Abstract
Exopolysaccharide (EPS) production and the activities of the enzymes involved in sugar nucleotide biosynthesis in Lactobacillus helveticus ATCC 15807 under controlled pH conditions were investigated. Batch fermentations using lactose as energy source showed higher EPS synthesis by L. helveticus ATCC 15807 at pH 4.5 with respect to pH 6.2, the enzyme α-phosphoglucomutase (α-PGM) being correlated with both total and specific EPS production. When glucose was used as carbon source instead of lactose, the lower EPS synthesis obtained was linked to a decrease in α-PGM and galactose 1-phosphate-uridyltransferase (GalT) activities, the reduction of the latter being more pronounced. Higher EPS production by L. helveticus ATCC 15807 at the acidic constant pH of 4.5 requires that both α-PGM and GalT activities are high. These enzymes are needed to synthesize UDP-glucose and UDP-galactose for supplying the corresponding monomers for EPS biosynthesis. Although differences are observed in EPS production by this strain regarding the energy source (lactose or glucose), the monomeric composition of the polymers produced is independent of the carbohydrate used. The obtained results contribute to a better understanding of the physiological factors that affect EPS biosynthesis by lactobacilli, which could help in the correct handling of the fermentation parameters within the fermented dairy industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the wide variety of exopolysaccharide (EPS)-producing microorganisms, lactic acid bacteria (LAB) have gained much attention because of their GRAS (generally recognized as safe) status, a sine qua non condition for the exploitation of food-grade fermented products. Either mesophilic (Lactobacillus casei, L. rhamnosus, L. paracasei, L. sakei, Lactococcus lactis subsp. lactis, Lcc. lactis subsp. cremoris) or thermophilic (Streptococcus thermophilus, S. macedonicus, Lactobacillus delbrueckii subsp. bulgaricus, L. acidophilus, L. helveticus) LAB are able to synthesize polysaccharides. These biopolymers have their most important applications in the dairy industry, by improving the texture, rheology and “mouthfeel” of fermented milks (especially in stirred and low-fat yoghurts) and eliminating the use of food additives to achieve new market demands (De Vuyst and Degeest 1999; Duboc and Mollet 2001). For many years, dairy processors have exploited the differences among ropy strains of EPS-producing LAB to produce a variety of fermented milks with unique textural properties (Duboc and Mollet 2001). Although yoghurt manufacture remains the most important commercial application of these polymers within the food industry some attempts have recently been made to introduce EPS-producing strains as culture adjuncts to improve low fat and partly skim milk cheeses (Broadbent et al. 2001). Thermophilic EPS-producing starters and/or adjunct cultures have been used in the manufacture of reduced-fat and low-fat Mozzarella cheeses in order to increase moisture retention (Perry et al. 1997). It has been observed that fat removal has undesirable effects on the physical properties of Mozzarella cheese, which becomes tough and rubbery, loses its pliability rapidly upon cooling and requires more heat for melting (McMahon and Oberg 1998). These undesirable changes, due to the lower moisture level in the cheese (Merrill et al. 1994), can be solved by including EPS-producing strains in the starter cultures. However, the presence of EPS in cheese increases the viscosity of whey and thereby retards the efficiency of membrane processing, slowing down whey protein concentration during the drying steps (Petersen et al. 2000). Therefore, it is important that the EPS produced by LAB adsorbs tightly onto the bacterial cells rather than being released into the whey. A rational selection of EPS-producing LAB is fundamental to improving the rheological and textural properties of fermented foods.
A large biodiversity of EPS from LAB exists, with respect to their yields, monomer compositions, molecular masses and functionalities (Vaningelgem et al. 2004), the EPS rheological properties being influenced not only by the amount of polymer produced but also by the structures and molecular mass (Faber et al. 1998; Ruas-Madiedo et al. 2002; Tuinier et al. 2001). These characteristics are dependent on specific and non-specific enzymes, encoded in eps gene clusters—either plasmid- or chromosome-located—and in chromosomal housekeeping genes. The enzymes involved in the biosynthesis of the EPS precursors or building blocks, well known as sugar nucleotides, belong to the latter group. In recent years, several researchers have demonstrated a correlation between EPS production, the activity of the sugar nucleotide-biosynthesizing enzymes and the monomers present in the EPS repeating units, although differences among strains have been reported (Boels et al. 2001; Degeest et al. 2001a, b; Degeest and De Vuyst 2000; Escalante et al. 1998, 2002; Grobben et al. 1996; Levander and Radström 2001; Mozzi et al. 2001, 2003). Thus, the Leloir enzyme UDP-galactose 4-epimerase plays an essential role in the EPS synthesis by L. casei CRL 87 in batch fermentations at constant pH (5.0) when galactose is used as sugar source (Mozzi et al. 2003). Degeest and De Vuyst (2000) reported a correlation between the ∝-phosphoglucomutase, UDP-galactose 4-epimerase and UDP-glucose pyrophosphorylase activities and EPS production in the strain S. thermophilus LY03.
