Abstract
Acetate esters and higher alcohols greatly influence the quality and flavor profiles of Chinese Baijiu (Chinese liquor). Various mutants have been constructed to investigate the interactions of ATF1 overexpression, IAH1 deletion, and BAT2 deletion on the production of acetate esters and higher alcohols. The results showed that the overexpression of ATF1 under the control of the PGK1 promoter with BAT2 and IAH1 double-gene deletion led to a higher production of acetate esters and a lower production of higher alcohols than the overexpression of ATF1 with IAH1 deletion or overexpression of ATF1 with BAT2 deletion. Moreover, deletion of IAH1 in ATF1 overexpression strains effectively increased the production of isobutyl acetate and isoamyl acetate by reducing the hydrolysis of acetate esters. The decline in the production of higher alcohol by the ATF1 overexpression strains with BAT2 deletion is due to the interaction of ATF1 overexpression and BAT2 deletion. Mutants with varying abilities of producing acetate esters and higher alcohols were developed by genetic engineering. These strains have great potential for industrial application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese Baijiu (Chinese liquor), one of the six most famous distillates worldwide, has been a traditional alcoholic beverage in China for thousands of years. The annual output of Chinese Baijiu has increased steadily in recent years and exceeds 10 million metric tons in China. Chinese Baijiu is typically made from grains, mainly sorghum, by soaking, steaming, fermenting, distilling, aging, and blending [36]. During fermentation, flavor compounds result from the metabolic activity of the microbial community [20, 27]. Higher alcohols and aromatic esters, two of the most abundant and most important groups of volatile flavor compounds in Chinese Baijiu, are significant parameters in determining the quality and flavor profiles of the beverage.
Higher alcohols markedly influence the smell and taste of alcoholic beverages [25]. However, high concentration of higher alcohols in Chinese Baijiu leads to fusel oil taste and is potentially harmful to human health. This characteristic may cause cerebral paralysis [31]. Flavor-active esters, which are responsible for the highly desired fruity aroma, are the most important compounds for the flavor of Chinese Baijiu [6]. Appropriate control of the higher alcohol content and increasing the production of beneficial esters are always considered during fermentation of Chinese Baijiu.
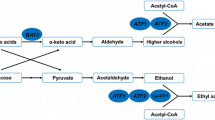
Higher alcohols are mostly produced by Saccharomyces cerevisiae in traditional Chinese Baijiu fermentation [29]. The flavor substance originates from the biosynthetic pathway [2] or the Ehrlich pathway in yeast cells [1]. In the Ehrlich pathway, branched-chain alcohols such as isobutanol, isoamylol, active amyl alcohol, and β-phenylethanol are products from the degradation of corresponding branched-chain amino acids (BCAAs) [14]. BCAAs are converted to the corresponding α-keto acids through the initial transamination step, which is catalyzed by the corresponding mitochondrial and cytosolic amino acid aminotransferases encoded by BAT1 and BAT2 [2–4, 13]. Lilly et al. [16] showed that the transaminases encoded by BAT1 and BAT2 significantly influenced the production of branched-chain alcohols. We previously showed that BAT2 deletion remarkably affected the formation of higher alcohols in Chinese rice wine; by contrast, BAT1 deletion had little impact [33].
Flavor-active esters are produced by the microbial community and synthesized by esterifying enzyme in natural mixed culture starters (Daqu, Xiaoqu, etc.) at later stages of the traditional fermentation process [5]. Esters are mainly biosynthesized using aroma-producing yeast, bacteria, and molds with high esterification abilities instead of ethanol-fermenting yeast S. cerevisiae [28, 35]. Implementing effective measures to increase the content of beneficial esters produced by S. cerevisiae is motivated. S. cerevisiae strains that can produce high amounts of beneficial esters can reduce grain expense, increase efficiency, and lower costs by shortening the fermentation period. In S. cerevisiae, alcohol acetyltransferase encoded by ATF1 (known as AATase I or Atf1p) is a key enzyme in acetate ester synthesis [37]. Acetate ester, such as ethyl acetate (solvent-like aroma), isoamyl acetate (banana-like aroma) and isobutyl acetate (fruity-like aroma), is one of the major beneficial esters [30]. Alcohols and acetyl-coenzyme A are catalyzed to acetate esters by Atf1p [21–23]. Previous studies have shown that the deletion or overexpression of ATF1 significantly affects the production of acetate esters in beer, wine, and sake [12, 15, 17, 26]. In addition, esterase encoded by IAH1 is one of the hydrolyzing enzymes that catalyzes the cleavage of ester bonds in S. cerevisiae [19]. Fukuda et al. [7] reported that, instead of enhancing the activity of AATases, the concentration of isoamyl acetate was significantly improved by deleting the acetate-hydrolyzing esterase gene IAH1 to avoid isoamyl acetate cleavage. The important balance activity between AATases and esterases for the ester accumulation by S. cerevisiae was further proved in [8].
