Abstract
Penicillium echinulatum was evaluated as a cellulolytic enzyme producer in shaking flasks and bioreactor submerged culture using sugarcane bagasse as carbon source. Sodium hydroxide delignified steam-exploded pretreated bagasse (SDB) and hydrothermal pretreated bagasse had a maximum filter paper activity (FPase) of 2.4 and 2.6 FPU/mL, respectively. Delignified acid pretreated bagasse and Celufloc 200TM (CE) carbon sources displayed maximum FPase of 1.3 and 1.6 FPU/mL while in natura bagasse (INB) provided the lowest enzyme activity, ca. 0.4 FPU/mL. Measurement of surface specific area of lignocellulosic material and scanning electron microscopic images showed a possible correlation between fungal mycelia accessibility to lignocellulosic particles and obtained cellulolytic enzyme activity of fermentation broth. Fed-batch experiments performed in a controlled bioreactor attained the highest value of FPase of 3.7 FPU/mL, enzyme productivity of 25.7 FPU/L h, and enzyme yield from cellulose equal to 134 FPU/g with SDB. Enzyme hydrolysis of steam-pretreated bagasse accomplished with the obtained supernatant of fermentation broth (10 FPU/g of biomass and 5 % w/v) performed better than commercial cellulose complex. The results showed that P. echinulatum has potential to be used as an on-site enzyme platform aiming second bioethanol production from sugarcane lignocellulosic residue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over 40,000,000 tons of bagasse and leaves from sugarcane are generated every season in Brazil, and this material has become recognized worldwide as one of the most important lignocellulosic wastes for second generation bioethanol production. Its utilization could increase Brazilian ethanol production by 40 % for the same area of sugarcane crop [1].
Enzymatic hydrolysis is the preferred technique for release of fermentable sugars from lignocellulosic biomass. However, the main bottleneck for wider application of cellulases in second generation ethanol production is their cost, especially considering that large quantities of the enzymes are required [2]. The cost is a function of enzyme productivity (the amount of enzyme produced in a defined bioreactor volume according to time), enzyme yield from the carbon source (Y P/S, the amount of enzyme produced according to the amount of carbon source consumed), enzyme product recovery, formulation, and transportation, as well as its tax for commercialization.
As a low-cost feedstock [3], cellulosic biomass is an ideal carbon source for enzyme production. It is therefore logical to try to produce, on-site, tailor-made enzymatic mixtures that are optimized for the specific feedstock to be processed. Furthermore, the use of the same material for enzyme production and hydrolysis at the same location should help to reduce the production costs of second generation ethanol since both steps of the operation could be undertaken in the same place and benefit from the installed infrastructure (including the provision of water, electricity, and steam). Other advantages are avoidance of downstream operations including unnecessary product formulations, transport from the manufacturer to the consumer, and storage for long periods [3, 4].
Trichoderma reesei secreted the most studied cellulase complexes, and it is currently used as an important microorganism for the cellulase enzyme industry. On the other hand, fungi of the genus Penicillium, such as Penicillium echinulatum, have shown the ability to produce a complete set of cellulolytic enzymes with high filter paper activity (FPase), a satisfactory β-glucosidase to FPase ratio, and the capacity to hydrolyze pretreated sugarcane bagasse with very high yields [5, 6, 7, 8]. It is therefore clear that these fungi are potential alternative candidates for industrial cellulase enzyme complex production. Nonetheless, a better understanding is needed concerning the response of P. echinulatum to sugarcane bagasse as carbon source.
The present paper reports on efforts to understand the type of sugarcane bagasse pretreatment that might be used to generate a suitable carbon source for enzyme production. The behavior of P. echinulatum was investigated in batch and fed-batch submerged bioreactor fermentation using this type of carbon source, and the potential of the resulting enzyme complex to perform enzymatic biomass hydrolysis was evaluated. The following pretreatments were tested: milled in natura bagasse (INB), steam explosion followed by NaOH delignification (DSB), acid treatment followed by NaOH delignification (DAB), and hydrothermal pretreatment (HB). The results were compared with those obtained using Celufloc 200™ (CE), a pure microcrystalline cellulose supplied by Celufloc Ind. Com. (São Paulo, Brazil). The chemical composition, specific surface area, and morphology (using scanning electron microscopy) of the different types of pretreated bagasse were also assessed in order to better understand the enzyme yields obtained.
Materials and Methods
Organism and Inoculum Preparation
The P. echinulatum 9A02SI (DSM18942) strain was obtained by mutation and selection [9], and has already been identified for its potential to produce cellulases and related enzymes [5, 6]. Stock cultures were stored at 8 °C on potato dextrose agar (PDA) slants. The fungus was grown in PDA (in 250-mL Erlenmeyer flasks containing 50 mL of medium) at 29 °C for 7 days. A conidia suspension, obtained by adding 20 mL of sterilized distilled water and Tween 80, was transferred to the inoculum media (Table 1).
