianus genes were also checked for presence in the Uniprot database [54]. For each reaction, its Enzyme Commission number (E.C. number) and reaction directionality were checked and validated. The IntEnz [22] (available at http://www.ebi.ac.uk/intenz/) database was the main reference source for mass and charge balance validation. To represent the K. marxianus biomass growth reaction, we used the S. cerevisiae biomass composition as described by Gombert et al. [31].
Metabolites
Metabolite names, their neutral and charged formulas and InChI (International Chemical Identifier) strings [22] were taken from the CheBi database [13] (available at http://www.ebi.ac.uk/chebi/), and the yeast-specific Metacyc [7]. The PubChem database [96] (available at http://pubchem.ncbi.nlm.nih.gov/) was used to get additional information about metabolites [22].
Kluyveromyces marxianus strains and cultivation conditions
The results of original experiments carried out by us to provide data for model development are marked in Table 1 as “this study”. K. marxianus strain DSM 5422 was cultivated in semi-synthetic medium containing (g/l) KHPO4 (1.0), CaCl2 (0.1), MgSO4·7H2O (0.5), NaCl (0.5), (NH4)2SO4 (5.0) KH2PO4 (0.1) yeast extract (Biolife) (0.5). Different carbon sources (lactose or inulin) were added at concentrations of 5 or 10% w/v. All fermentations were carried out in 1 litre Infors 2HT or 0.4 litre Sartorius Biostat Qplus 6-fold system fermenters at 35 °C and 400 rpm.
Metabolite and biomass analyses
Extracellular lactose, ethanol, acetate and glycerol contents were measured simultaneously using an Agilent 1100 HPLC system with a Shodex Asahipak SH1011 column. Metabolites were quantitated with a refractive index detector (RI detector RID G1362A). The flow of the mobile phase (0.01 N H2SO4) was 0.6 ml min−1; the sample injection volume was 5 μL.
Biomass growth was estimated by absorbance measurements at 600 nm (OD600). The conversion coefficient of K. marxianus DSM 5422 strain OD600 to culture dry weight was determined gravimetrically: OD600 1.0 was equivalent to 0.3 g dw.L−1.
Results and Discussion
Model construction and properties
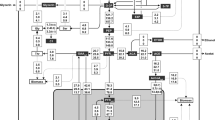
The model is shown diagrammatically in Fig. 1 and is supplied in SBML [34] (Online Resource 1) format and in the form of a COBRA [79] MS Excel input file (Online Resource 2). Our K. marxianus metabolism model contains 113 reactions and 101 metabolites organised in 3 compartments: extracellular, cytoplasm and mitochondria. There are 72 cytosolic reactions (central metabolism pathways), 28 transmembrane transport reactions, 11 mitochondrial reactions, one extracellular and one biomass reaction (24 components).
Specific assumptions for our K. marxianus FBA model included the following:
-
ammonium sulphate was the sole nitrogen and sulphur source and was available in excess;
-
extracellular product accumulation had no effect on intracellular reactions;
-
inorganic phosphate was available in excess;
-
NADH and NADPH were assumed not to freely exchange between mitochondria and cytoplasm. Instead, redox equivalents could be translocated across the mitochondrial membrane by specific transport systems (shuttles). A malate–aspartate shuttle [17] and a 2-oxo glutarate–citrate carrier [8] were included to model NAD- and NADP-dependent redox exchange between cytosol and mitochondria.
To allow for succinate exchange across the mitochondrial membrane, a succinate–malate carrier was introduced [1, 64]. An electron transport chain was included in the model as a lumped reaction with the P/O ratio set to 1.2 [32].
AcetylCoA transport across the inner mitochondrial membrane occurs via a carnitine shuttle that is related to fatty acid metabolism [102]. Since the main fluxes for many biotechnologically important products stem directly from short chain carbon metabolites, we decided not to include a representation of fatty acid metabolism and described AcetylCoA transport across the mitochondrial membrane as a simple transport reaction (model reaction ACCOA_DIFF).
Kluyveromyces marxianus is an example of Crabtree negative yeast. Its physiology is believed to be closely related to its sister species K. lactis [95]. It is reported that ethanol production in K. lactis coincides with decreased oxygen supply [41]. It is assumed that flux regulation around pyruvate bypass is the reason for Crabtree negative yeasts to choose between fermentation or oxidative growth. The cytoplasmic pyruvate bypass in K. lactis consists of pyruvate decarboxylase, NADP-dependent acetaldehyde dehydrogenase and acetyl-CoA synthetase. The first step of the pyruvate bypass in K. lactis is strongly upregulated during fermentative growth thus increasing the cytoplasmic production of AcetylCoA [41]. In the case of disturbed functioning of the mitochondrial pyruvate dehydrogenase complex, oxygen limitation or blockage of respiration chain, this bypass can supply enough cytoplasmic acetylCoA to support growth [103, 104].
Acetyl-CoA production in K. marxianus mitochondria occurs via the pyruvate dehydrogenase complex (model reaction ed5). In the model, cytoplasmic AcetylCoA synthesis was catalysed by acetyl-CoA synthetase (model reaction ACS). To model anaerobic or semi-anaerobic fermentations, an AcetylCoA (reaction ACCOA_DIFF) transport reaction from cytoplasm to mitochondria was included.
Model validation
Data sources for validation/calibration
The main carbon fluxes for model validation in K. marxianus were as follows: substrate uptake, CO2, ethanol, glycerol, acetate and biomass production. For a K. marxianus batch cultivation with limited oxygen supply these fluxes can account for up to 100% of total carbon [77]. Therefore, this set of fluxes is sufficient to validate this medium-scale model. A similar set of fluxes has been successfully applied to validate the medium-scale carbon metabolism model of Pichia pastoris [87].
Here, the model outputs were compared with previously published and original experimental data. Metabolite and biomass data from the exponential growth phase were extracted from numerous published studies involving K. marxianus batch cultivations on various substrates (Table 1).
Model validation on lactose as substrate
Lactose is the main carbohydrate in cheese whey. In K. marxianus, lactose is split by the enzyme β-galactosidase into glucose and galactose, then each of these monosaccharides enters glycolysis at different levels: glucose is converted to glucose-6P, but galactose is converted to glucose-1 phosphate by the Leloir pathway. Strains of K. marxianus differ with respect to the first steps of lactose metabolism—some strains have intracellular and some extracellular β-galactosidase [6]. We modelled K. marxianus lactose uptake with transport reaction lactD (lactose permease) and breakdown by reaction GALSID (β-galactosidase). The model was able to achieve a steady-state solution for all the experimentally measured flux distributions (Table 1).
In addition to the published studies, we performed aerobic fermentations (semi-synthetic broth with 7 or 10% lactose, aeration 0.2 or 1 vol vol−1 min−1 of fermentation volume). The measured fluxes of the extracellular metabolites are presented in Table 1 as “this study”; ethanol was the major product with glycerol and acetate as the main byproducts.
