Abstract
Fusaricidin, a lipodepsipeptide isolated from Paenibacillus polymyxa, has high antimicrobial activity against fungi and Gram-positive bacteria. Through mutagenesis, we obtained two mutant strains, N1U7 and N17U7, which produce 6.2- to 7.9-fold more fusaricidin than their parent strain. Causal mutations were identified by whole-genome sequencing, and the two strains each contained at least eleven point mutations, including four common mutations. A mutation in the PPE04441 gene (pgm), encoding an α-phosphoglucomutase, was found to be an important factor in fusaricidin overproduction by complementation experiments. Null mutation of pgm in the parental strain increased fusaricidin production by 5.2-fold. Increased growth and cell viability in stationary phase, reduced exopolysaccharide production, and increased fusA expression were observed in the pgm mutant strains, which might be related to fusaricidin overproduction. This is the first report revealing that PGM deficiency leads to an overproduction of fusaricidin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclic lipodepsipeptides (CLDPs) containing one or more ester bonds, in addition to amide bonds, have been isolated from natural organisms such as fungi and bacteria, and are promising compounds for the development of new antibiotics [3, 34]. The biosynthesis of these peptides proceeds non-ribosomally through the catalytic function of nonribosomal peptide synthetases (NRPSs) [33]. Daptomycin, ramoplanin, and fusaricidin (or LI-F) are well-known examples of CLDPs that have high antimicrobial activity. Daptomycin was the first lipopeptide agent to be released onto the market for the treatment of infections caused by multidrug-resistant Gram-positive organisms [1, 35, 37]. Ramoplanin is a promising agent for the treatment of Clostridium difficile-associated disease [10, 11, 37]. Fusaricidin has antimicrobial activity against fungi and Gram-positive bacteria, and shows potential as a new antibacterial drug [4, 17, 21].
Fusaricidins are a group of antibiotics isolated from Paenibacillus polymyxa (formerly Bacillus polymyxa), and have a cyclic structure composed of six amino acid residues in addition to a 15-guanidino-3-hydroxypentadecanoic acid moiety. Various analogs of fusaricidins, called LI-F03a, LI-F03b, LI-F04a, LI-F04b, LI-F05a, LI-F05b, LI-F06a, LI-F06b, LI-F07a, LI-F07b, LI-F08a, and LI-F08b [9, 19–21], as well as fusaricidins A, B, C, and D [15–17], have been isolated and characterized. Fusaricidins have high antimicrobial activity against fungi such as Aspergillus nidulans, Candida albicans, Fusarium oxysporum, Leptosphaeria maculans, and Penicillium expansum [2, 19, 21], and Gram-positive bacteria such as Staphylococcus aureus, Mycobacterium smegmatis, and Micrococcus luteus [17, 21]. The toxicity of the fusaricidins in mice, however, is low [21]. In 2012, Quinn et al. [29] reported that fusaricidin B is an important component of a lipopeptide complex with biofilm inhibition activity. Lee et al. [23] recently reported that fusaricidin can induce systemic resistance against bacterial and fungal infections at concentrations as low as 0.1 parts per million in tobacco and red pepper plants. These reports show that fusaricidins, and perhaps the entire LI-F family of natural products, have a unique structure, high antimicrobial activity, and low toxicity, and are an attractive source of candidates for the development of new antimicrobial agents to combat multidrug-resistant bacteria, and for the development of novel crop protection agents.
We previously reported the identification and functional analysis of the fusaricidin synthetase gene, fusA, from P. polymyxa E681 using whole-genome sequencing [7, 18]. Because fusaricidin production in wild-type strains is low, development of a fusaricidin-overproducing strain is necessary for industrial purposes. However, no such strain has been developed, although it was recently reported that metal ions such as Zn2+, Mg2+, Mn2+, Ni2+, and Cu2+ significantly affect the production of fusaricidin-type antifungal compounds [30, 31], and that the global transition state regulator, AbrB, plays a negative role in fusaricidin synthesis by directly binding onto the promoter region of the fus gene cluster [25]. In this study, we used both targeted and random mutagenesis to construct mutant strains that can overproduce fusaricidin, and analyzed the mutations of two high-producing strains using whole-genome sequencing. We found that a null mutation of the phosphoglucomutase (PGM) gene is an important factor in the overproduction of fusaricidin.