Therefore, S. cerevisiae that can produce high amounts of acetate esters and low concentration of higher alcohols is very important in the Chinese Baijiu industry. Most studies that examined the effects of ATF1, IAH1, and BAT2 on the production of flavor compounds have investigated one or two genes and focused especially on beer and wine fermentation. Moreover, ATF1 overexpression, IAH1 deletion, and BAT2 deletion have different effects on the flavor compound production in various yeasts [7, 8, 15, 17, 24, 26, 32–34]. In S. cerevisiae used for Chinese Baijiu fermentation, the effects of ATF1 overexpression with BAT2 deletion and/or IAH1 deletion on the production of acetate esters and higher alcohols remain unclear. Furthermore, comparison of combined mutations of ATF1 overexpression, BAT2 deletion, and IAH1 deletion remains unclear.
In this study, we constructed the ATF1 overexpression Chinese Baijiu yeast strains with BAT2 and/or IAH1 deletion. In addition, the ATF1 overexpression strain, deletion mutant strains of IAH1 and/or BAT2, and ATF1 double-overexpression strain with BAT2 and IAH1 double-gene deletion were successfully constructed. The effects of ATF1 overexpression, BAT2 deletion, and IAH1 deletion on the flavor compounds were investigated by corn hydrolysate fermentation and Chinese Baijiu fermentation. The interactions of ATF1 overexpression, BAT2 deletion, and IAH1 deletion were further investigated. The results suggest that, compared with other mutant strains, overexpression of ATF1 with double-gene deletion of BAT2 and IAH1 in yeast cells could further increase the ratio of ester to higher alcohol. Combined mutations of ATF1 overexpression, BAT2 deletion, and IAH1 deletion showed diverse effects on the flavor compounds. This study introduced a method for the future optimization of yeast strains for Chinese Baijiu production. In addition, this study has great reference value for future optimization of yeast strains for other alcoholic beverages.
Materials and methods
Strains and plasmids
All strains and plasmids as well as their relevant genotypes used in this study are listed in Table 1.
Media and culture conditions
Escherichia coli cells were grown at 37 °C in Luria–Bertani medium (0.5% yeast extract, 1% tryptone, and 1% NaCl), supplemented with 100 μg/mL ampicillin to select positive E. coli transformants.
Saccharomyces cerevisiae cells were cultured at 30 °C in a yeast extract peptone dextrose (YEPD) medium (1% yeast extract, 2% peptone, and 2% glucose). To select the positive yeast transformants, 1000 μg/mL G418 (Promega, Madison, USA) was added to the YEPD plates for the yeast culture. To select Zeocin-resistant yeast strains, 500 mg/L Zeocin (Promega, Madison, USA) was added to the YEPD plates. Then, the YEPD medium was used for the Cre expression in the yeast transformants.
All solid media contained 2% agar powder (Solarbio, Beijing, China).
Plasmid constructions
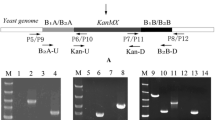
The plasmid pUC19 was used as the backbone to construct recombinant plasmid pUC-PAK. The plasmid pUG6 was used as the template to amplify the KanMX gene for G418 resistance and transformation fragments. Plasmid pPGK1 which contained the promoter (PGK1 P ) and terminator (PGK1 T ) was utilized to prepare the overexpression cassette. The plasmid Yep352 was used as the backbone to construct recombinant plasmid Yep352-A. The plasmid was prepared from DH5α using a Plasmid Mini Kit II (D6945, Omega, Norcross, USA). Genomic yeast DNA was prepared from the industrial yeast strain α5 using a yeast DNA kit (D3370-01, Omega, Norcross, USA). The polymerase chain reaction (PCR) primers used in this study are listed in Online Resource 1.
The plasmid pUC-PAK was constructed as follows. The yeast phosphoglycerate kinase I gene PGK1 fragment (1771 bp) was amplified via PCR from the plasmid pPGK1 with PGK-F and PGK-R primers. The PGK1 fragment was inserted into the XbaI site of pUC19 to generate plasmid pUC-P. The ATF1 fragment (1578 bp), which was amplified via PCR from genomic yeast DNA of α5 with ATF1-F and ATF1-R primers, was inserted into the XhoI site of plasmid pUC-P, between PGK1 P and PGK1 T . The pUC-PA plasmid was then constructed. The PCR-generated loxP-KanMX-loxP fragment (1613 bp) from plasmid pUG6 with Kan-F and Kan-R primers was inserted into the KpnI site of plasmid pUC-PA to construct the final recombinant plasmid pUC-PAK.
The plasmid Yep352-A, which is an episomal plasmid with ATF1 under the control of PGK1 P and PGK1 T , was constructed as follows. The fragment PGK1 P -ATF1-PGK1 T -loxP-KanMX-loxP was amplified via PCR from the plasmid pUC-PAK with PGK-U and KAN-D primers. The fragment was inserted into the EcoRI and SphI sites of Yep352 to generate plasmid Yep352-A.