Depending on the oxygen supply, K. marxianus lactose fermentation is biomass (aerobic) or ethanol (anaerobic) orientated. Sansonetti et al. [77] demonstrated results for K. marxianus DSM 5422 strain lactose fermentation in “self anaerobic” mode reaching 3.33 units of ethanol per unit of lactose. In this case, biomass growth was slow (μ = 0.07 h−1) and glycerol was produced as the main byproduct. On the other hand, rapid biomass production by K. marxianus strain CBS 6556 from lactose has been described under fully aerobic mode with comparatively low ethanol flux [51].
Interestingly, none of above-mentioned cases reported acetate accumulation, which seems to be related either to slow cytoplasmic consumption of AcetylCoA (as in the case of low μ), or sufficient AcetylCoA supply by mitochondria (in the aerobic case). Longhi et al. [51] reported possible accumulation of acetate during fermentation, albeit they did not report exact concentrations.
Model validation on glucose, sucrose and inulin as substrates
Glucose, fructose and their derived glucose and fructose oligo- and polysaccharides form an important group of substrates for industrial applications. Sugarcane or sugar beet molasses, starch, sucrose and inulin are typical examples [66].
Kluyveromyces marxianus is able to hydrolyze inulin directly due to its extracellular inulinase activity [75]. We performed fermentations with strain DSM 5422 in semi-synthetic broth with inulin as a sole carbon source; results are depicted in Table 1.
For strain DSM 5422, extracellular inulinase activity by far exceeded the uptake of released monosaccharides (data not shown). An ample amount of free fructose in the media due to extracellular inulinase activity was also demonstrated by other authors [101]. In the model we assumed that only fructose is produced after inulin hydrolysis; glucose is released in negligible amount and has no effect on fructose uptake. Similarly, when simulating the data of Etchmann et al. [20], we assumed that sucrose is split outside the cell and invertase activity exceeds the rate of monosaccharide uptake [75]. Subsequent simultaneous consumption of glucose and fructose happens when sucrose is hydrolyzed by invertase [23]. Fructose uptake (model reaction inulin_t) followed by fructose kinase (model reaction onoHLK) was considered as a starting point for inulin consumption. All results from inulin, glucose and sucrose fermentations described in Table 1 were replicated by the model.
Model validation on xylose as substrate
Kluyveromyces marxianus is able to ferment xylose. As for many yeasts and fungi, in K. marxianus xylose is taken up and converted to xylulose-5 phosphate (pentose phosphate pathway intermediate) via three sequential reactions: xylose reductase (reaction XYL1), xylitol dehydrogenase (reaction XDH) and xylulose kinase (reaction pengluc3). Moreover, xylose reductase in K. marxianus is exclusively NADPH dependent [106]. Xylose reductase reaction in our model was represented as exclusively NADPH dependent.
There are many reports of xylose fermentation by K. marxianus. We chose three example fermentations [14, 56, 82] to extract data for model validation. All three xylose fermentations yielded slow biomass growth with μ varying from 0.007 to 0.08 h−1. Interestingly enough, the experimental μ values correlated with oxygen supply: increased oxygen supply led to increased μ [82].
Reaction and gene essentiality
In this study, we linked gene (or reaction) essentiality to the inability to form biomass (maximal biomass flux = 0) on deletion of all reactions catalysed by that gene product. Reaction deletion was performed by setting a zero flux for the reaction in model. Mostly, there were one-to-one relations between genes and reactions, but in some cases there were (1) redundant genes when each of alternative genes encoded enzyme (OR relationship), (2) two or more genes encoded polypeptides that form functional enzyme (AND relationship) and (3) one gene encoded more than one reaction. According to the analysis results (Online Resource 3), the model contained 38 essential reactions (Fig. 2). The 26 reactions (23%) essential for all analysed substrates belonged to central carbon metabolism. Due to the small model size (113 reactions), there were not many redundant or parallel pathways included. Large-scale experimental deletion studies with S. cerevisiae report 17% [99] and 19% [29] essential proteins for viability in rich medium which is close to our medium-scale model-based prediction. For comparison, 2–66% of reactions are essential across different eukaryotes [9].
Model reaction essentiality for biomass production depending on substrate. Analyses revealed 26 essential reactions for all substrates, 5 reactions exclusively essential for xylose, and 4 for lactose. Glucose and inulin are shown as one substrate. Fructose kinase and inulinase were essential reactions for inulin consumption, while hexokinase was essential for both lactose and glucose consumption
Model optimisation
The model was optimised by FBA for production of ethanol, acetate, lactate, glycerol, ethylacetate, succinate, glutamate phenylethanol and phenylalanine at fixed substrate uptake rate (glucose/inulin, lactose and xylose) at 10 mM g−1 DW h−1 and μ at 0.4 h−1 as a compromise between different substrate consumption and growth rates. The substrate uptake flux was set high to make flux distribution and yield calculations more practical. The maximal percentage of substrate carbon atoms converted into product (Fig. 3a) always was below the maximal theoretical yield (Fig. 3b).
a Maximal percentage of substrate carbon atoms converted into product at biomass growth μ = 0.4 h−1. b Difference between theoretical yield and maximal carbon flux to product at biomass growth μ = 0.4 h−1. c Maximal percentage of substrate carbon atoms converted into product at low oxygen consumption and biomass growth fixed at μ = 0.4 h−1. d Maximal percentage of substrate carbon atoms converted into product at high oxygen consumption and biomass growth fixed at μ = 0.4 h−1
Kluyveromyces marxianus metabolism is sensitive to the oxygen consumption rate. To assess the opportunities of metabolic control by variable oxygen supply, optimisation (FBA) and variability analysis (FVA) were performed for two extreme respiration cases—low (necessary for biomass production) (Fig. 3c) and high (Fig. 3d) oxygen consumption rates at fixed μ = 0.4 h−1 in the following steps:
-
1.
maximal and minimal oxygen consumption was determined minimised/maximised by FVA at μ = 0.4 h−1 for each substrate;
-
2.
90 and 100% of maximum oxygen consumption rate (determined in step 1) were set as lower and upper oxygen consumption rate bounds FBA analysis at high oxygen consumption;
-
3.
minimal and three minimal oxygen consumption rates (determined in step 1) were set as lower and upper oxygen consumption rate bounds FBA analysis at low oxygen consumption;
-
4.
maximal product rate at low (Fig. 3c)/high (Fig. 3d) oxygen consumption was determined by FBA.
In the case of no constraints on oxygen consumption, high values of carbon flux to product (Fig. 3a) were predicted for lactate, glutamate and phenylalanine. Ethanol, acetate and ethyl acetate yields were identical and close to their theoretical maxima (Fig. 3b). The lowest fractions of carbon flux to product were in the cases of succinate, phenylethanol and glycerol. All other cases attained at least 75% of their theoretical yields. Succinate was the only product that had higher yields on xylose as substrate compared to other substrates (Fig. 3a). At minimal oxygen consumption (Fig. 3c) for most products the carbon flux to products was lower in the case of xylose as substrate. With lactose, inulin and glucose as substrates, ethanol and lactate yields were very close to the theoretical maxima. Carbon flux to products at maximal oxygen (Fig. 3d) consumption still was relatively close to maximum in case of ethanol and acetate with lactose, inulin and glucose as substrates while lactate production had become very low.