Materials and methods
Bacterial strains, plasmids, and growth conditions
All bacterial strains and plasmids used in this study are described in Table 1. Unless otherwise described, the P. polymyxa strains described herein were grown in tryptic soy broth (TSB; Difco, Detroit, MI, USA) or on tryptic soy agar (TSA) at 30 °C. To analyze fusaricidin production, the P. polymyxa strains were grown in a TSBG medium (TSB, 8 g; glucose, 30 g; (NH4)2SO4, 2 g; K2HPO4, 0.1 g; Na2HPO4, 0.1 g; MgSO4, 0.01 g; and yeast extract, 1 g/L). Escherichia coli strains were grown in Luria–Bertani (LB; USB, Cleveland, OH, USA) broth or on LB agar at 37 °C. M. luteus was grown in TSB at 30 °C. When required, the media were supplemented with ampicillin (100 µg/mL), erythromycin (0.5 µg/mL), or spectinomycin (100 µg/mL).
Null mutation of trdA
All primers used in this study are listed in Table 1. To knock out the putative tridecaptin synthetase gene (trdA) in P. polymyxa E681, an E. coli fosmid clone containing the putative trdA region in a pCC1FOS vector (called pTrdA) was isolated from a library which was constructed for genome sequencing in our previous study [18]. A 1.3-kb DNA fragment containing the entire spectinomycin resistance gene (spc) was amplified from plasmid pDG1730 [12] using primers trdspF and trdspR, giving 70-bp arms homologous to the target site at each end. Then, the amplified spc-containing DNA fragment was used to disrupt the putative trdA in the pTrdA by means of the lambda Red recombination system mediated by the pKD46 plasmid [8]. The resulting recombinant fosmid, called pDtrdA, was introduced into P. polymyxa E681ΔpmxE which cannot produce polymyxin by knockout of the pmxE [6] by homologous recombination to generate a tridecaptin-defective mutant. P. polymyxa was transformed by electroporation, as previously described [7].
Mutagenesis using N-methyl-N′-nitro-N-nitrosoguanidine (NTG)
NTG (Sigma-Aldrich Company, St Louis, MO, USA) was used for mutagenesis. Fresh P. polymyxa cells were grown in TSB at 30 °C with shaking to an OD600 of 0.7–1.0. To generate the mutant strains, a stock solution of NTG (2 mg/mL) was added to 1 mL of the P. polymyxa culture to a final concentration of 400 µg/mL. Following 10 min of incubation at room temperature without shaking, the cells were harvested through centrifugation (4 °C for 90 s), the mutagen was removed, and then the cells were washed with an equal volume of cold TSB and re-suspended in 1 mL of TSB. The cell suspension was diluted appropriately and then spread onto TSA plates. The plates were incubated at 30 °C for 1–2 days, and the colonies were assayed for fusaricidin production by bioassay against M. luteus as described below.
Mutagenesis using ultraviolet (UV) light
UV-induced mutants were obtained by exposing agar plates containing bacterial cells to UV light. A P. polymyxa culture freshly grown in TSB with an OD600 of 0.7–1.0 was serially diluted with a 0.1 M MgSO4 solution, and three dilutions (10−4, 10−5, and 10−6) were then used to make plates for UV irradiation by spread plating 0.1 mL of the diluted cell suspensions onto TSA plates. The plates were placed on an adjustable platform under a UV lamp (G30T8 UV lamp, 30 W; Sankyo Denki, Tokyo, Japan) fixed in a chamber. The UV irradiation was carried out by adjusting the distance between the UV lamp and culture plate from 40 to 80 cm, with exposure times of 10–60 s. The plates were incubated at 30 °C for 48 h, and viable colonies were counted to determine the appropriate irradiation conditions.