Yeast transformation and screening
The transformation fragments IA-PGK1 P -ATF1-PGK1 T -loxP-KanMX-loxP-IB and BA-PGK1 P -ATF1-PGK1 T -loxP-KanMX-loxP-BB were amplified by PCR from the recombinant plasmid pUC-PAK with II-F/IK-R and BB-F/BK-R primers, respectively. The transformation fragments IF-loxP-KanMX-loxP-IB and BF-loxP-KanMX-loxP-BB were amplified by PCR from the plasmid pUG6 with IK-F/IK-R and BK-F/BK-R primers, respectively. The fragments and plasmid Yep352-A were then transferred to yeast cells using the lithium acetate/PEG procedure [9]. The recombinant strains were selected using the YEPD medium supplemented with G418 (α-type haploid, 1000 μg/mL; a-type haploid, 1400 μg/mL; and diploid, 1200 μg/mL) and verified through PCR with the primers listed in Online Resource 1. The marker gene KanMX in the engineered strain was removed using the Cre/loxP recombination system [11].
Construction of the recombinant diploid yeast strains
Recombinant diploid yeast strain was constructed by hybridizing a-type and α-type haploid recombinants. Haploid a- and α-type cells were cultured in 5 mL of YEPD medium at 30 °C for 24 h, respectively. Then, they (0.5 mL each) were added to a tube containing 5 mL of YEPD. Yeast cells were cultivated and hybridized at 30 °C for 24 h. The recombinant diploid strain was selected using the MacConkey medium (0.1% glucose, 0.18% NaCl, 0.82% sodium acetate, 0.25% yeast extract, and 2% agar) and verified through PCR with the primers MAT-F/MAT-α/MAT-a. The resulting diploid yeast strains with spore formation were verified under a microscope (Olympus, Tokyo, Japan).
Growth curve determination
Yeast cells were precultured in 5 mL of YEPD medium at 30 °C for 12 h and then transferred into the YEPD medium and cultured at 30 °C for 16 h. The optical density (OD 600) was determined every 1 h using a Bioscreen Automated Growth Curves analysis system (OY Growth Curves Ab Ltd., Helsinki, Finland).
Real-time quantitative PCR (RT-qPCR)
The total RNA of yeast was extracted using a Yeast RNAiso Kit (Takara Biotechnol, Dalian, China) and then reverse-transcribed using a PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara Biotechnol, Dalian, China). Changes in gene expression level were assessed by RT-qPCR using an SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara Biotechnol, Dalian, China). The PCR primers used are listed in Online Resource 1. The PCR program was composed of pre-denaturation for 30 s at 95 °C, amplification using 40 cycles of denaturation for 5 s at 95 °C and annealing and polymerization for 30 s at 60 °C, and melt curve stage for 15 s at 95 °C and 1 min at 60 °C. The result was quantitatively analyzed using the 2−ΔΔCt method. The actin gene (ACT1) was used as a housekeeping gene. The PCR primers used are listed in Online Resource 1.
Fermentation experiments
The corn hydrolysate medium was prepared by gelatinizing a mixture of 60 g of corn flour and 130 mL of water at 65 °C for 20 min in a 250-mL conical flask. Then the mixture was liquefied at 90 °C for 90 min with thermostable α-amylase (10 U/g corn weight, 2 × 105 U/mL; Novozymes, Copenhagen, Denmark) and subsequently saccharified at 60 °C for 30 min with a saccharifying enzyme (150 U/g corn weight, 10 × 105 U/mL; Novozymes, Copenhagen, Denmark). The resulting medium was cooled to 30 °C at room temperature. Yeast cells were precultured in 4 mL of 8 Brix degree (°Bx) corn hydrolysate medium at 30 °C for 24 h. Then, the yeasts were transferred into 36 mL of 12°Bx corn hydrolysate in a 100-mL conical flask and cultured at 30 °C for 16 h. The second precultured yeast (15 mL) was transferred to the prepared corn hydrolysate medium. Moreover, the recombinant strains which harbored Yep352-A were cultured using medium supplemented with G418 (α-type haploid, 1000 μg/mL; a-type haploid, 1400 μg/mL; diploid, 1200 μg/mL). The mixture was fermented at 30 °C until the weight loss of CO2 after interval 12 h was less than 1 g.
For the solid fermentation of sorghum, 80 g of sorghum was immersed in 200 mL of water at 60 °C in a 500-mL conical flask. The mixture was liquefied at 90 °C for 60 min with thermostable α-amylase (5 U/g sorghum weight), and subsequently sterilized at 0.1 MPa for 30 min. Then the mixture was saccharified at 60 °C for 30 min with a saccharifying enzyme (50 U/g sorghum weight), and acid proteinase (15 U/g sorghum weight; 2 × 105 U/g, Novozymes, Copenhagen, Denmark) was added at 40 °C for 30 min. The resulting medium was cooled to 30 °C at room temperature. Yeast cells were precultured in 4 mL of YEPD medium at 30 °C for 24 h and then transferred into 36 mL of YEPD medium at 30 °C for 16 h. The natural mixed culture starter Daqu (20 g) and 20 mL of the second precultured yeast were transferred to the prepared sorghum medium. Moreover, the strain AY15+ATF1 was cultured using the medium supplemented with G418 (1200 μg/mL). The mixture was fermented at 30 °C for 1 day and then at 35 °C until the weight loss of CO2 after an interval of 12 h was less than 1 g.
The CO2 weight loss, residual sugar, and ethanol production were determined using an analytical balance, Brix hydrometer, and oenometer, respectively. The production of volatile flavor compounds including higher alcohols and esters was determined using gas chromatography (GC).