Ethanol production
Kluyveromyces marxianus is able to convert lactose, inulin (fructose), glucose and xylose into ethanol. Maximum theoretical ethanol-to-substrate ratios are for lactose 4, for inulin/glucose 2, but for xylose 1.6. The model predicted the maximum ratio of lactose conversion into ethanol to be 3.93, inulin/glucose 1.91 and xylose 0.96. While the model predictions for lactose and glucose/inulin were close to the theoretical maximum, the low ethanol:xylose ratio came as a surprise. This most probably relates to the carbon flux re-routing through the glucose-6P dehydrogenase reaction to generate the NADPH needed for xylose reduction. Additionally, according to the model, a notable portion (0.5 units per unit of xylose) was directed to acetate production.
Many authors have noted the need for an oxygen supply for K. marxianus growth on respiration when fermenting xylose as a sole carbon source [82]. K. marxianus respiration mutants are not able to ferment xylose [49]. This dependence might be related to different reasons/factors.
Firstly, to metabolise xylose, a K. marxianus cell needs enough resources of cytoplasmic NAD + which is consumed by xylitol dehydrogenase (reaction XDH). The cytoplasmic demand for NAD + is fulfilled by the alcohol dehydrogenase reaction (reaction Alcohol4) producing ethanol and NAD + [95] or through the activity of the mitochondrial malate–aspartate shuttle [17, 36]. The model predicted that the latter is not active in the case of a poor oxygen supply.
Secondly, the model predicted acetate accumulation, up to 50% of the ethanol flux, if oxygen consumption was kept low. A similar effect was demonstrated in xylose fermentation by S. cerevisiae with engineered XYLT, XDH and XK (reaction pengluc3) reactions [44, 70]. According to our model, a decrease in acetate production was observed if the xylose reductase (XYLT) cofactor specificity was changed from NADPH to NADH; in this case, the ethanol-to-xylose ratio reaches 1.6. This in silico result complements in vivo results from various authors, who engineered the xylose reductase cofactor (enzyme preference to use NADH or NADPH) specificity in S. cerevisiae [44] or explored cofactor specificity of wild-type xylose reductases of various yeast species [5].
A third reason why respiration activity is crucial for xylose utilisation is because of increased ATP consumption to maintain cytoplasmic pH. Stambuk et al. [83] found that xylose uptake in K. marxianus is symport. Each xylose is imported together with a proton. In this process cytoplasmic pH drops. To maintain cytoplasmic pH unchanged, cell membrane ATPases export protons at the expense of ATP hydrolysis.
The model predicted the maximum ratio of fructose conversion into ethanol to be 1.9 units of ethanol per unit fructose or glucose. Flux variability analyses revealed that if the maximum ethanol flux changes by 10%, then 4–17% of incoming carbon flux is always routed to glycerol production. Within the limits of error, our in vivo data on inulin fermentation were in good correlation with this model prediction. We observed ethanol-to-monosaccharide flux ratio to be 1.72 ± 10% (comprising 90% of maximum) while the rest of the carbon was distributed between glycerol and acetate.
Acetate
Acetic acid (ethanoic acid) is among the first chemicals to have been industrially produced by microorganisms. Traditionally bacterial producers (Clostridium sp. or Acetobacter sp.) are used. Potentially K. marxianus can produce acetic acid, though its commercial value is low. Acetic acid accumulation during exponential growth phase is typical for K. marxianus fermentations. This has been demonstrated by us as well as other authors [53, 82]. Acetate, as with glycerol, is perceived as an unwanted fermentation side product. In our model, acetate and acetylCoA reactions in our model were cofactor “entangled”, so when there was need for acetylCoA, extra acetate was produced; in parallel, there was a risk of cytoplasmic redox imbalance since acetate is produced along with NADH.
Maximum theoretical acetate/substrate ratios were calculated: for lactose 4, for inulin or glucose to acetate 2, but for xylose 1.6. The model predicted the maximum ratio of lactose conversion into acetate to be 3.94, inulin or glucose 1.94 and the maximum ratio of xylose conversion into ethanol to be 1.44 units of acetate per unit of xylose.
Acetate production can occur without formation of significant byproducts, but strong aeration must be supplied (optimal oxygen consumption up to 2 units of O2 per unit of substrate). Based on our model results, cytoplasmic acetate accumulation occurred in two situations—if there was need for extra NADH (as in succinate production) or need for cytoplasmic acetylCoA (in the case of ethyl acetate or biomass production).
Lactate
Lactic acid is widely used acid in the food industry and has potential application as a monomer for biodegradable plastics. In both of those applications l-lactic acid is used. Microbial (bacterial, yeast or fungi) fermentation is one of the options for l-lactic acid isomer synthesis in industrial amounts [35]. Kluyveromyces sp. has been proposed as a prospective lactic acid producer due to its fast production rates and GRAS status [15]. Yeasts do not have lactate dehydrogenase; therefore, for lactate production recombinant strains harbouring LDH of eukaryotic origin (mammals, moulds) are used.
The theoretical molar yield of l-lactate from mole of glucose was 2, from lactose 4, but from xylose 1.6. Our model predicted l-lactate formation with the following molar ratios: 3.9 from lactose, 1.9 from glucose and 1.6 from xylose.
Introduction of heterologous lactate dehydrogenase alone does not lead to maximal l-lactate production in vivo. Carbon flow towards lactate or ethanol was divided at the level of pyruvate by pyruvate decarboxylase (reaction in the model PDC) or the pyruvate dehydrogenase complex in mitochondria. If the pyruvate gets decarboxylated, direct lactate production from pyruvate was not possible, instead carbon was routed to acetaldehyde and ethanol or acetate formation. Flux variability analyses revealed that this was the case—when simulating a decrease in lactate flux, an equimolar increase in CO2 and ethanol fluxes was observed.
Kluyveromyces sp., unlike Saccharomyces, contain just one PDC gene; therefore, preparation of pdc functional knockouts is comparatively easy. Lactate dehydrogenase overexpression in a K. lactis pdc strain has proven to be an efficient strategy yielding a lactic acid: consumed glucose ratio up to 0.5 [72]. Lactate production close to the theoretical maximum was achieved when both pyruvate consuming branches (pyruvate decarboxylase and dehydrogenase) were inactivated. A molar lactate/glucose ratio close to 2 in K. lactis pdc pdh knockouts was obtained by Bianchi and colleagues [4]. Alternatively, additional heterologous expression of lactate dehydrogenase by increasing gene copy numbers can be a strategy to increase lactate production [67].
Glycerol production
Glycerol is a typical byproduct of yeast ethanol fermentation that forms in response to the need to balance cytoplasmic NADH oxidation. Glycerol formation as an NADH sink becomes crucial when NADH oxidation via the electron transport chain is not possible (limited oxygen supply). Although glycerol synthesis by microbial producers per se has no applications in biotechnology, we included this metabolite in our analyses since this is one of the major carbon and redox sinks in the K. marxianus metabolism.