Antimicrobial activity assay and electrospray ionization–liquid chromatography/mass spectrometry (ESI–LC/MS) analysis
Antimicrobial activity against M. luteus was assayed as follows. M. luteus KCTC 2177 cells grown overnight in 3 mL of TSB at 30 °C were mixed with 300 mL of TSA, autoclaved, and cooled to <50 °C to prepare the bioassay plate (ML plate). To test the antimicrobial activity of thousands of mutants derived from the P. polymyxa E681ΔpmxEΔtrdA strain (hereafter called E681Δpt) which has a decreased antibacterial activity by double knockout of polymyxin and tridecaptin synthetase genes, the colonies were transferred onto the ML plate using toothpicks, and the plate was incubated at 30 °C for 24 h. Mutants showing larger growth inhibition zones than that of E681Δpt were selected for further analysis. The selected mutant strains were grown in TSBG, the cells were harvested by centrifugation (4 °C for 10 min) and extracted using equal volumes of methanol, and 10 µL of each extract was then loaded onto 6-mm paper disks, dried, and placed on an ML plate for observation of the growth inhibition zone. The methanol-extracted fusaricidin was analyzed using ESI–LC/MS (Thermo Electron Co., San Jose, CA, USA), as previously described by Choi et al. [7]. The fusaricidin produced by P. polymyxa strains was quantitatively analyzed by electrospray ionization–liquid chromatography/mass spectrometry (ESI–LC/MS). ESI–LC/MS was operated under the XCALIBUR software system using a Finnigan Surveyor™ Modular HPLC System with XTerra MS C18 column (5 μm; 2.1 × 150 mm; Waters, Milford, MA, USA) and a Finnigan LCQ Advantage MAX ion trap mass spectrometer equipped with a Finnigan electrospray source (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The mobile solvents were water (solvent A) and acetonitrile (solvent B), both containing 0.1 % formic acid. A gradient elution at a flow rate of 0.3 mL/min was performed as follows: 0–15 min, 5–80 % B (linear gradient), followed by 15–20 min of 100 % B (isocratic). Selected ion-monitoring mass spectra were obtained at m/z 883, 897, 911, and 925 [M + H]+ using three microscans and a maximum ion injection time of 200 ms. The amount of fusaricidin was calculated by measuring the peak area.
Genome sequencing
The genome sequences of P. polymyxa strains N1U7 and N17U7 were determined using a whole-genome shotgun strategy and an Illumina HiSeq 2000 instrument (Illumina, San Diego, CA, USA). Pretreatment, reference mapping and variant detection analysis of Illumina paired-end reads (101 nt × 2) were performed using the CLC Genomics Workbench ver. 4.8. Quality-trimmed reads were aligned to the genome sequence of parental strain E681 (GenBank accession number CP000154) with length fraction cutoff of 0.8 and similarity cutoff of 0.9, which was more stringent than default mapping condition. Genomic variants as single nucleotide polymorphisms and deletion/insertion polymorphisms were then automatically reported from the CLC Genomics Workbench.
Complementation of mutations in P. polymyxa
To perform a complementation test on the four mutated genes in P. polymyxa N17U7, each gene was amplified using primer sets containing SmaI and BamHI restriction enzyme sites. To generate an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible system, a pHPspac plasmid vector was constructed using pHPS9 [13] and pHCMC05 [27]. A 1.7-kb region including the Pspac promoter and lacI was obtained from pHCMC05 by cleaving with SmaI and EcoRI (Roche, Basel, Switzerland), and then inserted into pHPS9 treated with the same enzymes. The target genes, PPE00036, PPE01127, PPE01377, and PPE04441, were cloned into the BamHI and SmaI sites of pHPspac. The resulting plasmids were introduced into N17U7, and the antimicrobial activity and fusaricidin production of the transformants were analyzed following culture in TSBG supplemented with IPTG for 30 h.
Construction of P. polymyxa mutant strains 4441p and 4441d
Point mutation and deletion of the PPE04441 were conducted using an overlap extension PCR technique. To construct a P. polymyxa mutant strain with only the PPE04441 point mutation and no other mutations, the PPE04441 gene and its downstream region (1.8 kb) were amplified from N17U7 genomic DNA using primer pairs of 4441F-4441R′ and 4441BF-4441BR, respectively (4441R′ and 4441BF contained 20 bp of nucleotides complementary to the 3′ and 5′ ends, respectively, of the erythromycin resistance gene, erm). The erm was amplified from the pDG1664 [12] plasmid using a primer pair of emF–emR, and used as a selectable marker. These three PCR products were purified and joined through an overlap extension PCR. The joined cassette was cloned into a pGEM-T Easy vector (Promega, Madison, USA) to construct pT4441p, and the cassette was then integrated into the P. polymyxa E681Δpt chromosome by double cross-over recombination to obtain 4441p strain. To generate a PPE04441 deletion mutation, part of the PPE04441 gene (1 kb) and its upstream region (0.4 kb) were amplified from P. polymyxa E681 using primers 4441FF and 4441FR. The erm and the downstream region of the PPE04441 gene were obtained in the same manner as described above. These three fragments were joined through an overlap extension PCR, cloned into a pGEM-T Easy vector to construct pT4441d, and then integrated into the P. polymyxa E681Δpt chromosome using a double cross-over recombination to obtain 4441d strain.