All fermentations were performed in triplicate.
Gas chromatography analysis
GC has been widely used in the analysis of volatile compounds in Chinese Baijiu [5]. Samples from the corn hydrolysate and sorghum media were distilled after fermentation and then used for GC.
The volatile compounds were analyzed on an Agilent 7890C GC with an HP-INNOWax polyethylene glycol column (30 m × 320 μm internal diameter and 0.5 μm coating thickness; Lab Alliance). GC was equipped with an Agilent G4513A autosampler, injector, and a flame ionization detector (FID). Nitrogen was used as carrier gas at a constant flow rate of 2 mL/min. The injector temperature was 200 °C, split ratio was 10:1, and injection volume was 1 μL. The FID was operated at 200 °C. The oven temperature program was as follows: 50 °C for 8 min and then the temperature was increased to 120 °C at 5 °C/min. The final temperature was maintained for 5 min. N-butyl acetate was used as the internal standard. An internal calibration curve was constructed for each compound measured using a specific amount of authentic standards. These chemicals were purchased from Merck.
Results
Overexpression of ATF1 and deletion of IAH1 increased acetate ester production and reduced higher alcohol production
IAH1 genes of α5 strain were, respectively, replaced with the constructed cassette IA-PGK1 P -ATF1-PGK1 T -loxP-KanMX-loxP-IB and the fragment IF-loxP-KanMX-loxP-IB. We obtained engineered strains α5-IAH1+ATF1 and α5-IAH1. In addition, the engineered strain α5+ATF1, which harbored the plasmid Yep352-A, was constructed. The effects of ATF1 overexpression and IAH1 deletion on ester and higher alcohol productions were investigated in the liquid fermentation of corn hydrolysate. The data of fermented samples analyzed by GC are presented in Fig. 1.
No significant distinction was observed between the engineered strain α5-IAH1 and parental strain α5. The production of ethyl acetate by α5-IAH1+ATF1 was ~44.93-fold higher than that by α5. The concentration of higher alcohols produced by α5-IAH1+ATF1 was 40.58% less than that produced by α5. The productions of isobutanol, isoamylol (isoamyl alcohol and active amyl alcohol), and β-phenylethanol by α5-IAH1+ATF1 were significantly lower than those produced by the parental strain. Moreover, the productions of ethyl acetate and higher alcohols by α5+ATF1 were similar to that of α5-IAH1+ATF1. The productions of isobutyl acetate and isoamyl acetate by α5-IAH1+ATF1 increased to 9.66 and 70.37 mg/L, respectively. The productions of isobutyl acetate and isoamyl acetate by α5+ATF1 were 54.04 and 24.93% less than those of α5-IAH1+ATF1. By contrast, no isobutyl acetate or isoamyl acetate was detected in the fermentation sample of α5.
Overexpression of ATF1 and deletion of BAT2 increased acetate ester production and further reduced higher alcohol production
Thus, BAT2 genes of α5 strain were replaced with the constructed cassette BA-PGK1 P -ATF1-PGK1 T -loxP-KanMX-loxP-BB, and the fragment BF-loxP-KanMX-loxP-BB. The engineered strains α5-BAT2+ATF1 and α5-BAT2 were obtained. Fermentation data presented in Fig. 1a suggest that the production of ethyl acetate by the engineered strain α5-BAT2+ATF1 was ~54.03-fold higher than that by α5. The production of isobutyl acetate and isoamyl acetate also increased to 4.98 and 59.17 mg/L, respectively. Meanwhile, the concentration of higher alcohols produced by α5-BAT2+ATF1 was 57.02, 39.59, 30.09, and 27.67% less than those produced by α5, α5-BAT2, α5+ATF1, and α5-IAH1+ATF1 (Fig. 1b).
These results indicate that the production of higher alcohols (isobutanol, isoamylol, and β-phenylethanol) remarkably declined because of the overexpression of ATF1 or the deletion of BAT2. Moreover, compared with the overexpression of ATF1 without BAT2 deletion, overexpression of ATF1 with BAT2 deletion could further reduce higher alcohol production and increase ethyl acetate production in yeast cells. In addition, overexpression of ATF1 with IAH1 deletion produced higher concentrations of isobutyl acetate and isoamyl acetate than overexpression of ATF1 without IAH1 deletion. To construct the strain with ATF1 overexpression and both IAH1 and BAT2 deletion, the kan r marker gene was removed from the engineered strain α5-BAT2+ATF1 using the Cre/loxP recombination system to produce the mutant strain α5-BAT2+ATF1-1.
Overexpression of ATF1 and double-gene deletion of BAT2 and IAH1 further increased the ratio of ester to higher alcohol
The IAH1 gene of α5-BAT2+ATF1-1 was replaced with the fragment IF-loxP-KanMX-loxP-IB to further increase ester production and reduce higher alcohol production. The engineered strain α5-IAH1-BAT2+ATF1 was obtained. In addition, the engineered strain α5-IAH1-BAT2 was constructed, using the fragment BF-loxP-KanMX-loxP-BB amplified via PCR from the plasmid pUG6. Figure 1a shows that the concentration of ethyl acetate produced by α5-IAH1-BAT2+ATF1 was 1267.04 mg/L, which was nearly the same as that produced by α5-BAT2+ATF1. The concentrations of isobutyl acetate and isoamyl acetate were 9.46 and 72.11 mg/L, which were nearly the same as those produced by α5-IAH1+ATF1. Meanwhile, negligible differences in higher alcohol contents were found between the fermentation samples of α5-IAH1-BAT2+ATF1 and α5-BAT2+ATF1 (Fig. 1b).