Erlenmeyer Flask Experiments
The Erlenmeyer flasks were incubated for 72 h at 200 rpm at 29 °C. A volume equal to 10 % of the grown culture was then transferred to 1-L Erlenmeyer flasks containing 200 mL of the production media (Table 1). The concentrated saline solution used [10] contained (g/L) KH2PO4 (20.0), (NH4)2SO4 (14.0), urea (3.0), MgSO4·7H2O (3.0), CaCl2 (3.0), FeSO4·7H2O (0.05), ZnSO4·7H2O (0.014), MnSO4·H2O (0.016), and CoCl2 (0.02). The compositions of the carbon sources tested are provided in Table 2. The pH of the culture medium was adjusted to 5.3 before sterilization in an autoclave at 121 °C for 20 min.
The assays were performed in triplicate, with incubation of the shaker flasks for 7 days at 29 °C and 200 rpm in an Excella E24N incubator (New Brunswick Scientific, USA), with samples being periodically removed and analyzed to determine the concentrations of cellulase and protein. Hemicellulases and β-glucosidase were analyzed after 144 h of fermentation, as described below.
Bioreactor Experiments
Batch experiments were performed using 1.5 L or 3.0 L working volume bioreactors (BioFlo 115, New Brunswick Scientific, USA) equipped with three flat blades Rushton-type impellers. The experimental conditions were a temperature of 29 °C, a dissolved O2 concentration equivalent to 30 % of air saturation, and a pH of 5.0, adjusted using 0.4 M H2SO4 or aqueous NH4OH solution (1:3). Foaming was controlled by the manual addition of previously sterilized polypropylene glycol antifoaming agent (P2000; Dow Chemical, Brazil). Samples were periodically removed and centrifuged at 10,000×g and 10 °C for 20 min. The cellulase, xylanase, and glucosidase activities, as well as the protein concentration, were determined as described below. The same culture media were used in the batch and shaker flask experiments.
Fed-batch experiments were performed using an initial batch under the conditions described above, followed by periodic additions of dry CE or DSB in order to maintain an unlimited carbon source throughout the experiment. Different bioreactor protocols were tested, as follows:
-
Protocol A:
batch with an initial CE concentration of 10 g/L.
-
Protocol B:
fed batch with an initial CE concentration of 10 g/L, followed by two further additions at 29 and 54 h of fermentation, to give a total of 31 g/L of CE.
-
Protocol C:
fed batch with an initial CE concentration of 10 g/L, followed by four additions at 43, 69, 94, and 143 h, to give a total of 49 g/L of CE.
-
Protocol D:
batch with an initial DSB concentration of 10 g/L.
-
Protocol E:
fed batch with an initial DSB concentration of 10 g/L, followed by four additions at 24, 48, 72, and 96 h, to give a total of 46 g/L of DSB.
Hydrolysis Assays
The hydrolysis was performed in 250-mL shaker flasks containing a liquid volume of 50 mL, using steam-pretreated bagasse as substrate. The steam pretreatment was performed at Largo do Rosario mill (Orlândia, São Paulo, Brazil), using 800 kg of in natura bagasse in a 5-m3 reactor. After adding 22 bar of superheated steam, the reaction temperature was raised to 200 °C, followed by sudden decompression of the bagasse bed down to normal pressure. The resulting mash was removed from the reactor, separated from the hydrolysis liquor in a press filter, washed with water until neutral pH was reached, dried at room temperature, milled, and classified using a 35 mesh screen. Hydrolysis of the steam-pretreated bagasse was performed using a concentration of 10 % (w/v, dry basis), and the added enzyme loading corresponded to 10 FPU/g substrate. The supernatant of a 144 h P. echinulatum culture grown on DSB was used for the hydrolysis studies, and the commercial cellulase enzyme complex Accelerase 1500 (Genencor, Palo Alto, CA, USA) was used as a control under the same hydrolysis conditions. Sodium azide was added to a concentration of 2 % (0.2 g/L) in order to inhibit microbial contamination. The volume was adjusted with 50 mM sodium citrate (pH 4.8), and the hydrolysis was carried out at 50 °C and 150 rpm. Samples were periodically withdrawn, centrifuged at 12,000×g for 5 min, and stored at −20 °C. The supernatant was purified using Sep-Pak C18 cartridges, after which glucose, xylose, cellobiose, and arabinose were measured using a Dionex Ultimate 3000 HPLC fitted with an Aminex HPX-87H column kept at 50 °C. A Shodex infrared detector was operated at 40 °C, and the mobile phase was 0.005 M H2SO4, at a flow rate of 0.5 mL/min.