L. helveticus is an industrially important starter microorganism used for the elaboration of semi-hard cheeses, such as Grana and Provolone (Fortina et al. 1998). For the manufacture of Mozzarella cheese, this microorganism is used in combination with S. thermophilus (Broadbent et al. 2001; Perry et al. 1997). The ropy strain L. helveticus ATCC 15807 is able to synthesize a high molecular mass EPS (1.8×106 Da) composed of glucose and galactose (2:1) when grown in milk cultures (Torino et al. 2000). This microorganism produces higher amounts (247–549 mg ml−1) of polymer under acidic culture conditions (pH 4.5–5.0) with respect to the values obtained at a more alkaline pH (6.2) when grown at 37°C in milk (Torino et al. 2001).
In order to better understand the biochemical basis determining EPS synthesis in L. helveticus ATCC 15807 under optimum growth conditions, the aim of this work was to investigate polymer production in a simplified culture medium and to determine its relation with the sugar nucleotide-biosynthesizing enzymes at constant pH.
Materials and methods
Microorganism and culture conditions
L. helveticus ATCC 15807 was obtained from the culture collection of the Centro de Referencia para Lactobacilos (San Miguel de Tucumán, Argentina). Before experimental use, cultures were propagated (2%, v/v) in basal medium (BM) containing (per liter distilled water): 10.0 g peptone (Britania), 15.0 g triptone (Oxoid, New York, N.Y.), 5.0 g yeast extract (Britania), 1.0 g Tween 80, 0.05 g MnSO4·4H2O, 3.5 g CaCl2 and either lactose or glucose (Britania, Buenos Aires, Argentina). The strain was sub-cultured on each carbohydrate at least twice just prior to experimental use. The microorganism was stored at −20°C in reconstituted skim milk (10%, w/v) containing 10% (v/v) glycerol, 0.5% (w/v) yeast extract and 1% (w/v) glucose.
Fermentation conditions
Batch cultures were carried out in a 2.0-l BioFlo fermentor (New Brunswick Scientific Co., Edison, N.J.) with a working volume of 1.8 l. Agitation was applied at 100 rpm, the temperature was maintained at 37°C and the pH was kept automatically at 4.5 or 6.2 with either 1 M HCl or NH4OH (both sterile). Lactose, glucose and CaCl2·2H2O were sterilized separately (20 min at 121°C) and then added to the fermentor vessel aseptically. The 16-h culture was washed twice with sterile saline solution (NaCl 0.85%, w/v), suspended in 10 ml saline solution and added to the fermentation vessel until an optical density at 560 nm (OD560) of 0.1 [about 107 colony-forming units (cfu) ml−1] was reached. Fermentation was allowed to proceed for 30 h; and samples were aseptically withdrawn from the fermentation vessel at 0, 8, 12, 16, 24 and 30 h incubation and cooled immediately on ice before the following assays were performed: cell growth (OD560), cell viability [cfu ml−1; using the plate dilution method in MRS agar plates (MRS: Britania, Argentina; plus agar, 13 g l−1) incubated at 37°C for 48 h] and EPS production (see below). The enzyme activities were determined at 12 h and 24 h fermentation.
All fermentations were carried out in duplicate independent experiments and the shown results represent the mean of the obtained data.
Isolation, purification and characterization of EPS
EPS were isolated by the two-step deproteination–precipitation technique described by de Vuyst et al. (1998), but ethanol was used instead of acetone. The isolated EPS were re-dissolved in distilled water, neutralized to pH 7.0–7.5 with 0.1 M NaOH and dialyzed against distilled water at 4°C for 48 h. The amount of polysaccharides (mg l−1) was estimated by the phenol/sulfuric acid method (Dubois et al. 1956), subtracting the amount of EPS-reacting material from the non-inoculated culture medium. Specific EPS production (Yp/x) was calculated dividing the EPS production (p) by the dry cell biomass produced (x), and was expressed as mg EPS mg−1 biomass.
The EPS were lyophilized, re-suspended in 1 ml distilled water and applied to a Sepharose 4B (Sigma, Mo.) column for purification and determination of their molecular mass (MM). For this purpose, the column was calibrated with a mixture of dextrans (Sigma) with MM of 39, 73, 515 and 2,000 kDa, at a concentration of 0.25 mg ml−1 each. The MM of the EPS was determined by plotting the log of the dextrans MM against the elution volume (Mozzi et al. 1996). The EPS were eluted with 0.05 M Tris-HCl buffer (pH 7.0) and 2-ml fractions were collected at a flow rate of 0.1 ml min−1.