The results indicate that compared with other engineered and parental strains, the strain with overexpression of ATF1 and double-gene deletion of BAT2 and IAH1 could produce higher ratio of ester to higher alcohol. This strain combines the advantages of α5-IAH1+ATF1 and α5-BAT2+ATF1.
We confirmed that overexpression of the ATF1 gene could enhance the synthesis of acetate ester. Thus, IAH1 gene of α5-BAT2+ATF1-1 was replaced with the fragment BA-PGK1 P -ATF1-PGK1 T -loxP-KanMX-loxP-BB. The engineered strain α5-IAH1+ATF1-BAT2+ATF1 was obtained. However, no obvious differences in the productions of ester and higher alcohol were observed between α5-IAH1-BAT2+ATF1 and α5-IAH1+ATF1-BAT2+ATF1.
mRNA levels
It is noteworthy that there were significant differences in acetate ester and higher alcohol productions among the engineered strains. We quantified the mRNA expression levels of ATF1, BAT2, and IAH1 to clarify the relationship among these genes (Fig. 2a, b, c). The results of RT-qPCR suggest that the ATF1 expression levels of α5-IAH1+ATF1, α5-BAT2+ATF1, α5-IAH1-BAT2+ATF1, and α5-IAH1+ATF1-BAT2+ATF1 were 23.62-, 26.29-, 27.99-, and 29.13-fold higher than that of α5, respectively (Fig. 2a). The overexpression of ATF1 gene under the control of the PGK1 promoter in S. cerevisiae significantly increased the gene expression level. In addition to α5-IAH1+ATF1, which showed the lowest mRNA level, other ATF1 overexpression strains with different ATF1 expression levels showed similar capacities of producing ethyl acetate. The synthesis pathways of acetate esters catalyzed by ATF1-coded alcohol O-acetyltransferase may reach the highest efficiency by overexpressing ATF1 with the PGK1 promoter. Compared with α5-BAT2+ATF1, the lower mRNA level of ATF1 in α5-IAH1+ATF1 may be due to the gene locus where the fragment was inserted. Moreover, the mRNA level of ATF1 in α5-IAH1+ATF1-BAT2+ATF1 was similar to that in α5-BAT2+ATF1, which may be restricted by the concentration of substrate acetyl-CoA.
Compared with the parental strain, the BAT2 expression levels of BAT2 deletion strains and IAH1 expression levels of IAH1 deletion strains were nearly zero. Furthermore, no obvious differences in the BAT2 expression levels were observed among IAH1 one-gene deletion and parental strains (Fig. 2b). It is noteworthy that the IAH1 expression level of α5-BAT2+ATF1 was ~1.56-fold higher than that of other BAT2 one-gene deletion strains and the parental strain (Fig. 2c). In addition, the BAT2 expression level was not affected by ATF1 overexpression or IAH1 deletion. The results indicate that the expression level of IAH1 gene was relatively upregulated because of the ATF1 overexpression.
Growth curve and fermentation properties
The growth abilities of engineered strains showed a slight difference compared with their parental strains (Fig. 3a). Fermentation properties, including residual sugar and weight loss of CO2, were further monitored (Table 2). The ATF1 overexpression strains produced 5.03–7.79% less ethanol contents than the parental strain in the liquid fermentation. This result may be due to more synthesis of ethyl acetate from ethanol and acetyl-CoA in the ATF1 overexpression strains.
Growth curves of recombinant strains and their parental strains. a The growth curves of α mating-type mutant strains and α5; b the growth curves of a mating-type mutant strains and a8; c the growth curves of diploid mutant strains and AY15. Growth curves (in triplicate) were monitored at 30 °C by measuring the optical density (OD600) of the cultures every 1 h
Construction of recombinant diploid yeast strains and Chinese Baijiu fermentation
Different results on flavor compounds were obtained by α5, α5-BAT2, α5+ATF1, α5-IAH1+ATF1, α5-BAT2+ATF1, and α5-IAH1-BAT2+ATF1. Then, a-type haploid recombinants were constructed. The engineered strains a8-BAT2, a8-IAH1+ATF1, a8-BAT2+ATF1, and a8-IAH1-BAT2+ATF1 were obtained. Recombinant diploid yeast strain was constructed by hybridizing a-type and α-type haploid recombinants. The engineered diploid strains AY15-BAT2, AY15-IAH1+ATF1, AY15-BAT2+ATF1, and AY15-IAH1-BAT2+ATF1 were obtained successfully. In addition, the engineered strains a8+ATF1 and AY15+ATF1 were constructed. The recombinant yeast strains showed slightly weaker growth abilities than their parental strains (Fig. 3b, c). The data of volatile flavor compounds produced by these engineered strains during the liquid fermentation of corn hydrolysate are presented in Fig. 4.