Microscopy Examination
After the fermentation period, the contents of the flasks were centrifuged at 4,000×g and 12 °C for 20 min (Eppendorf 5810R centrifuge). Blank flasks were prepared as described above, without addition of inoculum. After 96 and 144 h (for blank and fermented flasks, respectively) in a shaker at 200 rpm and 29 °C, the contents of the flasks were centrifuged at 3,000×g and 12 °C for 20 min. The pellets formed were lyophilized (Labconco Freezone 6 Benchtop) and stored at −80 °C prior to examination using scanning electron microscopy (SEM). The lyophilized samples were coated with a thin layer of gold in a sputter coater and were then observed with a JSM-5900 LV microscope operated using an acceleration voltage of 10 kV.
Specific Surface Area Measurements
The specific surface areas (SSA) of the substrates used as carbon sources were measured with a surface area and porosity analyzer (Assay 2020; Micromeritics). The samples were dried for about 25 h at 70 °C, using a temperature gradient of 1 °C/min, an evacuation rate of 1 mm Hg/s, a vacuum set point of 10 μm Hg, and an evacuation time of 60 min. Nitrogen was used to analyze the samples, and helium was used to calculate the dead volume. The standard deviation was calculated from at least five measurements at increasing pressures. The SSA was calculated using the Asap 2020 program, based on single and multipoint BET surface areas. The values were further corrected to account for the influence of the sample tube.
Analytical Methods
Filter paper activity (FPase) in the centrifugation supernatant was determined as described by Ghose [11], with modifications so that the scale of the reaction was reduced 10-fold. Reducing sugars were measured by the dinitrosalicylic acid (DNS) method, using glucose as standard.
The xylanase activity measurement was adapted from the literature [12]: 10 μL of diluted centrifugation supernatant was mixed with 40 μL of 50 mM sodium citrate buffer (pH 4.8) and 50 μL of birchwood xylan or beechwood xylan (Sigma-Aldrich, St. Louis, MO, USA) at 0.5 % (w/v), and incubated at 50 °C for 10 min. The concentration of the carbohydrate released was measured by the DNS method, using xylose as standard.
β-Glucosidase activity was measured using p-nitrophenol-β-d-glucoside (Sigma-Aldrich), as described in the literature [13]. The assay contained 10 μL of diluted centrifugation supernatant, 30 μL of 50 mM citrate buffer (pH 4.8), and 20 μL of 12 mM p-nitrophenol-β-d-glucoside, and incubation was carried out at 50 °C for 10 min. The reaction was stopped by adding 120 μL of 1 M Na2CO3, and the absorbance was measured at 412 nm. One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of reducing sugar per minute under the assay conditions.
Protein concentrations were measured in micro plates using Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, USA). Bovine serum albumin was used as standard.
The concentration of residual cellulose was estimated as described previously [14]. A 5-mL volume of fermentation broth was centrifuged in a 15-mL Falcon tube (3,000×g, 20 min, 12 °C). The pellet was suspended in 1.5 mL of reagent (consisting of a mixture of 40 mL of acetic acid, 10 mL of water, and 5 mL of nitric acid) and boiled in a water bath for 30 min. The supernatant was removed, and 5 ml of water was added. The mixture was then centrifuged (3,000×g, 20 min, 12 °C), the supernatant was discarded, and the mass of residue was dried at 104 °C. The concentration of residual cellulose was expressed as the mass of dry residue per volume (5 mL) of fermentation broth.
Composition of the Carbon Sources
The different carbon sources were chemically characterized in terms of their contents of cellulose, hemicellulose, and lignin, using the methodologies described elsewhere [15]. All the analyses were performed in triplicate. A representative 200 mg of sample powder was hydrolyzed with sulfuric acid in two steps. The primary hydrolysis was carried out with 72 % H2SO4 at 45 °C for 5 min, with stirring, followed by dilution of the acid concentration to 2.5 % and heating in an autoclave at 121 °C for 30 min. The samples were rapidly cooled to room temperature on ice and then filtered through a porous crucible plate under vacuum. The quantities of acid-soluble and insoluble (Klason) lignin were determined spectrophotometrically and gravimetrically, respectively. The cellulose and hemicellulose contents were analyzed by high performance anion exchange–pulsed amperometric detection (HPAE–PAD) using a Dionex ICS 3000 instrument fitted with CarboPac PA1 analytical and guard columns (4 mm × 250 mm and 4 mm × 50 mm, respectively). The monosaccharides present in the hydrolysates of the original samples were converted to the percentage of polysaccharides (by converting glucose to glucan). The HPAE–PAD monosaccharide peak areas were converted to concentrations using calibration standards of l-fucose, l-rhamnose, l-arabinose, d-galactose, d-glucose, and d-xylose (Sigma-Aldrich). The monosaccharide weights were converted to hydrolysis factors considering the dry weight of the original sample.