Theoretical maximal glycerol production from different substrates in molar ratios was as follows: from lactose 4, from glucose 2, from xylose 1.66. Our medium-scale K. marxianus metabolic model predicted maximum molar yields from lactose 2.4 from glucose 1.2 and from xylose 1.2. The model predicted the need for a certain respiratory activity (up to 0.5 units of O2 per unit of substrate) for glycerol production to reach a maximum, and hence CO2 was the only byproduct in the case of optimal glycerol production.
We and other researchers have observed similar effects in vivo in inulin and lactose fermentations with K. marxianus—higher aeration leads to smaller ethanol and glycerol flux and vice versa [82]. Severe fermentation dependence on oxygen supply has also been demonstrated in the physiology of K. marxianus’ sister species K. lactis [58].
Ethyl acetate production
Ethyl acetate is a volatile, slightly polar molecule, used as an organic solvent. Nowadays, it has many applications in cosmetics (nail polish remover), electronics (cleaning circuit boards, etc.), and has a potential future application as an environmentally friendly acyl acceptor in biodiesel production instead of methanol. Currently, ethyl acetate is produced from petrochemical sources, but it can be produced through biotechnological synthesis by many yeasts. Currently, K. marxianus is regarded as the most productive ethyl acetate producer [52].
For ethyl acetate, the theoretical molar product/substrate yield, when considering pyruvate decarboxylation, was 2 for lactose, 1 for glucose and 1 for xylose. Our model predicted the maximum ethyl acetate-to-substrate ratio from lactose to be 1.97, 0.72 from xylose, and 0.97 for inulin or glucose. FVA results revealed strong effects of aeration on ethyl acetate formation. Most ethyl acetate was produced at increased aeration. However, the most effective ethyl acetate formation was not during growth with maximal respiration (Fig. 3c). Additionally, FVA revealed a notable increase in glycerol production during oxygen limitation, which indicated the necessity of cytoplasmic NADH reoxidation to support acetate production. In the case of respiration, cytoplasmic NADH could be reoxidised through the electron transport chain and mitochondrial shuttle activity.
Careful fine-tuning of oxygen consumption might be a strategy for maximum ethyl acetate production. A similar strategy was applied when limiting K. marxianus access to metal ions [91, 92]. Metal ion (Fe, Cu, Zn) limitation was found to affect ethyl acetate production. Amongst them, Fe limitation had the most effect. K. marxianus culture starving for Fe produced ethyl acetate at close to 50% of theoretical maximum. Fe limitation lowered the activities of Fe-dependent mitochondrial aconitase and succinate dehydrogenase; this subsequently led to accumulation of acetylCoA, which was used to increase ethyl acetate production [92].
Succinate production
Succinate is one of the 12 most recognised sugar-derived chemical precursors. There is biotechnological potential for succinate due to its wide application spectrum, since it can serve as a precursor for tetrahydrofuran, butanediol, succinonitrile etc. Cheap microbial production of succinate has huge market potential [97]. There are already several examples of microbial succinate production at industrial scale (Reverdia, Myriant, BioAmber, BASFPurac, etc.). At least one of the processes is yeast based (Reverdia, S. cerevisiae) [12]. Although succinate yields close to the theoretical stoichiometric maximum are reached by bacterial cells, yeast offer several advantages over bacteria: they are not obligately anaerobic; they are robust, acid and osmotically tolerant, and non-pathogenic organisms [73].
The theoretical maximal molar ratio for succinate production from lactose was 3, from glucose 1.5 and xylose 1.25. Our K. marxianus carbon metabolism model predicted the maximum succinate production ratio from glucose to be 0.55, from lactose 1.1 but from xylose 0.78. From here, it seems, that xylose might be the most potent substrate for succinate production; however, there are not many in vivo results on succinate production by Kluyveromyces sp. from xylose. Interestingly, a xylose/ethanol mixture is suggested as a prospective substrate for glyoxylate production along with succinate (isocitrate lyase reaction) in S. cerevisiae and K. lactis isocitrate lyase overexpressed strains [45].
Succinate can be produced via the tricarboxylic acid cycle or the glyoxylate shunt. It is not a redox neutral product with respect to carbohydrate substrates—theoretically, 2 NAD + are consumed per each molecule of succinate. Reduced cofactors can be oxidised in the electron transport chain or by production of glycerol or ethanol—NAD regenerating pathways. The model predicted accumulation of at least one byproduct when optimised for succinate production. FVA results demonstrated that, depending on oxygen supply, many byproducts were formed. Interestingly, the model predicted glycerol formation in the case of poor aeration, independent of substrate. The compensatory NADH reoxidation through increased glycerol production in S. cerevisiae strains, optimised for succinic acid production, was demonstrated in vivo [73].
Based on our medium-scale model, phenylalanine can also be formed as a byproduct in rather large amounts (0.3–0.6 units of phenylalanine per unit of lactose) if oxygen is supplied in surplus (3.7 units of oxygen per unit of lactose). In this case, production of a relatively large amount of phenylalanine is possible, since our medium-scale model is not nitrogen (ammonia) restricted (see model assumptions). In real applications, however, nitrogen bioavailability might prevent such high levels of phenylalanine production being reached.
Deletion of the genes for succinate dehydrogenase subunits is a popular strategy for yeast-based succinate production [73]. Succinate accumulation in the case of KlSDH1 (succinate dehydrogenase subunit) deletion was observed in the case of Kluyveromyces lactis [76]. Our model predicted that inactivation of the aspartate malate shuttle in combination with increased oxygen consumption (up to 1.3 per unit of glucose) would give maximum succinate yield, while inactivation of succinate dehydrogenase together with inactivated glyoxylate shunt would be preferable in the case of fermentation of xylose.
Glutamate production
In our central metabolism model, the K. marxianus biomass reaction consisted of 24 metabolites, excluding the amino acids, although phenylalanine and glutamate are included in the model as desired products. The amino acid content of yeast biomass is of particular industrial interest, since some of them (like glutamic acid) are responsible for developing of umami taste [37]. Random mutations are the typical method for generating yeast strain with increased glutamic acid content [61]. Here, we provide model-based theoretical analysis of possible scenarios for increasing glutamic acid yield from substrate in K. marxianus.
The theoretical maximal glutamate production from different substrates in molar ratios would be as follows: from lactose 2.4, from glucose 1.2, from xylose 1. Our medium-scale K. marxianus metabolic model predicted maximum molar yields from lactose 1.97, from glucose 0.97, and from xylose 0.8. Glutamate in K. lactis and, most probably also in K. marxianus, can be produced by either of two reactions: NADP-dependent glutamate dehydrogenase (EC 1.4.1.4 reaction GLUDE_nadp) or by GOGAT (EC 1.4.1.13, reaction Glude_NAD) [74]. Our model predicted the larger carbon flux to be routed through NADPH-dependent glutamate dehydrogenase. To recover enough cytoplasmic oxoglutarate an NADP—oxoglutarate—citrate shuttle was used (see Fig. 1). At the same time, a high respiration rate was needed to reach maximum glutamate production if glucose or lactose was used as substrates (approx. 1.5 O2/glucose). Interestingly, a higher fractional of molar yield of glutamate was achieved by xylose fermentation (80% from theoretical) and less oxygen needed to be supplied per substrate moiety (1.2). In addition, the main glutamate synthesis flux in K. marxianus consuming xylose was predominantly, through the GOGAT reaction, unlike when lactose or glucose was consumed (see above).