Preparation of crude extract from P. polymyxa cells
For the PGM activity assay, an overnight culture of the P. polymyxa strain was inoculated into 3 mL of TSB broth. After growing for 9 h, the cells were harvested by centrifugation at 15,000×g for 10 min at 4 °C. The cell pellet was suspended in a buffer containing 50 mM Tris–HCl (pH 7.4) and 0.5 M NaCl, and then disrupted by sonication for 3 min (duty cycle 50 %, output control 4). Following centrifugation at 15,000×g for 15 min at 4 °C, the supernatant was collected into new 1.5-mL microcentrifuge tubes and used for a PGM assay.
Purification of the PPE04441 gene product
The PPE04441 gene of E681Δpt was amplified using primers 4441X and 4441N. The PCR product was cleaved with XhoI and NdeI and cloned into pET22b (Novagen, Inc., Madison, WI, USA) to construct pET4441. The plasmid was introduced into E. coli BL21(DE3) by CaCl2-mediated transformation, and the transformant was grown in LB broth supplemented with ampicillin and IPTG at 25 °C. The cells were disrupted by sonication for 1 min (duty cycle 50 %, output control 4) and the PPE04441 protein was purified using a His-Spin Protein Miniprep kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s instructions.
Assay of PGM activity
PGM activity was assayed using a coupling enzyme reaction [38]. The standard reaction mixture for measuring the PGM activity contained 50 mM Tris–HCl (pH 7.4), 5 mM MgCl2, 10 μM glucose 1,6-diphosphate, 1 mM NADP+, 1 U of phosphoglucose dehydrogenase (coupling enzyme), and 1 mM glucose-1-phosphate. A 1-mL aliquot of a mixture containing 50 µL of crude extract or purified PPE04441 protein was used to measure the formation of nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm using an Ultrospec 7000 spectrophotometer (GE Healthcare Life Sciences, Uppsala, Sweden). The activity of the crude extract was expressed in arbitrary units ((ΔA 340/OD600) × 100) ± SEM, and the activity of the purified protein was expressed in units/mg protein ± SEM. One unit of enzyme activity is defined as the amount required for the formation of 1.0 μmol of NADPH per minute under the conditions described above.
Statistical analysis
Each assay was repeated three times and the data were analyzed using Analysis Of Variance (ANOVA). Comparison of data was made using Fisher’s protected least significant difference (LSD) at p = 0.05. A p value <0.05 was considered statistically significant. All statistical tests were performed using JMP software version 5.0 (SAS Institute, Cary, NC, USA).
Results and discussion
Mutagenesis for fusaricidin-overproducing mutants
We used both targeted and random mutagenesis methods to develop P. polymyxa strains that could produce high levels of fusaricidin. We initially constructed a mutant P. polymyxa E681 strain that could not produce either polymyxin or a tridecaptin homolog, called E681Δpt, to remove any possible interference caused by the co-production of unrelated antibiotics. Specifically, one of the putative tridecaptin synthetase-encoding genes, trdA, was knocked out by replacing it with spc in the P. polymyxa E681ΔpmxE. This strain was constructed in our previous study to block the biosynthesis of polymyxin by knockout of pmxE, and the resulting strain now defective in both trdA and pmxE was designated P. polymyxa E681Δpt [6]. Tridecaptin, a lipopeptide antibiotic produced by P. polymyxa, was reported to have bactericidal activity against Gram-negative and -positive bacteria [32], and the putative tridecaptin synthetase genes, trdA and trdB, were discovered in the genome of the E681 strain (unpublished data). The resulting mutant, P. polymyxa E681Δpt, showed very low antibacterial activity against E. coli, and slightly decreased activity against M. luteus KCTC 2177 compared to wild type, but maintained its antifungal activity (data not shown). The E681Δpt strain was subjected to NTG treatment (six times) and UV irradiation (five times) to generate the fusaricidin-overproducing mutants. The overall mutagenesis procedure conducted in this study is shown in Fig. 1a. Fusaricidin-overproducing mutants were screened using a bioassay against M. luteus KCTC 2177 (ML), a Gram-positive bacterium that can grow on TSA and is sensitive to fusaricidin. After the first round of NTG mutagenesis, approximately 5,000 colonies grown on TSA plates were screened for antibacterial activity using an ML plate to isolate mutants showing increased antimicrobial activity. Then, a mutant strain showing the highest activity was selected and used for further mutagenesis. The second round of NTG mutagenesis was conducted in the same way, and after the third round mutagenesis, two mutant strains, N1 and N17, were selected for further development. In the next step, the two mutant strains were mutagenized using UV irradiation and NTG treatment. Finally, two mutant strains, N1U7 and N17U7, were selected as the highest fusaricidin producers in each line. An analysis of the LC/MS data showed that N1U7 and N17U7 strains produced 6.2- and 7.9-fold more fusaricidin, respectively, than the E681Δpt strain (Fig. 1b).