Results
Shaker Flask Experiments
Table 2 summarizes the cellulose, hemicellulose, and lignin contents of the pretreated sugarcane bagasse and the commercial Celufloc microcrystalline cellulose used as carbon sources. The amounts of secreted protein and the activities of the cellulolytic enzymes were influenced by the type of bagasse pretreatment, and generally increased up to 144 h then reached a plateau (Fig. 1). In natura bagasse gave the lowest enzyme and protein production, with a maximum filter paper activity of less than 0.5 FPU/mL, while the HB and DSB carbon sources provided the highest cellulase production by the fungus. An initial carbon source concentration of 10 g/L resulted in equal performance of DSB and HB, with FPase activity of almost 2.5 FPU/mL at 144 h (Fig. 1a). Celufloc™ 200, which is currently used as carbon source for cellulase production [5, 7], and DAB showed intermediate performance, with FPase activities of 1.6 and 1.3 FPU/mL, respectively (Fig. 1a).
Time course of filter paper activity, FPase (a), and protein concentration (b) for shaking flasks experiments with P. echinulatum grown on different carbon sources: Celufloc™ 200 (CE), in natura bagasse (IN), delignified acid-pretreated bagasse (DAB), delignified steam-explosion pretreated bagasse (DSB), and hydrothermal pretreated bagasse (HB). Initial dry mass carbon source content of all flasks was 10 g/L
Protein secretion appeared to correlate with FPase activity, and maximum values of around 1 g/L were again obtained for DSB and HB. The in natura bagasse provided the lowest protein concentration (<0.5 g/L) (Fig. 1b), while intermediate values were obtained for DAB and CE.
Hemicellulase production, expressed as xylanase activity, was measured after a fermentation period of 144 h. Maximum xylanase activity was achieved using DSB and HB, with slight differences according to the type of substrate employed: 61.4 U/mL (DSB) and 59.4 U/mL (HB), using beechwood xylan as substrate; and 55.3 U/mL (DSB) and 69.6 U/mL (HB), using birchwood xylan as substrate. The xylanase activities obtained for the other carbon sources using beechwood and birchwood xylans, respectively, were (IU/mL) 51.1 and 50.8 (DAB), 30.0 and 25.2 (CE), and 16.1 and 29.3 (INB).
Maximum production of β-glucosidase (3.1 IU/mL after 144 h fermentation) was obtained using CE as carbon source. The values for the other carbon sources were (IU/mL) 1.7 (DAB), 1.2 (DSB), 0.9 (HB), and 0.3 (INB). Table 3 summarizes the enzyme activities obtained for these materials used as carbon sources. There was a tendency towards higher cellulase activity (as measured by filter paper activity) as the specific surface area increased. Hence, the lowest and highest cellulase activities (0.39 and 2.46 FPU/mL, respectively) corresponded to the lowest and highest SSA (0.65 and 1.30 m2/g, respectively). Typical structures of in natura bagasse and DSB, before and after fungal growth, are shown in the scanning electron microscopy images presented in Fig. 2. These reveal that there was vigorous growth in the pretreated bagasse.
Bioreactor Experiments
A set of experiments were performed in order to evaluate the performance of P. echinulatum in submerged bioreactor fermentation with CE and DSB as substrates.
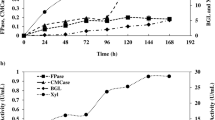
Biosynthesis of cellulase was related to cellulose consumption, and a peak value was obtained with exhaustion of the carbon source (Fig. 3a, c). There was almost complete consumption of the CE used as carbon source for P. echinulatum grown in batch and fed-batch modes according to protocols A, B, and C (10, 31, and 49 g/L of CE, respectively) (Fig. 3c). Maximum FPase activities were 0.8 FPU/mL for the batch experiment and about 1.5 FPU/mL at approximately 140–160 h for the fed-batch procedure, irrespective of the amount of cellulose added and consumed (Fig. 3a, c). The results showed that there was no consistent ratio between the amounts of carbon source delivered and consumed. The resulting enzyme protein concentration increased from 0.5 g/L for the batch experiment up to a maximum of 0.7–0.8 g/L in the fed-batch procedure, regardless of the amount of added and consumed cellulose (Fig. 3b, c).