In K. marxianus glutamate synthesis is tightly product-regulated by feedback inhibition. As in S. cerevisiae, glutamate synthesis via NADPH-dependent glutamate dehydrogenase is subject to nitrogen catabolite repression [59]. To analyse these types of product–substrate interactions, kinetic modelling would need to be applied.
Phenylethanol and phenylalanine as products
Phenylalanine is the precursor metabolite for many industrial fragrances as well as an ingredient of the artificial sweetener aspartame. Phenylethanol is a rose flavour used in food and pharmaceutical industries. It is usually extracted from rose petals. The global demand for phenylethanol continues to increase, and it cannot be fulfilled by traditional extraction methods. Bacterial synthesis might be an sustainable alternative for phenylethanol production [84]. Traditionally, the yeast S. cerevisiae is considered as a vehicle for phenylethanol production, though there have been attempts to use other yeasts, including K. maxianus [19].
Central K. marxianus carbon metabolism was extended to phenylethanol production. Phenylethanol production in the model was introduced as a linear, 11 reaction chain from PEP and E4P. This chain consumed additional NADPH, NADH, ATP and PEP.
The final steps of phenylethanol production were introduced according to Uzunov et al. [93]. The model supported two instances of phenylethanol production by K. marxianus: from sucrose and glucose, it predicted the maximum phenylethanol-to-substrate ratio from lactose to be 1.1 and around 0.5 from xylose, inulin, sucrose or glucose. That is far above in vivo experiments where phenylethanol-to-substrate ratio ranges around 0.02–0.1 were typically observed [28, 100].
Conclusions
We developed a mass and charge balanced stoichiometric model of central K. marxianus carbon metabolism including biomass production. The model is published in forms ready for simulation (SBML and COBRA toolbox formats). Most of the model information was based on recent K. marxianus genome annotations [48]. Mitochondrial shuttles were included to describe proton and molecule allocation between mitochondria and cytoplasm. The model is able to reproduce the experimentally observed mix of industrially valuable products, as well as explaining formation of unwanted side products (acetate and glycerol).
Our modelling results imply that oxygen control can be used to influence product yield and flux distributions. Also cofactor swapping (NADPH to NADH and/or vice versa) can significantly improve xylose conversion to products. Interestingly, xylose turned out to be a biotechnologically promising substrate for K. marxianus with yet unused potential linked to fine-tuned redox engineering. Succinate is a commercially appealing product that could potentially be produced from xylose in a more efficient way compared to other substrates analysed.
The model predicted that ethanol, acetate, l-lactate and ethyl acetate can be produced at close to their theoretical yield and without the need for genetic engineering in the cases of lactose, glucose and inulin as substrates. At the same time, the model predicted that a high fraction of the phenylethanol and succinate theoretical yields could not be achieved without metabolic engineering, for which the proposed model is a powerful tool.
Our model can be used for analysis of K. marxianus and its metabolic engineering as well as basis of larger scale models. It would be valuable to extend the model to include precise characteristics of transport reactions (proton symport and antiport), together with the plasma membrane H+-ATPase system.
References
Aliverdieva D, Mamaev DM, Lagutina LS, Bondarenko DI (2010) Transport of dicarboxylates in Saccharomyces cerevisiae. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol 2:1611–1620
Behera S, Sharma NK, Arora R, Kumar S (2016) Effect of evolutionary adaption on xylosidase activity in thermotolerant yeast isolates Kluyveromyces marxianus NIRE-K1 and NIRE-K3. Appl Biochem Biotechnol 179:1143–1154. doi:10.1007/s12010-016-2055-2
Benson DA, Cavanaugh M, Clark K et al (2013) GenBank. Nucleic Acids Res 41:36–42. doi:10.1093/nar/gks1195
Bianchi MM, Brambilla L, Protani F et al (2001) Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl Environ Microbiol 67:5621–5625. doi:10.1128/AEM.67.12.5621-5625.2001
Bruinenberg PM, de Bot PHM, van Dijken JP, Scheffers WA (1984) NADH-linked aldose reductase: the key to anaerobic alcoholic fermentation of xylose by yeasts. Appl Microbiol Biotechnol 19:256–260. doi:10.1007/BF00251847
Carvalho-Silva M, Spencer-Martins I (1990) Modes of lactose uptake in the yeast species Kluyveromyces marxianus. Antonie Van Leeuwenhoek 57:77–81
Caspi R, Altman T, Dreher K et al (2012) The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 40:D742–D753. doi:10.1093/nar/gkr1014
Castegna A, Scarcia P, Agrimi G et al (2010) Identification and functional characterization of a novel mitochondrial carrier for citrate and oxoglutarate in Saccharomyces cerevisiae. J Biol Chem 285:17359–17370. doi:10.1074/jbc.M109.097188
Chen WH, Minguez P, Lercher MJ, Bork P (2012) OGEE: an online gene essentiality database. Nucleic Acids Res 40:901–906. doi:10.1093/nar/gkr986
Cheon Y, Kim J-S, Park J-B et al (2014) A biosynthetic pathway for hexanoic acid production in Kluyveromyces marxianus. J Biotechnol 182–183:30–36. doi:10.1016/j.jbiotec.2014.04.010
Chi ZM, Zhang T, Cao TS et al (2011) Biotechnological potential of inulin for bioprocesses. Bioresour Technol 102:4295–4303. doi:10.1016/j.biortech.2010.12.086
Cok B, Tsiropoulos I, Roes AL, Patel MK (2014) Succinic acid production derived from carbohydrates: an energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels, Bioprod Biorefining 8:16–29. doi:10.1002/bbb.1427
Degtyarenko K, de Matos P, Ennis M et al (2008) ChEBI: a database and ontology for chemical entities of biological interest. Nucleic Acids Res 36:D344–D350. doi:10.1093/nar/gkm791
Delgenes J, Moletta R, Navarro J (1986) The effect of aeration on d-xylose fermentation by Pachysolen tannophilus, Pichia stipitis, Kluyveromyces marxianus and Candida shehatae. Biotechnol Lett 8:7–14