Flavor compound productions of mutant and parental strains in the liquid fermentation of corn hydrolysate. a Flavor compound productions of a mating-type strains and a8; b flavor compound productions of diploid mutant strains and AY15. Data are average values and standard deviations from at least three independent tests
We investigated the acetate ester and higher alcohol productions of the obtained recombinant diploid strains and the parental strain AY15 in Chinese Baijiu fermentation with natural mixed culture starter Daqu. The data of fermentation samples analyzed by GC are presented in Fig. 5. Compared with the liquid fermentation of corn hydrolysate, similar results of the difference between the obtained mutants and the parental strain were measured for the Chinese Baijiu fermentation. The concentrations of ethyl acetate produced by AY15+ATF1, AY15-IAH1+ATF1, AY15-BAT2+ATF1, and AY15-IAH1-BAT2+ATF1 were 3.29-, 3.48-, 3.89-, and 3.91-fold higher than that produced by AY15, respectively. The concentrations of higher alcohols produced by AY15-BAT2, AY15+ATF1, AY15-IAH1+ATF1, AY15-BAT2+ATF1, and AY15-IAH1-BAT2+ATF1 were 27.35, 17, 18.01, 38.22, and 39.41% less than that by AY15, respectively. Moreover, the productions of isoamyl acetate by the ATF1 overexpression mutants increased to 2.14–6.23 mg/L. By contrast, no isoamyl acetate was detected in the fermentation sample of AY15.
Discussion
A yeast strain capable of producing high amounts of esters and low concentration of higher alcohols is very important and necessary in the Chinese Baijiu industry. In the present study, we showed for the first time that overexpression of ATF1 and double-gene deletion of BAT2 and IAH1 in yeast cells could further increase the ratio of ester to higher alcohol. Moreover, the interactions of ATF1 overexpression, BAT2 deletion, and IAH1 deletion were investigated. Mutants with varying abilities of producing acetate esters and higher alcohols were constructed.
BAT2, ATF1, and IAH1 are key genes in the metabolic pathways related to higher alcohols and acetate esters. The amino acid aminotransferase encoded by BAT2 converts BCAAs to α-keto acids, which are precursors of branched-chain alcohols in the Ehrlich pathway [2–4, 14]. Alcohols and acetyl-coenzyme A are catalyzed to acetate esters by ATF1-encoded alcohol acetyltransferase [21–23]. Several acetate esters, such as isoamyl acetate, are hydrolyzed to corresponding alcohols by IAH1-encoded esterase [19]. The overexpression of ATF1 gene under the control of the PGK1 promoter in S. cerevisiae could significantly improve the production of acetate esters (ethyl acetate, isobutyl acetate, and isoamyl acetate) in Chinese Baijiu fermentation. The result is consistent with those of previous studies [15–17, 26] and our previous studies in other alcoholic beverages [24, 34]. The IAH1 expression level was markedly upregulated because of the overexpression of ATF1 (Fig. 2c). Hence, the deletion of IAH1 in ATF1 overexpression strains may effectively increase the productions of isobutyl acetate and isoamyl acetate by reducing the hydrolysis of acetate esters. The results support the hypothesis that the expression of ATF1 and IAH1 encoding opposite enzymatic activities is finely regulated [8]. Furthermore, higher alcohol production in the ATF1 overexpression strains with BAT2 deletion decreased due to the interaction of ATF1 overexpression and BAT2 deletion. The production of isobutanol was affected more obviously by the deletion of BAT2, and the overexpression of ATF1 contributed more in the reduction of isoamylol and β-phenylethanol productions. The overexpression of ATF1 with the deletion of BAT2 could further decrease the production of isobutanol and isoamylol. Moreover, no obvious interaction between BAT2 and IAH1 was observed in the study.
Studies on the interactions of the three genes introduce methods for future optimization of yeast strains. Different genetic engineering strategies can be applied based on the distinct requirements on the productions of acetate esters and higher alcohols. In addition, the studies provide novel ideas to investigate the metabolism of esters and higher alcohols. Similar to IAH1-encoded esterase, additional other related enzymes involved in the hydrolysis of acetate esters may be more produced because of ATF1 overexpression, since it was proved that the two insertions of PGK1 P -ATF1-PGK1 T cassettes showed no obvious difference with one insertion in the production of esters (Figs. 1a, 2a). Further increase in acetate ester production may be derived from the deletion of these ester hydrolytic enzymes.
Stable performance of the industrial strain is significant for fermentation application. In this study, industrial strains were used as parent strains for genetic engineering. We constructed diploid mutants with varying abilities of producing acetate esters and higher alcohols. The recombinant strains showed slightly weaker growth abilities and similar fermentation properties to the parental strain in Chinese Baijiu fermentation (Fig. 3c). These strains with satisfactory performance exhibit broad application prospects in industrial production.