Time course for bioreactor experiments with P. echinulatum grown on Celufloc™ 200 (CE): FPase (a), protein concentration (b), and cellulose concentration (c) according to protocol A (filled triangles), B (open circles), and C (filled squares). Protocol A: batch with CE at initial concentration of 10 g/L. Protocol B: fed batch with CE initial concentration of 10 g/l followed by two additions at 29 and 54 h of fermentation up to CE = 31 g/L. Protocol C: fed batch with CE initial concentration of 10 g/L followed by four additions at 43, 69, 94, and 143 h up to CE = 49 g/L
In the experiments using DSB, the added carbon source (10 and 46 g/L for protocols D and E, respectively) had been totally consumed at about 150 h. Maximum FPase values were 1.5 and 3.7 FPU/mL, respectively, at 144 h (Fig. 4a), with up to 4 g/L of secreted protein produced in the fed-batch procedure (Fig. 4b). In contrast to the CE experiments, in the case of DSB there was therefore a close association between the amount of carbon source delivered and the maximum FPase activity observed. The highest enzyme productivity was achieved for fed-batch protocol E (25.7 FPU/L h at 144 h fermentation time).
Time course for bioreactor experiments with P. echinulatum grown on delignified steam-explosion pretreated bagasse (DSB) according to protocol D (filled triangles) and E (filled squares) for FPase (a); protein concentration (b); xylanase (c), and β-glucosidase (d). Protocol D: batch with initial concentration of DSB of 10 g/L. Protocol E: fed batch with initial concentration of DSB of 10 g/L followed by four additions at 24, 48, 72, and 96 h up to DSB = 46 g/L
Similarly, the activities of xylanase and β-glucosidase were higher for the fed-batch procedure (protocol E) than for the batch procedure (protocol D), with values of 95.0 and 3.9 IU/mL, respectively, for the former and 69.6 and 1.0 IU/mL, respectively, for the latter (Fig. 4c, d).
Hydrolysis of Pretreated Bagasse Using the P. echinulatum Enzyme Complex
The performance of the cellulase complex produced by P. echinulatum was evaluated for the hydrolysis of bagasse subjected to conventional steam pretreatment (200 °C, 7 min). The composition of the pretreated bagasse (SB) is shown in Table 2. The hydrolysis assays were performed using the P. echinulatum cellulase cocktail produced with DSB, and the results were compared with those obtained for the commercial Accelerase 1500 cellulase enzyme complex. The experiments were performed in triplicate, and the values obtained are shown in Fig. 5.
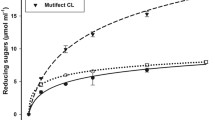
Time course for concentration of glucose (a), cellobiose (b) and xylose (c) during the enzymatic hydrolysis of steam explosion pretreated bagasse (SB) (concentration of 10 % w/v; enzyme load of 10 FPU/g) using the cellulase enzyme cocktail produced by P. echinulatum grown on DSB (filled diamonds); and using the cellulase enzyme cocktail Accelerase 1500 (open squares)
In terms of maximum glucose concentration, the P. echinulatum enzyme complex showed much better performance than Accelerase 1500, with yields of 15.5 and 6.1 g/L, respectively, after 72 h of hydrolysis (Fig. 5a). On the other hand, accumulation of cellobiose, a hydrolysis intermediate, was always lower for the commercial complex compared to the P. echinulatum enzyme complex (Fig. 5b). In terms of pentose recovery from the pretreated biomass, use of the P. echinulatum complex and the commercial enzyme gave similar low values of xylose concentration with time (Fig. 5c), and arabinose concentrations were always below detectable levels for both enzyme complexes.
Discussion
Pretreated sugarcane bagasse was evaluated as carbon source for P. echinulatum enzyme production in shaker flasks and compared to the commercial Celufloc200™ cellulose used as carbon source.
The different bagasse pretreatments produced a diversity of materials for use as carbon sources (Table 2). Although the structural lignin content of HB was much higher than that of DSB (Table 2), experiments performed with the same amounts of carbon (Fig. 1a) resulted in similar enzyme activities (in terms of FPase, xylanase, and β-glucosidase). Furthermore, DAB, for which the lignin content was also low (Table 2), provided lower enzyme activities than HB or DSB. The amount of lignin did not, therefore, appear to be an important chemical property of the carbon source in terms of enzyme production, in contrast to the findings of previous work [16] that reported a delay in enzyme production caused by the presence of lignin. This apparently did not occur in the present case. Additionally, it is possible that DAB was a less satisfactory carbon source for P. echinulatum due to the presence of inhibitors released during the pretreatment, such as furfural and weak acids [17]. It should be noted that the possibility of pH effects on enzyme biosynthesis can be ignored since for all the carbon sources the pH was corrected to 5.3 prior to sterilization of the culture media (see “Materials and Methods” section), and variation of pH during the course of the experiments was virtually identical for all the carbon sources.