Genome-wide analysis of two mutant strains
The two fusaricidin-overproducing mutants, N1U7 and N17U7, were analyzed to identify mutations using whole-genome sequencing (Illumina HiSeq 2000; Illumina, San Diego, CA, USA). By comparing the genomic sequences of the mutants with that of the parental strain, E681, we found that the N1U7 strain had eleven point mutations, including seven missense mutations and three synonymous substitutions in coding regions and one mutation in a non-coding region, and that the N17U7 strain had 22 point mutations, including eleven missense mutations and seven synonymous substitutions in coding regions and four mutations in non-coding regions. The missense mutations in coding regions of the two strains are shown in Table 2. Through a comparative analysis of the mutations, five genes with mutations in both the N1U7 and N17U7 strains were found and these mutations might be involved in enhancing fusaricidin production. Among the five mutations, the four missense mutations in PPE00036, PPE01127, PPE01377, and PPE04441 were confirmed by PCR and sequencing and selected for further analysis to identify the effective mutations. One mutation in a 651-bp non-coding region was excluded because the point mutation seemed to be a silent mutation in our preliminary analysis of the mutation region. Among the four common missense mutations, one point mutation, which causes an amino acid change from glycine to valine at position 329 of the PPE01377, was in a sporulation histidine kinase [28]. The second point mutation causes a valine to isoleucine substitution at position 235 of the PPE00036, which has high similarity to the N-acetylglucosamine-1-phosphate uridyltransferases of Bacillus subtilis 168 (61.2 % amino acid identity; GenBank accession number CAB11826.1) and E. coli K12 (41.4 % amino acid identity; GenBank accession number AAC76753.1). The other two mutations were found in PPE01127 (Ser73Phe) and PPE04441 (Gln170Pro), which showed high similarities to the glycosyltransferase WecG/TagA superfamily and PGM, respectively.
Effect of complementation of the four point mutations on fusaricidin production
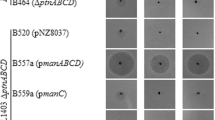
To investigate the effects of the four selected mutations on fusaricidin production in P. polymyxa E681, complementation plasmids were generated for the PPE00036, PPE01127, PPE01377, and PPE04441 mutated genes using an IPTG-inducible vector pHPspac as listed in Table 1. The plasmids were introduced into N17U7 strain to prepare four complementation strains, and the strains were used for growth inhibition assays against M. luteus. Three transformants of N17U7, containing the complementation plasmids of pHR00036, pHR01127, and pHR01377, showed the same level of antimicrobial activity as that of N17U7 (data not shown). However, the antimicrobial activity of N17U7(pHR04441) was significantly lower than N17U7 (Fig. 2a, b). Quantitative analysis of fusaricidin produced by N17U7 and N17U7(pHR04441) using ESI–LC/MS (Fig. 2c) showed that the latter strain had 65 % lower production than the former. These results suggest that the point mutation in the PPE04441 gene, which encodes a PGM homolog, has a significant effect on fusaricidin production in P. polymyxa E681.
Antibacterial activities of the N17U7 and N17U7(pHR04441) strains against Micrococcus luteus (a and b), and quantitative analysis of fusaricidin produced by the strains (c). Cells harvested from 30-h cultures of the two strains were extracted with equal volumes of methanol, and 10 µL of the extract was dropped onto a 6-mm paper disk, dried, and placed on an M. luteus plate. The means with different letters are significantly different (p < 0.05). The error bars indicate standard deviations (n = 3)
Effect of null mutation of the PPE04441 gene on the fusaricidin production of P. polymyxa E681
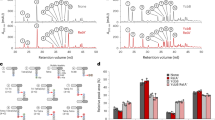
P. polymyxa N17U7, which produces a high level of fusaricidin, has 22 point mutations, including four that are also seen in the N1U7 strain. With the complementation of the PPE04441 gene in the N17U7 strain, we observed a significant decrease in antimicrobial activity, and therefore hypothesized that PPE04441 mutation plays an important role in fusaricidin overproduction. To support this finding, we generated two different P. polymyxa E681Δpt strains with PPE04441 gene defects: a mutant containing a point mutation at position 509 of the PPE04441 gene, mimicking the N17U7 PPE04441 gene, and a mutant with deletion of PPE04441, to completely disrupt its function. The resulting mutant strains were designated P. polymyxa 4441p and P. polymyxa 4441d, respectively. The antibacterial activity and fusaricidin production of the two strains are shown in Fig. 3. An approximately fivefold increase in fusaricidin production was observed in both P. polymyxa 4441p and P. polymyxa 4441d by ESI–LC/MS quantitative analysis (Fig. 3c).