In Chinese Baijiu industry, the contradiction between increasing liquor yield and enriching flavor substance is particularly prominent. A yeast strain capable of producing high amounts of acetate esters and low concentration of higher alcohols is highly important and necessary to simplify the traditional technological process, shorten the traditional fermentation period and reduce costs. The results of this study introduced a method for future optimization of yeast strains that produce high amounts of acetate esters and low concentration of higher alcohols in Chinese Baijiu production. Overexpression of ATF1 and double-gene deletion of BAT2 and IAH1 in yeast cells could further increase the ratio of ester to higher alcohol. Moreover, the interactions of ATF1 overexpression, BAT2 deletion, and IAH1 deletion were preliminarily investigated. These interactions are significant in the investigation of the metabolic mechanism of esters and higher alcohols. Hence, these recombinant yeast strains with varying abilities of producing acetate esters and higher alcohols can be remarkably applied to industrial application.
References
Derrick S, Large PJ (1993) Activities of the enzymes of the Ehrlich pathway and formation of branched-chain alcohols in Saccharomyces cerevisiae and Candida utilis grown in continuous culture on valine or ammonium as sole nitrogen source. J Gen Microbiol 139:2783–2792. doi:10.1099/00221287-139-11-2783
Dickinson JR, Norte V (1993) A study of branched-chain amino acid aminotransferase and isolation of mutations affecting the catabolism of branched-chain amino acids in Saccharomyces cerevisiae. FEBS Lett 326:29–32. doi:10.1016/0014-5793(93)81754-N
Dickinson JR, Salgado LEJ, Hewlins MJE (2003) The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J Biol Chem 278:8028–8034. doi:10.1074/jbc.M211914200
Eden A, Nedervelde LV, Drukker M, Benvenisty N, Debourg A (2001) Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl Microbiol Biotechnol 55:296–300. doi:10.1007/s002530000506
Fan W, Qian MC (2005) Headspace solid phase microextraction and gas chromatography-olfactometry dilution analysis of young and aged Chinese “Yanghe Daqu” liquors. J Agric Food Chem 53:7931–7938. doi:10.1021/jf051011k
Fan W, Qian MC (2006) Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J Agric Food Chem 54:2695–2704. doi:10.1021/jf052635t
Fukuda K, Kuwahata O, Kiyokawa Y, Yanagiuchi T, Wakai Y, Kitamoto K, Inoue Y, Kimura A (1996) Molecular cloning and nucleotide sequence of the isoamyl acetate-hydrolyzing esterase gene (EST2) from Saccharomyces cerevisiae. J Ferment Bioeng 82:8–15. doi:10.1016/0922-338X(96)89447-5
Fukuda K, Yamamoto N, Kiyokawa Y, Yanagiuchi T, Wakai Y, Kitamoto K, Inoue Y, Kimura A (1998) Balance of activities of alcohol acetyltransferase and esterase in Saccharomyces cerevisiae is important for production of isoamyl acetate. Appl Environ Microbiol 64:4076–4078. doi:10.1016/0003-2697(71)90136-9
Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96. doi:10.1016/S0076-6879(02)50957-5
Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH (2002) A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucl Acids Res 30:88–94. doi:10.1093/nar/30.6.e23
Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucl Acids Res 24:2519–2524. doi:10.1093/nar/24.13.2519
Hirosawa I, Aritomi K, Hoshida H, Kashiwagi S, Nishizawa Y, Akada R (2004) Construction of a self-cloning sake yeast that overexpresses alcohol acetyltransferase gene by a two-step gene replacement protocol. Appl Microbiol Biotechnol 65:68–73. doi:10.1007/s00253-004-1563-0
Kispal G, Steiner H, Court DA, Rolinski B, Lill R (1996) Mitochondrial and cytosolic branched-chain amino acid transaminases from yeast, homologs of the myc oncogene-regulated Eca39 protein. J Biol Chem 271:24458–24464. doi:10.1074/jbc.271.40.24458
Kobayashi M, Shimizu H, Shioya S (2008) Beer volatile compounds and their application to low-malt beer fermentation. J Biosc Bioeng 106:317–323. doi:10.1263/jbb.106.317
Lilly M, Bauer FF, Lambrechts MG, Swiegers JH, Cozzolino D, Pretorius IS (2006) The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 23:641–659. doi:10.1002/yea.1382
Lilly M, Bauer FF, Styger G, Lambrechts MG, Pretorius IS (2006) The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res 6:726–743. doi:10.1111/j.1567-1364.2006.00057.x
Lilly M, Lambrechts MG, Pretorius IS (2000) Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl Environ Microbiol 66:744–753. doi:10.1128/AEM.66.2.744-753.2000
Lu J, Dong J, Wu D, Chen Y, Guo X, Shi Y, Sun X, Xiao D (2012) Construction of recombinant industrial brewer’s yeast with lower diacetyl production and proteinase A activity. Eur Food Res Technol 235:951–961. doi:10.1007/s00217-012-1821-9