On the other hand, it appeared that the observed enzyme activities could have been influenced by differences in the ease of access of fungal mycelia to the pretreated bagasse. Pretreatment results in well-known changes in the chemical composition and physical structure of the substrate, such as redistribution of the hemicellulose and lignin fractions, increased surface area, and formation of channels and pores, among other effects [18]. The data indicated that pretreatment caused an increase of the specific surface area (SSA), compared to the untreated in natura bagasse. The measured SSA values were (m2/g) 0.65 ± 0.11 (INB), 0.78 ± 0.10 (DAB), 0.80 ± 0.15 (CE), 1.07 ± 0.16 (HB), and 1.30 ± 0.15 (DSB) (Table 2).
The in natura bagasse showed a very smooth and ordered fibrous surface, while the delignified steam-exploded bagasse (DSB) presented a dismantled and fractured structure with various cracks and void spaces (Fig. 2a, b). This was probably an important determining factor for the differences in fungal growth observed for these substrates. SEM images of the in natura bagasse and DSB after P. echinulatum cultivation using these materials as carbon source revealed that the former was poorly colonized by fungal mycelia (Fig. 2c), while the DSB substrate was totally occupied by mycelial biomass (Fig. 2d).
From the present results, it can be hypothesized that aggressive pretreatment, such as steam explosion followed by hot NaOH delignification, provides greater accessibility for penetration of fungal mycelia into the substrate, promoting better growth of P. echinulatum and increasing FPU yields. This hypothesis is corroborated by the observed differences in the specific surface areas and morphologies of the substrates.
Batch and fed-batch bioreactor experiments with increased levels of delivered carbon sources (CE and DSB) were carried out to evaluate the performance of P. echinulatum enzyme biosynthesis in controlled submerged fermentation. There was almost complete consumption of CE used according to protocols A, B, and C (Fig. 3c), but an increase in the amount of the carbon source delivered to the system was unable to guarantee an increase in enzyme yield. It should be pointed out that in these experiments the level of dissolved O2 always exceeded 30 % of saturation, so that there was little risk of oxygen transfer limitation. It is possible that catabolite repression of cellulase biosynthesis could have occurred, as proposed previously for T. reesei [19]. Mitigation of this effect would require a more sensitive carbon source input protocol. This phenomenon probably did not occur when DSB was used as substrate since in this case a correlation was observed between the amount of carbon source added and the FPase yield.
Interestingly, in both CE and DSB experiments, the secreted protein concentration in the supernatant was correlated with the FPase activity of the fermented broth, indicating a high selectivity during biosynthesis of the cellulolytic enzyme complex (Figs. 3a, b and 4a, b). The values obtained for the bioreactor fermentations were lower than for the shaker flask experiments, indicating that stress effects on the fungal mycelia might have been occurred due to the Rushton impeller used in the bioreactor, as found for T. reesei [20].
Cellulase production by P. echinulatum has been studied previously using Celufloc as carbon source in submerged shaker flask cultivations. An FPase activity of 1.6 FPU/mL was obtained after 168 h of fermentation using a 10 g/L mixture of lactose and CE [5], and an activity of around 3 FPU/mL was achieved for a CE culture medium supplemented with glucose and methylxanthine [8]. In another work, a maximum FPase yield of 30–40 FPU/g of solid phase and β-glucosidase activity of up to 60 IU/g of solid phase were obtained using solid-state fermentation and a culture medium composed of a mixture of acid-pretreated bagasse and wheat flour [21].
However, considering a bioreactor solid phase density of about 0.1–0.2 g/mL of reactor volume, the FPase titer would diminish to 3–6 FPU/mL, which is almost the same as that achieved using the fed-batch protocol E employing DSB (∼4 FPU/mL). In terms of enzyme titer and FPase productivity, the present results are the highest yet reported for submerged cultivation of P. echinulatum. Nonetheless, there have been previous reports of the use of low-cost pretreated biomass as carbon source for the production of glycohydrolase using filamentous fungi other than P. echinulatum. Bigelow and Wyman [16] reported cellulase production of 1.2 FPU/mL by T. reesei RUT-C30 grown on bagasse pretreated with hot water. Kovacs et al. [22] described the production of cellulase by a mutant strain of Trichoderma atroviride, using willow pretreated using steam (205 °C for 3 min with 3 % SO2) as the carbon source in submerged fermentation, and achieved values of up to 0.7 FPU/mL for FPase and 8.0 IU/mL for β-glucosidase after 76 h of cultivation. Ahmed et al. [23] cultivated Trichoderma harzianum E-58 using pretreated poplar and obtained a β-glucosidase activity of 0.63 IU/mL. More recently, Castro et al. [24] reported activities of 0.097 FPU/mL (FPase) and 0.75 IU/mL (β-glucosidase) after 166 h of cultivation of T. harzianum IOC-4038, using sugarcane bagasse that had been pretreated with acid followed by delignification using NaOH. Delabona et al. [25] reported 1.3 FPU/mL at 76 h in submerged bioreactor cultivation with a wild strain of T. harzianum grown in a culture medium consisting of DSB and sucrose in a ratio of 3:1 (w/w). Compared to the values reported in the literature, the present results obtained for P. echinulatum grown on a low-cost carbon source are therefore superior in terms of enzyme titer and productivity.