Dequin S, Barre P (1994) Mixed lactic acid–alcoholic fermentation by Saccharomyes cerevisiae expressing the Lactobacillus casei L(+)–LDH. Bio/Technology 12:173–177. doi:10.1038/nbt0294-173
Dias O, Pereira R, Gombert AK et al (2014) iOD907, the first genome-scale metabolic model for the milk yeast Kluyveromyces lactis. Biotechnol J 9:776–790. doi:10.1002/biot.201300242
Easlon E, Tsang F, Skinner C et al (2008) The malate–aspartate NADH shuttle components are novel metabolic longevity regulators required for calorie restriction-mediated life span extension in yeast. Genes Dev 22:931–944. doi:10.1101/gad.1648308
van den Ende W (2013) Multifunctional fructans and raffinose family oligosaccharides. Front Plant Sci 4:1–11. doi:10.3389/fpls.2013.00247
Etschmann M, Bluemke W, Sell D, Schrader J (2002) Biotechnological production of 2-phenylethanol. Appl Microbiol Biotechnol 59:1–8. doi:10.1007/s00253-002-0992-x
Etschmann MMW, Sell D, Schrader J (2003) Screening of yeasts for the production of the aroma compound 2-phenylethanol in a molasses-based medium. Biotechnol Lett 25:531–536. doi:10.1023/A:1022890119847
Fell DA, Poolman MG, Gevorgyan A (2010) Building and analysing genome-scale metabolic models. Biochem Soc Trans 38:1197–1201. doi:10.1042/BST0381197
Fleischmann A, Darsow M, Degtyarenko K et al (2004) IntEnz, the integrated relational enzyme database. Nucleic Acids Res 32:D434–D437. doi:10.1093/nar/gkh119
Fonseca GG, de Carvalho NMB, Gombert AK (2013) Growth of the yeast Kluyveromyces marxianus CBS 6556 on different sugar combinations as sole carbon and energy source. Appl Microbiol Biotechnol 97:5055–5067. doi:10.1007/s00253-013-4748-6
Fonseca GG, Heinzle E, Wittmann C, Gombert AK (2008) The yeast Kluyveromyces marxianus and its biotechnological potential. Appl Microbiol Biotechnol 79:339–354. doi:10.1007/s00253-008-1458-6
Gabardo S, Pereira GF, Rech R, Ayub MAZ (2015) The modeling of ethanol production by Kluyveromyces marxianus using whey as substrate in continuous A-Stat bioreactors. J Ind Microbiol Biotechnol 42:1243–1253. doi:10.1007/s10295-015-1661-2
Galindo-Leva LÁ, Hughes SR, López-Núñez JC et al (2016) Growth, ethanol production, and inulinase activity on various inulin substrates by mutant Kluyveromyces marxianus strains NRRL Y-50798 and NRRL Y-50799. J Ind Microbiol Biotechnol 43:927–939. doi:10.1007/s10295-016-1771-5
Gao J, Yuan W, Li Y et al (2015) Transcriptional analysis of Kluyveromyces marxianus for ethanol production from inulin using consolidated bioprocessing technology. Biotechnol Biofuels. doi:10.1186/s13068-015-0295-y
Garavaglia J, Flôres SH, Pizzolato TM et al (2007) Bioconversion of l-phenylalanine into 2-phenylethanol by Kluyveromyces marxianus in grape must cultures. World J Microbiol Biotechnol 23:1273–1279. doi:10.1007/s11274-007-9361-3
Giaever G, Chu AM, Ni L et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391. doi:10.1038/nature00935
Gombert AK, Madeira JV, Cerdán M-E, González-Siso M-I (2016) Kluyveromyces marxianus as a host for heterologous protein synthesis. Appl Microbiol Biotechnol. doi:10.1007/s00253-016-7645-y
Gombert AK, Moreira dos Santos M, Christensen B, Nielsen J (2001) Network identification and flux quantification in the central metabolism of Saccharomyces cerevisiae under different conditions of glucose repression. J Bacteriol. doi:10.1128/JB.183.4.1441-1451.2001
Groeneveld P (1999) Control of specific growth rate and physiology of the yeast Kluveromyces marxianus: a BioThermoKinetic approach. Free University of Amsterdam, Amsterdam
Hong SJ, Kim HJ, Kim JW et al (2015) Optimizing promoters and secretory signal sequences for producing ethanol from inulin by recombinant Saccharomyces cerevisiae carrying Kluyveromyces marxianus inulinase. Bioprocess Biosyst Eng 38:263–272. doi:10.1007/s00449-014-1265-7
Hucka M, Finney A, Sauro HM et al (2003) The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics 19:524–531. doi:10.1093/bioinformatics/btg015
Inkinen S, Hakkarainen M, Albertsson AC, Södergård A (2011) From lactic acid to poly(lactic acid) (PLA): characterization and analysis of PLA and Its precursors. Biomacromol 12:523–532. doi:10.1021/bm101302t
Inokuma K, Ishii J, Hara KY et al (2015) Complete genome sequence of Kluyveromyces marxianus NBRC1777, a nonconventional thermotolerant yeast. Genome Announc 3:1–2. doi:10.1128/genomeA.00389-15.Copyright
Jinap S, Hajeb P (2010) Glutamate. Its applications in food and contribution to health. Appetite 55:1–10. doi:10.1016/j.appet.2010.05.002
Johnson M, Zaretskaya I, Raytselis Y et al (2008) NCBI BLAST: a better web interface. Nucleic Acids Res 36:5–9. doi:10.1093/nar/gkn201
Kalnenieks U, Pentjuss A, Rutkis R et al (2014) Modeling of Zymomonas mobilis central metabolism for novel metabolic engineering strategies. Front Microbiol 5:42
Kerkhoven EJ, Lahtvee P-J, Nielsen J (2014) Applications of computational modeling in metabolic engineering of yeast. FEMS Yeast Res. doi:10.1111/1567-1364.12199
Kiers J, Zeeman AM, Luttik M et al (1998) Regulation of alcoholic fermentation in batch and chemostat cultures of Kluyveromyces lactis CBS 2359. Yeast 14:459–469
Kim JS, Park JB, Jang SW, Ha SJ (2015) Enhanced xylitol production by mutant Kluyveromyces marxianus 36907-FMEL1 due to improved xylose reductase activity. Appl Biochem Biotechnol 176:1975–1984. doi:10.1007/s12010-015-1694-z
Kim T-Y, Lee S-W, Oh M-K (2014) Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzyme Microb Technol 61–62:44–47. doi:10.1016/j.enzmictec.2014.04.011
Klimacek M, Krahulec S, Sauer U, Nidetzky B (2010) Limitations in xylose-fermenting Saccharomyces cerevisiae, made evident through comprehensive metabolite profiling and thermodynamic analysis. Appl Environ Microbiol 76:7566–7574. doi:10.1128/AEM.01787-10
Koivistoinen OM, Kuivanen J, Barth D et al (2013) Glycolic acid production in the engineered yeasts Saccharomyces cerevisiae and Kluyveromyces lactis. Microb Cell Fact 12:82. doi:10.1186/1475-2859-12-82
Lane MM, Morrissey JP (2010) Kluyveromyces marxianus: a yeast emerging from its sister’s shadow. Fungal Biol Rev 24:17–26. doi:10.1016/j.fbr.2010.01.001