Ma J, Lu Q, Yuan Y, Ge H, Li K, Zhao W, Gao Y, Niu L, Teng M (2011) Crystal structure of isoamyl acetate-hydrolyzing esterase from Saccharomyces cerevisiae reveals a novel active site architecture and the basis of substrate specificity. Proteins 79:662–668. doi:10.1002/prot.22865
Meng X, Wu Q, Wang L, Wang D, Chen L, Xu Y (2015) Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with Bacillus licheniformis for Chinese maotai-flavor liquor making. J Ind Microbiol Biotechnol 42:1–8. doi:10.1007/s10295-015-1647-0
Nordström K (1962) Formation of ethyl acetate in fermentation with brewer’s yeast. III. Participation of coenzyme A. J I Brewing 68:398–407. doi:10.1002/j.2050-0416.1962.tb01882.x
Nordström K (1963) Formation of ethyl acetate in fermentation with brewer’s yeast: IV. Metabolism of acetyl-coenzyme A. J Inst Brew 69:142–153. doi:10.1002/j.2050-0416.1963.tb01910.x
Nordström K (1964) Formation of esters from alcohols by brewer’s yeast. J Inst Brew 70:328–336. doi:10.1002/j.2050-0416.1964.tb01999.x
Stribny J, Querol A, Pérez-Torrado R (2016) Differences in enzymatic properties of the Saccharomyces kudriavzevii and Saccharomyces uvarum alcohol acetyltransferases and their impact on aroma-active compounds production. Front Microbiol 7:897. doi:10.3389/fmicb.2016.00897
Swiegers JH, Pretorius IS (2005) Yeast modulation of wine flavor. Adv Appl Microbiol 57:131–175. doi:10.1016/S0065-2164(05)57005-9
Verstrepen KJ, Van Laere SD, Vanderhaegen BM, Derdelinckx G, Dufour JP, Pretorius IS, Winderickx J, Thevelein JM, Delvaux FR (2003) Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl Environ Microbiol 69:5228–5237. doi:10.1128/AEM.69.9.5228-5237.2003
Wu Q, Kong Y, Xu Y (2015) Flavor profile of Chinese liquor is altered by interactions of intrinsic and extrinsic microbes. Appl Environ Microbiol 82:422–430. doi:10.1128/AEM.02518-15
Wu XH, Zheng XW, Han BZ, Vervoort J, Nout MJ (2009) Characterization of Chinese liquor starter, “Daqu”, by flavor type with 1H NMR-based nontargeted analysis. J Agric Food Chem 57:11354–11359. doi:10.1021/jf902881p
Xiao M, Wang G, Liu F (1996) Study on higher alcohols production by microbes in the course of sorghum solid-state Chinese-liquor fermentation. Food Ferment Ind 19:65–85 (in Chinese)
Xiao ZB, Yu D, Niu YW, Chen F, Song SQ, Zhu JC, Zhu GY (2014) Characterization of aroma compounds of Chinese famous liquors by gas chromatography-mass spectrometry and flash GC electronic-nose. J Chromatogr B 945–946:92–100. doi:10.1016/j.jchromb.2013.11.032
Yang D, Luo X, Wang X (2014) Characteristics of traditional Chinese shanlan wine fermentation. J Biosci Bioeng 117:203–207. doi:10.1016/j.jbiosc.2013.07.010
Zhang CY, Liu YL, Qi YN, Zhang JW, Dai LH, Lin X, Xiao DG (2013) Increased esters and decreased higher alcohols production by engineered brewer’s yeast strains. Eur Food Res Technol 236:1009–1014. doi:10.1007/s00217-013-1966-1
Zhang CY, Qi YN, Ma HX, Li W, Dai LH, Xiao DG (2015) Decreased production of higher alcohols by Saccharomyces cerevisiae, for Chinese rice wine fermentation by deletion of Bat aminotransferases. J Ind Microbiol Biotechnol 42:617–625. doi:10.1007/s10295-015-1583-z
Zhang JW, Zhang CY, Dai LH, Dong J, Liu YL, Guo XW, Xiao DG (2012) Effects of overexpression of the alcohol acetyltransferase-encoding gene ATF1 and disruption of the esterase-encoding gene IAH1 on the flavour profiles of Chinese yellow rice wine. Int J Food Sci Technol 47:2590–2596. doi:10.1111/j.1365-2621.2012.03140.x
Zheng XW, Tabrizi MR, Nout MJR, Han BZ (2011) Daqu—a traditional Chinese liquor fermentation starter. J Inst Brew 117:82–90. doi:10.1002/j.2050-0416.2011.tb00447.x
Zheng XW, Yan Z, Nout MJR, Boekhout T, Han BZ, Zwietering MH, Eddy JS (2014) Characterization of the microbial community in different types of Daqu samples as revealed by 16s rRNA and 26s rRNA gene clone libraries. World J Microbiol Biotechnol 31:199–208. doi:10.1007/s11274-014-1776-z
Zhu J, Lin J, Palomec L, Wheeldon I (2015) Microbial host selection affects intracellular localization and activity of alcohol-o-acetyltransferase. Microb Cell Fact 14:1–10. doi:10.1186/s12934-015-0221-9
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31471724) and the Major Project of Research Program on Applied Fundamentals and Advanced Technologies of Tianjin (14JCZDJC32900).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Wang, JH., Zhang, CY. et al. Regulation of Saccharomyces cerevisiae genetic engineering on the production of acetate esters and higher alcohols during Chinese Baijiu fermentation. J Ind Microbiol Biotechnol 44, 949–960 (2017). https://doi.org/10.1007/s10295-017-1907-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1907-2