The performance of the cellulase complex produced by P. echinulatum grown on DSB was compared with that of the commercial Accelerase 1500 enzyme complex in experiments involving the hydrolysis of bagasse that had been subjected to conventional steam pretreatment (at 200 °C for 7 min). The P. echinulatum enzyme complex performed best in terms of maximum glucose concentration. On the other hand, accumulation of the hydrolysis intermediate, cellobiose, was always lower for the commercial enzyme complex (Fig. 5b), while similar low levels of xylose production were obtained for the two different enzyme complexes (Fig. 5c).
Primary plant cell walls are composed mainly of cellulose microfibrils embedded in a matrix of complex polysaccharides (pectins and hemicelluloses) [26]. Cellulase, hemicellulase, and pectinase enzymes are responsible for the depolymerization of these structures [27]. Cell wall depolymerase enzymes are rarely, if ever, completely specific, and some structurally related xylanases and cellulases show reciprocal activities [27]. The enzymatic hydrolysis of lignocellulosic material can be considerably enhanced by supplementation of cellulase enzyme complexes with xylanases [28]. This is attributed to partial removal of the hemicellulose coating of the cell wall and consequent increased access of cellulase enzymes to the cellulose fibers, resulting in greater glucose release. It has also been found that xyloligomers derived from xylan hydrolysis play an important role in cellulase inhibition, and that xylanase activity could alleviate this inhibition by promoting the hydrolysis of xyloligomers to xylose, which is thought to be less inhibitory [29].
The enzymatic activity of the cellulolytic complex used in the hydrolysis experiments is described in Table 3. The xylanase/FPase activity ratio for the P. echinulatum cocktail produced using DSB was 24.9 IU/FPU, while for Accelerase 1500 the ratio was about 20 times lower, at 2.2 IU/FPU. The specific xylanase activity of Accelerase 1500 was also very low (2.5 IU/mg protein) compared to the P. echinulatum cocktail produced with DSB (60 IU/mg protein). The hydrolysis performed with the P. echinulatum complex was much more efficient than achieved with Accelerase 1500, with the former delivering three times more glucose (Fig. 5a). The steam explosion pretreatment used in the present work was very aggressive and substantially reduced the hemicellulose content of the sugarcane bagasse from 25 % to 5 % by weight (Table 2). However, even at this low hemicellulose biomass content, the relative xylanase load influenced the effectiveness of the hydrolysis. It is therefore possible that the poorer hydrolysis achieved using Accelerase 1500 was due to its low net xylanase activity, compared to the P. echinulatum cellulase complex that had a much higher xylanase/FPase ratio. However, these hypotheses will require further investigation. Greater accumulation of cellobiose in the hydrolysis performed with the P. echinulatum enzymatic complex (Fig. 5b) could have been due to an imbalance between the activities of cellobiohydrolase and β-glucosidase. Supplementation of the P. echinulatum complex with β-glucosidase would probably benefit the hydrolysis.
Conclusions
Pretreated sugarcane bagasse provided a satisfactory carbon source for the production of FPase, β-glucosidase, and xylanase using P. echinulatum, with yields that were superior to those reported in the literature for other filamentous fungi. Enzyme activities did not seem to be related to the composition of the pretreated bagasse, but rather to access of the mycelium to the carbon source, since there was a correlation between enzyme activity and the specific surface area of the substrate. Submerged batch and fed-batch bioreactor fermentation experiments demonstrated the potential of P. echinulatum for use as a cellulolytic enzyme producer. The enzyme complex produced by P. echinulatum grown on steam-pretreated delignified bagasse was evaluated in hydrolysis experiments that also employed steam-pretreated bagasse. The better performance of the P. echinulatum complex, compared to a commercial cellulolytic enzyme cocktail, was probably due to the balance obtained between the FPase, xylanase, and β-glucosidase activities. The present results demonstrated that P. echinulatum is a promising organism for use in submerged bioreactor cultivation for on-site cellulolytic enzyme production in a sugarcane biorefinery, using pretreated bagasse as carbon source.