Antibacterial activities of E681Δpt, 4441p, and 4441d strains against Micrococcus luteus (a and b), and quantitative analysis of fusaricidin produced by the strains (c). Cells harvested from 30-h cultures of the three strains were extracted with equal volumes of methanol, and 10 µL of the extract was dropped onto a 6-mm paper disk, dried, and placed on an M. luteus plate. The means with different letters are significantly different (p < 0.05). The error bars indicate standard deviations (n = 3)
Functional analysis of the PPE04441 gene
The PPE04441 gene of P. polymyxa E681 was predicted to encode a 572 amino acid protein with high similarity to several known α-PGMs from other bacteria (B. subtilis pgcA, 53 %, GenBank accession number AJ784890.1; Streptococcus thermophilus pgmA, 46 %, GenBank accession number AJ243290.2; S. gordonii pgm, 46 %, GenBank accession number DQ234767.1) [5, 22, 24]. To confirm the function of the PPE04441 gene, PPE04441 protein purified from E. coli (pET4441) was analyzed for its PGM activity by measuring the NADPH generated during the coupling enzyme reaction [38]. As shown in Table 3, the PPE04441 protein of E681Δpt had specific PGM activity of 13.4 units/mg. P. polymyxa E681Δpt and the two mutant strains, 4441p and 4441d, were also analyzed for their PGM activity. A crude extract of E681Δpt, which has an intact PPE04441 gene, had PGM activity of 30.0 AU, whereas the PGM activity of the two mutant strains was nearly abolished. These results show that PPE04441 is the only gene encoding PGM, which was supported by our finding that there is no other ORF showing significant identity to known PGMs in the genome of P. polymyxa E681.
PGM catalyzes the interconversion of α-glucose 1-phosphate and glucose 6-phosphate, and plays an important role in synthesizing uridine diphosphate glucose, which is a precursor for the synthesis of cell envelope components, capsules, and exopolysaccharides [1, 14, 22, 24]. Many reports have shown that PGM is required for the biosynthesis of biofilm, capsular polysaccharide, and exopolysaccharides in Bacillus, Lactococcus, Streptococcus, and other bacterial species [14, 24, 26, 36]. In this study, we observed that cultures of the two mutant strains, 4441p and 4441d, had significantly lower viscosity than that of E681Δpt when the strains were grown in TSBG medium (Fig. S1), which suggests that EPS production was reduced by null mutation of pgm. Reduced culture viscosity caused by decreased EPS production is itself a desirable feature for a bacterial culture, aside from any effect on antibiotic production, because it enables higher oxygen transfer rates and low-cost agitation in industrial fermentations.
In other species, it has been reported either that pgm is an essential gene, or that pgm mutation reduces growth, reduces viability in the stationary phase, and increases autolytic activity [5, 24, 26]. However, pgm null mutations in P. polymyxa E681 produced no such traits. There was no reduced growth in 4441p and 4441d strains when compared with the parent strain, E681Δpt, in the logarithmic growth phase, and more interestingly, in stationary phase, the two mutant strains showed higher levels of growth and cell viability and lower autolysis than the parent strain. The OD600 values of pgm mutants increased continuously in TSBG medium for 24 h, but the OD600 of the parent strain decreased after 16 h (Fig. S2). We observed that fusaricidin production in the mutant strains was initiated in the early stationary growth phase and lasts for 12 h (data not shown); therefore, the higher levels of growth and cell viability of the mutant strains in stationary phase probably played a role in increasing productivity of fusaricidin.
We also found that the expression of the fusaricidin synthetase gene fusA was significantly higher in the pgm mutant strains. When the transcript level of fusA was analyzed by quantitative RT-PCR of a 10-h culture, the mutants showed about fourfold higher levels than the parent strain (Fig. S3). Although further studies are required to elucidate the mechanism involved in the regulation of gene expression, the increased transcript level of fusA might be related to fusaricidin overproduction.