Lee K, Kim T, Sohn S et al (2014) Genome-scale metabolic network model reconstruction of Kluyveromyces marxianus and strategies for engineering non-native pathways for 3-hydropropionate production in Kluyveromyces marxianus. Patent, Pub. No.: US 2014/0093901 A1
Lertwattanasakul N, Kosaka T, Hosoyama A et al (2015) Genetic basis of the highly efficient yeast Kluyveromyces marxianus: complete genome sequence and transcriptome analyses. Biotechnol Biofuels. doi:10.1186/s13068-015-0227-x
Lertwattanasakul N, Suprayogi Murata M et al (2013) Essentiality of respiratory activity for pentose utilization in thermotolerant yeast Kluyveromyces marxianus DMKU 3-1042. Antonie van Leeuwenhoek Int J Gen Mol Microbiol 103:933–945. doi:10.1007/s10482-012-9874-0
Liu S, Lu H, Hu R et al (2012) A sustainable woody biomass biorefinery. Biotechnol Adv 30:785–810. doi:10.1016/j.biotechadv.2012.01.013
Longhi LGS, Luvizetto DJ, Ferreira LS et al (2004) A growth kinetic model of Kluyveromyces marxianus cultures on cheese whey as substrate. J Ind Microbiol Biotechnol 31:35–40. doi:10.1007/s10295-004-0110-4
Löser C, Urit T, Keil P, Bley T (2014) Studies on the mechanism of synthesis of ethyl acetate in Kluyveromyces marxianus DSM 5422. Appl Microbiol Biotechnol 99:1131–1144. doi:10.1007/s00253-014-6098-4
Löser C, Urit T, Stukert A, Bley T (2013) Formation of ethyl acetate from whey by Kluyveromyces marxianus on a pilot scale. J Biotechnol 163:17–23. doi:10.1016/j.jbiotec.2012.10.009
Magrane M, Consortium U (2011) UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford) 2011:bar009. doi:10.1093/database/bar009
Mahadevan R, Schilling CH (2003) The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab Eng 5:264–276
Margaritis A, Bajpai P (1982) Direct fermentation of d-xylose to ethanol by Kluyveromyces marxianus strains. Appl Environ Microbiol 44:1039–1041
Martynova J, Kokina A, Kibilds J et al (2016) Effects of acetate on Kluyveromyces marxianus DSM 5422 growth and metabolism. Appl Microbiol Biotechnol. doi:10.1007/s00253-016-7392-0
Merico A, Galafassi S, Piškur J, Compagno C (2009) The oxygen level determines the fermentation pattern in Kluyveromyces lactis. FEMS Yeast Res 9:749–756. doi:10.1111/j.1567-1364.2009.00528.x
de Morais-Júnior MA (2003) The NADP+-dependent glutamate dehydrogenase of the yeast Kluyveromyces marxianus responds to nitrogen repression similarly to Saccharomyces cerevisiae. Braz J Microbiol 34:334–338. doi:10.1590/S1517-83822003000400009
Morrissey JP, Etschmann MMW, Schrader J, de Billerbeck GM (2015) Cell factory applications of the yeast Kluyveromyces marxianus for the biotechnological production of natural flavour and fragrance molecules. Yeast 32:3–16. doi:10.1002/yea.3054
Nakajo Y, Sano H (1998) Yeast extract composition, yeast for obtaining the same, and process for producing yeast extract composition. Patent, Pub. No.: US6344231 B1
Nitiyon S, Keo-oudone C, Murata M et al (2016) Efficient conversion of xylose to ethanol by stress-tolerant Kluyveromyces marxianus BUNL-21. Springerplus 5:185. doi:10.1186/s40064-016-1881-6
Orth JD, Thiele I, Palsson BO (2010) What is flux balance analysis? Nat Biotechnol 28:245–248. doi:10.1038/nbt.1614
Pallotta ML, Fratianni A, Passarella S (1999) Metabolite transport in isolated yeast mitochondria: fumarate/malate and succinate/malate antiports. FEBS Lett 462:313–316. doi:10.1016/S0014-5793(99)01535-5
Panesar PS, Kennedy JF (2012) Biotechnological approaches for the value addition of whey. Crit Rev Biotechnol 32:327–348. doi:10.3109/07388551.2011.640624
Patelski P, Berlowska J, Dziugan P et al (2015) Utilisation of sugar beet bagasse for the biosynthesis of yeast SCP. J Food Eng. doi:10.1016/j.jfoodeng.2015.03.031
Pecota DC, Rajgarhia V, Da Silva NA (2007) Sequential gene integration for the engineering of Kluyveromyces marxianus. J Biotechnol 127:408–416. doi:10.1016/j.jbiotec.2006.07.031
Pentjuss A, Odzina I, Kostromins A et al (2013) Biotechnological potential of respiring Zymomonas mobilis: a stoichiometric analysis of its central metabolism. J Biotechnol 165:1–10. doi:10.1016/j.jbiotec.2013.02.014
Pfeiffer T, Sanchez-Valdenebro I, Nuno J et al (1999) METATOOL: for studying metabolic networks. Bioinformatics 15:251–257. doi:10.1093/bioinformatics/15.3.251
Pitkänen J-P, Aristidou A, Salusjärvi L et al (2003) Metabolic flux analysis of xylose metabolism in recombinant Saccharomyces cerevisiae using continuous culture. Metab Eng 5:16–31. doi:10.1016/S1096-7176(02)00012-5
Poolman MG (2006) ScrumPy: metabolic modelling with Python. IEE Proc Syst Biol 153:375. doi:10.1049/ip-syb:20060010
Porro D, Bianchi MM, Brambilla L et al (1999) Replacement of a metabolic pathway for large-scale production of lactic acid from engineered yeasts. Appl Environ Microbiol 65:4211–4215
Raab AM, Gebhardt G, Bolotina N et al (2010) Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab Eng 12:518–525. doi:10.1016/j.ymben.2010.08.005
Romero M, Guzmán-León S, Aranda C et al (2000) Pathways for glutamate biosynthesis in the yeast Kluyveromyces lactis. Microbiology 146:239–245
Rouwenhorst RJ, Ritmeester WS, Scheffers WA, Dijken JPVAN (1990) Localization of inulinase and invertase in Kluyveromyces species. Appl Environ Microbiol 56:3329–3336
Saliola M, Bartoccioni PC, De Maria I et al (2004) The deletion of the succinate dehydrogenase gene KlSDH1 in Kluyveromyces lactis does not lead to respiratory deficiency. Eukaryot Cell 3:589–597. doi:10.1128/EC.3.3.589-597.2004
Sansonetti S, Hobley TJ, Calabrò V et al (2011) A biochemically structured model for ethanol fermentation by Kluyveromyces marxianus: a batch fermentation and kinetic study. Bioresour Technol 102:7513–7520. doi:10.1016/j.biortech.2011.05.014
Santharam L, Samuthirapandi AB, Easwaran SN, Mahadevan S (2016) Modeling of exo-inulinase biosynthesis by Kluyveromyces marxianus in fed-batch mode: correlating production kinetics and metabolic heat fluxes. Appl Microbiol Biotechnol 101:1877. doi:10.1007/s00253-016-7971-0
Schellenberger J, Que R, Fleming RMT et al (2011) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2.0. Nat Protoc 6:1290–1307. doi:10.1038/nprot.2011.308
Schuster S, Fell DA, Dandekar T (2000) A general definition of metabolic pathways useful for systematic organization and analysis of complex metabolic networks. Nat Biotechnol 18:326–332. doi:10.1038/73786