References
Macedo IC (organizer) (2007) Sugarcane’s energy—twelve studies on Brazilian sugarcane agribusiness and its sustainability. 2nd edition, Editora Berlendis, São Paulo, SP, Brazil (http://sugarcane.org/resource-library/books/Sugar%20Canes%20Energy%20-%20Full%20book.pdf)
Zhang YHP, Himmel ME, Mielenz JR (2006) Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv 24:452–481
Tolan JS (2002) Iogen’s process for producing ethanol from cellulosic biomass. Clean Techn Environ Policy 3:339–345
Sorensen A, Teller PJ, Lubeck PS, Ahring BK (2011) Onsite enzyme production during bioethanol production from biomass: screening for suitable fungal strains. Appl Biochem Biotechnol 164:1058–1070
Martins LF, Kolling D et al (2008) Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour Technol 19:1417–1424
Camassola M, Bittencourt LR et al (2004) Characterization of the cellulase complex of Penicillium echinulatum. Biocatal Biotransform 22:391–396
Sehnem NT, Bittencourt LR et al (2006) Cellulase production by Penicillium echinulatum on lactose. Appl Microbiol Biotechnol 72:163–167
Camassola M, Dilon AJP (2007) Effect of methylxanthines on production of cellulases by Penicillium echinulatum. J Appl Microbiol 102:478–485
Dillon AJP, Zorgi C (2006) Use of 2-deoxyglucose in liquid media for the selection of mutant strains of Penicillium echinulatum producing increased cellulase and b-glucosidase activities. Appl Microbiol Biotechnol 70:740–746
Mandels M, Reese ET (1957) Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol 73:269–278
Ghose TK (1987) Measurement of cellulose activities. Pure Appl Chem 59:257–268
Bailey MJ, Poutanen K (1989) Production of xylanolytic enzymes by strains of Aspergillus. Appl Microbiol Biotechnol 30:5–10
Zhang Y-HP, Hong J, Ye X (2009) Cellulase assays. Methods Mol Biol 581:213–231
Ahamed A, Vermette P (2008) Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem Eng J 40:399–407
Gouveia ER, Nascimento RT, Souto-Maior AM, Rocha GJM (2009) Validation of the methodology for the chemical characterization of the sugarcane bagasse. Quím Nova 32:1500–1503
Bigelow M, Wyman CE (2002) Cellulase production on bagasse pretreated with hot water. Appl Biochem Biotechnol 98:921–934
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Grethlein HE (1985) The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Biotechnology 3:155–160
Ilmén M, Thrane C, Penttilä ME (1996) The glucose repressor gene cre1 of Trichoderma: isolation and expression of a full-length and a truncated mutant form. Mol Gen Genet 251:451–460
Lejeune R, Baron GV (1995) Effect of agitation on growth and enzyme production of Trichoderma reesei in batch fermentation. Appl Microbiol Biotechnol 43:249–258
Camassola M, Dillon AJP (2007) Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugarcane bagasse and wheat bran in solid state fermentation. J Appl Microbiol 103:2196–2204
Kovács K, Szacks G, Zacchi G (2009) Comparative enzymatic hydrolysis of pretreated spruce by supernatants, whole fermentation broth and washed mycelia of Trichoderma reesei and Trichoderma atrovide. Bioresour Technol 100:1350–1357
Ahmed S, Bashir A, Saleem H, Saadia M, Jamil A (2009) Production and purification of cellulose degrading enzymes from a filamentous fungus Trichoderma harzianum. Pak J Bot 41:1411–1419
Castro AM, Ferreira MC, Cruz JC, Nascimento KC, Rodrigues P, Carvalho DF, Leite SGF, Pereira N Jr (2010) High-yield endoglucanase production by Trichoderma harzianum IOC-3844 cultivated in pretreated sugarcane mill byproduct. Enzym Res. doi:10.4061/2010/854526
Delabona PS, Farinas CS, Silva MR, Azzoni SF, Pradella JGC (2012) Use of a new Trichoderma harzianum strain isolated from the Amazon rainforest with pretreated sugarcane bagasse for on-site cellulase production. Bioresour Technol 107:517–521
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Walton JD (1994) Deconstructing the cell wall. Plant Physiol 104:1113–1118
Berlin A, Gilkes N, Kilburn D, Bura R, Markov A, Skomarovsky A, Okunev O, Gusakov A, Greg D, Sinitsyn A, Saddler J (2005) Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates—evidence for the role of accessory enzymes. Enzyme Microb Technol 37:175–184
Kumar R, Wyman CE (2009) Effect of enzyme supplementation at moderate cellulase loadings on initial glucose and xylose release from corn stover solid pretreated by leading technologies. Biotechnol Bioeng 102:457–467
Acknowledgments
The authors would like to thank Dr. Aldo J. P. Dillon for providing the P. echinulatum, the technicians of the National Laboratory of Science and Technology of Bioethanol (CTBE) for assistance during the experiments, and LME/LNLS for technical support during the electron microscopy work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, B.M.P., Alvarez, T.M., da Silva Delabona, P. et al. Cellulase On-Site Production from Sugar Cane Bagasse Using Penicillium echinulatum . Bioenerg. Res. 6, 1052–1062 (2013). https://doi.org/10.1007/s12155-013-9340-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9340-5