Conclusions
Random mutagenesis conducted in combination with targeted mutagenesis to disrupt two unnecessary NRPS genes, pmxE and trdA, was successful to obtain fusaricidin-overproducing P. polymyxa strains, N1U7 and N17U7. Through functional analysis of four mutations shared by the two mutant strains, a mutation in pgm which plays an important role in fusaricidin overproduction could be easily detected. A null mutation in pgm led to 5.2-fold higher fusaricidin production than the parent strain and the mutation seems to be a major factor contributing to fusaricidin overproduction in the mutant strains. The increased growth and cell viability in stationary phase, reduced viscosity of the culture, and increased transcript level of fusA in the pgm mutant strains might be related to fusaricidin overproduction. This is the first study to address the relationship between α-PGM and fusaricidin production, which should be useful for developing strains for industrial purposes.
References
Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI (2004) The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 38:1673–1681
Beatty PH, Jensen SE (2002) Paenibacillus polymyxa produces fusaricidin-type antifungal antibiotics active against Leptosphaeria maculans, the causative agent of blackleg disease of canola. Can J Microbiol 48:159–169
Bionda N, Cudic P (2011) Cyclic lipodepsipeptides in novel antimicrobial drug discovery. Croat Chem Acta 84:315–329
Bionda N, Pitteloud JP, Cudic P (2013) Solid-phase synthesis of fusaricidin/li-f class of cyclic lipopeptides: guanidinylation of resin-bound peptidyl amines. Biopolymers 100:160–166
Bizzini A, Majcherczyk P, Beggah-Moller S, Soldo B, Entenza JM, Gaillard M, Moreillon P, Lazarevic V (2007) Effects of alpha-phosphoglucomutase deficiency on cell wall properties and fitness in Streptococcus gordonii. Microbiology 153:490–498
Choi S-K, Park S-Y, Kim R, Kim S-B, Lee C-H, Kim JF, Park S-H (2009) Identification of a polymyxin synthetase gene cluster of Paenibacillus polymyxa and heterologous expression of the gene in Bacillus subtilis. J Bacteriol 191:3350–3358
Choi S-K, Park S-Y, Kim R, Lee C-H, Kim JF, Park S-H (2008) Identification and functional analysis of the fusaricidin biosynthetic gene of Paenibacillus polymyxa E681. Biochem Biophys Res Commun 365:89–95
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Deng Y, Lu Z, Bi H, Lu F, Zhang C, Bie X (2011) Isolation and characterization of peptide antibiotics LI-F04 and polymyxin B6 produced by Paenibacillus polymyxa strain JSa-9. Peptides 32:1917–1923
Farver DK, Hedge DD, Lee SC (2005) Ramoplanin: a lipoglycodepsipeptide antibiotic. Ann Pharmacother 39:863–868
Fulco P, Wenzel RP (2006) Ramoplanin: a topical lipoglycodepsipeptide antibacterial agent. Expert Rev Anti Infect Ther 4:939–945
Guerout-Fleury AM, Frandsen N, Stragier P (1996) Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61
Haima P, van Sinderen D, Bron S, Venema G (1990) An improved beta-galactosidase alpha-complementation system for molecular cloning in Bacillus subtilis. Gene 93:41–47
Hardy GG, Caimano MJ, Yother J (2000) Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J Bacteriol 182:1854–1863
Kajimura Y, Kaneda M (1996) Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. J Antibiot 49:129–135
Kajimura Y, Kaneda M (1997) Fusaricidins B, C and D, new depsipeptide antibiotics produced by Bacillus polymyxa KT-8: isolation, structure elucidation and biological activity. J Antibiot 50:220–228
Kaneda M, Kajimura Y (2002) New antifungal antibiotics, bacillopeptins and fusaricidins. Yakugaku Zasshi 122:651–671
Kim JF, Jeong H, Park S-Y, Kim S-B, Park YK, Choi S-K, Ryu C-M, Hur C-G, Ghim S-Y, Oh TK, Kim JJ, Park CS, Park S-H (2010) Genome sequence of the polymyxin-producing plant-probiotic rhizobacterium Paenibacillus polymyxa E681. J Bacteriol 192:6103–6104
Kuroda J, Fukai T, Konishi M, Uno J, Kurusu K, Nomura T (2000) LI-F antibiotics, a family of antifungal cyclic depsipeptides produced by Bacillus polymyxa L-1129. Heterocycles 53:1533–1549