Sharma NK, Behera S, Arora R, Kumar S (2016) Enhancement in xylose utilization using Kluyveromyces marxianus NIRE-K1 through evolutionary adaptation approach. Bioprocess Biosyst Eng 39:835–843. doi:10.1007/s00449-016-1563-3
Signori L, Passolunghi S, Ruohonen L et al (2014) Effect of oxygenation and temperature on glucose-xylose fermentation in Kluyveromyces marxianus CBS712 strain. Microb Cell Fact 13:51. doi:10.1186/1475-2859-13-51
Stambuk BU, Franden MA, Singh A, Zhang M (2003) d-Xylose transport by Candida succiphila and Kluyveromyces marxianus. Biotechnology for fuels and chemicals. Humana Press, Totowa, pp 255–263
Stark D, Zala D, Münch T et al (2003) Inhibition aspects of the bioconversion of l-phenylalanine to 2-phenylethanol by Saccharomyces cerevisiae. Enzyme Microb Technol 32:212–223. doi:10.1016/S0141-0229(02)00237-5
Stephanopoulos G, Arisitidou A, Nielsen J (1998) Metabolic engineering: principles and methodologies. Academic Press, San Diego
Thiele I, Palsson BØ (2010) A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat Protoc 5:93–121. doi:10.1038/nprot.2009.203
Tortajada M, Llaneras F, Picó J (2010) Validation of a constraint-based model of Pichia pastoris metabolism under data scarcity. BMC Syst Biol 4:115. doi:10.1186/1752-0509-4-115
Trinh CT, Srienc F (2009) Metabolic engineering of Escherichia coli for efficient conversion of glycerol to ethanol. Appl Environ Microbiol 75:6696–6705. doi:10.1128/AEM.00670-09
Trinh CT, Unrean P, Srienc F (2008) Minimal Escherichia coli cell for the most efficient production of ethanol from hexoses and pentoses. Appl Environ Microbiol 74:3634–3643. doi:10.1128/AEM.02708-07
Unrean P, Trinh CT, Srienc F (2010) Rational design and construction of an efficient E. coli for production of diapolycopendioic acid. Metab Eng 12:112–122. doi:10.1016/j.ymben.2009.11.002
Urit T, Manthey R, Bley T, Löser C (2013) Formation of ethyl acetate by Kluyveromyces marxianus on whey: influence of aeration and inhibition of yeast growth by ethyl acetate. Eng Life Sci 13:247–260. doi:10.1002/elsc.201200077
Urit T, Stukert A, Bley T, Löser C (2012) Formation of ethyl acetate by Kluyveromyces marxianus on whey during aerobic batch cultivation at specific trace element limitation. Appl Microbiol Biotechnol 96:1313–1323. doi:10.1007/s00253-012-4107-z
Uzunov ZG, Petrova VY, Ivanov SL, Kujumdzieva AV (2014) In silico study of aro genes involved in the ehrlich pathway: comparison between Saccharomyces cerevisiae and Kluyveromyces Lactis. Biotechnol Biotechnol Equip 25:133–137. doi:10.5504/BBEQ.2011.0128
Varma A, Palsson BO (1994) Metabolic flux balancing: basic concepts, scientific and practical use. Bio/Technology 12:994–998. doi:10.1038/nbt1094-994
Walker GM (1998) Yeast physiology and biotechnology. Wiley, New York
Wang Y, Xiao J, Suzek TO et al (2012) PubChem’s BioAssay database. Nucleic Acids Res 40:D400–D412. doi:10.1093/nar/gkr1132
Werpy T, Petersen G, Aden A et al (2004) Top value added chemicals from biomass. Volume 1-Results of screening for potential candidates from sugars and synthesis gas (No. DOE/GO-102004-1992). Department of Energy Washington DC, Washington DC
Wilkowska A, Kregiel D, Guneser O, Karagul Yuceer Y (2014) Growth and by-product profiles of Kluyveromyces marxianus cells immobilized in foamed alginate. Yeast. doi:10.1002/yea.3044
Winzeler EA, Shoemaker DD, Astromoff A et al (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901–906. doi:10.1126/science.285.5429.901
Wittmann C, Hans M, Bluemke W (2002) Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast 19:1351–1363. doi:10.1002/yea.920
Yuan W, Zhao X, Chen L, Bai F (2013) Improved ethanol production in Jerusalem artichoke tubers by overexpression of inulinase gene in Kluyveromyces marxianus. Biotechnol Bioprocess Eng 18:721–727. doi:10.1007/s12257-013-0026-9
Zeeman AM, Luttik MAH, Pronk JT et al (1999) Impaired growth on glucose of a pyruvate dehydrogenase-negative mutant of Kluyveromyces lactis is due to a limitation in mitochondrial acetyl-coenzyme A uptake. FEMS Microbiol Lett 177:23–28. doi:10.1016/S0378-1097(99)00283-9
Zeeman AM, Luttik MAH, Thiele C et al (1998) Inactivation of the Kluyveromyces lactis KIPDA1 gene leads to loss of pyruvate dehydrogenase activity, impairs growth on glucose and triggers aerobic alcoholic fermentation. Microbiology 144:3437–3446. doi:10.1099/00221287-144-12-3437
Zeeman AM, Steensma HY (2003) The acetyl co-enzyme A synthetase genes of Kluyveromyces lactis. Yeast 20:13–23. doi:10.1002/yea.936
Zhang B, Zhang J, Wang D et al (2016) Simultaneous fermentation of glucose and xylose at elevated temperatures co-produces ethanol and xylitol through overexpression of a xylose-specific transporter in engineered Kluyveromyces marxianus. Bioresour Technol 216:227–237. doi:10.1016/j.biortech.2016.05.068
Zhang B, Zhang L, Wang D et al (2011) Identification of a xylose reductase gene in the xylose metabolic pathway of Kluyveromyces marxianus NBRC1777. J Ind Microbiol Biotechnol 38:2001–2010. doi:10.1007/s10295-011-0990-z
Zhang J, Zhang B, Wang D et al (2014) Xylitol production at high temperature by engineered Kluyveromyces marxianus. Bioresour Technol 152:192–201. doi:10.1016/j.biortech.2013.10.109
Zhang J, Zhang B, Wang D et al (2015) Improving xylitol production at elevated temperature with engineered Kluyveromyces marxianus through over-expressing transporters. Bioresour Technol 175:642–645. doi:10.1016/j.biortech.2014.10.150
Zhang J, Zhang B, Wang D et al (2015) Rapid ethanol production at elevated temperatures by engineered thermotolerant Kluyveromyces marxianus via the NADP(H)-preferring xylose reductase-xylitol dehydrogenase pathway. Metab Eng 31:140–152. doi:10.1016/j.ymben.2015.07.008
Acknowledgements
The research was supported by ERDF project Nr. 2DP/2.1.1.1.0/14/APIA/VIAA/043.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pentjuss, A., Stalidzans, E., Liepins, J. et al. Model-based biotechnological potential analysis of Kluyveromyces marxianus central metabolism. J Ind Microbiol Biotechnol 44, 1177–1190 (2017). https://doi.org/10.1007/s10295-017-1946-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1946-8