Kuroda J, Fukai T, Nomura T (2001) Collision-induced dissociation of ring-opened cyclic depsipeptides with a guanidino group by electrospray ionization/ion trap mass spectrometry. J Mass Spectrom JMS 36:30–37
Kurusu K, Ohba K, Arai T, Fukushima K (1987) New peptide antibiotics LI-F03, F04, F05, F07, and F08, produced by Bacillus polymyxa. I. Isolation and characterization. J Antibiot 40:1506–1514
Lazarevic V, Soldo B, Medico N, Pooley H, Bron S, Karamata D (2005) Bacillus subtilis alpha-phosphoglucomutase is required for normal cell morphology and biofilm formation. Appl Environ Microbiol 71:39–45
Lee SH, Cho YE, Park S-H, Balaraju K, Park JW, Lee SW, Park K (2013) An antibiotic fusaricidin: a cyclic depsipeptide from Paenibacillus polymyxa E681 induces systemic resistance against Phytophthora blight of red-pepper. Phytoparasitica 41:49–58
Levander F, Radstrom P (2001) Requirement for phosphoglucomutase in exopolysaccharide biosynthesis in glucose- and lactose-utilizing Streptococcus thermophilus. Appl Environ Microbiol 67:2734–2738
Li S, Zhang R, Wang Y, Zhang N, Shao J, Qiu M, Shen B, Yin X, Shen Q (2013) Promoter analysis and transcription regulation of fus gene cluster responsible for fusaricidin synthesis of Paenibacillus polymyxa SQR-21. Appl Microbiol Biotechnol 97:9479–9489
Neves AR, Pool WA, Castro R, Mingote A, Santos F, Kok J, Kuipers OP, Santos H (2006) The alpha-phosphoglucomutase of Lactococcus lactis is unrelated to the alpha-d-phosphohexomutase superfamily and is encoded by the essential gene pgmH. J Biol Chem 281:36864–36873
Nguyen HD, Nguyen QA, Ferreira RC, Ferreira LC, Tran LT, Schumann W (2005) Construction of plasmid-based expression vectors for Bacillus subtilis exhibiting full structural stability. Plasmid 54:241–248
Park S-Y, Park S-H, Choi S-K (2012) Characterization of sporulation histidine kinases of Paenibacillus polymyxa. Res Microbiol 163:272–278
Quinn GA, Maloy AP, McClean S, Carney B, Slater JW (2012) Lipopeptide biosurfactants from Paenibacillus polymyxa inhibit single and mixed species biofilms. Biofouling 28:1151–1166
Raza W, Wu H, Shen Q (2010) Use of response surface methodology to evaluate the effect of metal ions (Ca2+, Ni2+, Mn2+, Cu2+) on production of antifungal compounds by Paenibacillus polymyxa. Bioresour Technol 101:1904–1912
Raza W, Yang X, Wu H, Huang Q, Xu Y, Shen Q (2010) Evaluation of metal ions (Zn2+, Fe3+ and Mg2+) effect on the production of fusaricidin-type antifungal compounds by Paenibacillus polymyxa SQR-21. Bioresour Technol 101:9264–9271
Shoji J, Hinoo H, Sakazaki R, Kato T, Wakisaka Y, Mayama M, Matsuura S, Miwa H (1978) Isolation of tridecaptins A, B and C (studies on antibiotics from the genus Bacillus. XXIII). J Antibiot (Tokyo) 31:646–651
Sieber SA, Marahiel MA (2003) Learning from nature’s drug factories: nonribosomal synthesis of macrocyclic peptides. J Bacteriol 185:7036–7043
Stawikowski M, Cudic P (2007) Depsipeptide synthesis. Methods Mol Biol 386:321–339
Steenbergen JN, Alder J, Thorne GM, Tally FP (2005) Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother 55:283–288
Torino MI, Mozzi F, Font de Valdez G (2005) Exopolysaccharide biosynthesis by Lactobacillus helveticus ATCC 15807. Appl Microbiol Biotechnol 68:259–265
Woodford N (2003) Novel agents for the treatment of resistant Gram-positive infections. Expert Opin Investig Drugs 12:117–137
Ye RW, Zielinski NA, Chakrabarty AM (1994) Purification and characterization of phosphomannomutase/phosphoglucomutase from Pseudomonas aeruginosa involved in biosynthesis of both alginate and lipopolysaccharide. J Bacteriol 176:4851–4857
Acknowledgments
This study was supported by the New Industry R&D program funded by the Ministry of Trade, Industry, and Energy, and the KRIBB Research Initiative Program, Ministry of Science, ICT and Future Planning, Republic of Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, HR., Park, SY., Kim, SB. et al. Inactivation of the phosphoglucomutase gene pgm in Paenibacillus polymyxa leads to overproduction of fusaricidin. J Ind Microbiol Biotechnol 41, 1405–1414 (2014). https://doi.org/10.1007/s10295-014-1470-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1470-z