Collected purified samples were freeze-dried, re-suspended in distilled water and hydrolyzed with 2 N trifluoroacetic acid (final concentration) in sealed tubes at 100°C for 3 h. The hydrolyzed EPS were freeze-dried and re-dissolved in ultra-pure water (MilliQ water; Millipore, Cape Cod, Mass.). The impurity concentration was lower than 5 ppm at a concentration of 100 μg ml−1. Monomer analysis was carried out by high-performance anion exchange chromatography (HPAEC) with a CarboPac PA 10 column (Dionex). The monomers were eluted with 18 mM NaOH at a fixed flow rate of 1 ml min−1. Pulsed amperometry (electromechanical detector ED 40, Dionex) allowed simultaneous detection of sugars and sugar derivatives (fucose, rhamnose, galactosamine, glucosamine, galactose, glucose, mannose, fructose).
EPS isolation and characterization were performed in duplicate independent experiments. The estimated error of the EPS isolation technique was within a range of 10–20%.
Preparation of cell-free extracts
Batch cultures of L. helveticus ATCC 15807 grown in BM medium at pH 4.5 and pH 6.2 for 12 h and 24 h at 37°C (as stated above) were harvested by centrifugation at 10,000 g for 10 min at 4°C and the cells were washed twice and re-suspended in cold 10 mM potassium phosphate buffer (pH 6.8) containing 5 mM MgCl2. Cells were disrupted by glass beads, debris was removed by centrifugation (10,000 g, 10 min, 4°C) and the supernatant fluid was used as the cell-free extract. The amount of protein in cell extracts was estimated according to Bradford (1976), using bovine serum albumin as standard. Cell-free extracts were maintained on ice and immediately used for enzyme activity determinations.
Enzyme activities
All in vitro assays were performed in a volume of 1.0 ml in 1.0-ml polystyrene cuvettes at 37°C in a Cecil 2021 spectrophotometer (Cecil Instruments Ltd., Cambridge, UK) with freshly prepared cell-free extracts. The formation or disappearance of NAD(P)H was monitored by measuring the absorbance at 340 nm (ɛ340: 6.22 M−1 cm−1). The protein content of cell-free extracts was determined using the method of Bradford (1976). The specific enzyme activity was expressed as nmol changed min−1 mg−1 cell protein.
Galactose 1-phosphate-uridyltransferase activity (GalT) was determined by the method of Kuruhashi and Anderson (1958). Concentrations of phosphoglucomutase and glucose-6-phosphate dehydrogenase had to be increased by a factor of ten to give detectable enzyme activities (Bettenbrock and Alpert 1998).
The uridine diphosphate (UDP)-glucose pyrophosphorylase (GalU) and UDP-galactose 4-epimerase (GalE) assays were determined as described by Grobben et al. (1996).
The α-phosphoglucomutase (PGM) assay was performed as described by Looijesteijn et al. (1999).
All enzymatic measurements were carried out at least in triplicate and results were expressed as mean values with standard deviations.
Results
EPS production by L. helveticus ATCC 15807 at constant pH
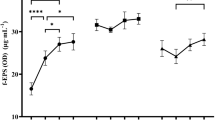
L. helveticus ATCC 15807 showed a similar growth behavior regarding the values of μmax (0.34–0.36 h−1), maximal cell viability (5×108–3×108 cfu ml−1) and OD560 (5.5–4.5) obtained in BM broth at constant pH 4.5 and pH 6.2, respectively (Fig. 1). The main difference between the cultures was the sudden decrease (two log units) in the cell viability detected after 30 h incubation at the more acidic pH (4.5).
EPS production by this strain started during the log growth phase at both pH values and reached the maximal amount during the stationary phase of growth. Polymer synthesis was 2.9-fold higher at pH 4.5 (280 mg l−1) than at pH 6.2 (97 mg l−1); and a slight decrease in the total EPS amount was observed after 24 h fermentation at pH 6.2.
EPS characterization: monomer composition and MM
In BM broth, L. helveticus ATCC 15807 produced a high MM EPS (1.2–1.9×106 Da) at both tested pH, a partial degradation being observed after 24 h incubation in both cases (Table 1). HPAEC analysis of the hydrolyzed samples showed that the EPS were composed of glucose and galactose in a molar ratio of 2.0:1.0, results that are in agreement with the 1H-NMR spectroscopy analysis of the polymer samples isolated from fermented milk (unpublished data). Moreover, the sugar composition of the EPS was not modified by the carbon source when L. helveticus ATCC 15807 grew in a chemically defined medium containing lactose, glucose or galactose as carbon source (unpublished data).
Sugar nucleotide-enzyme activities
The EPS produced by L. helveticus ATCC 15807 requires the sugar nucleotides UDP-glucose and UDP-galactose (Fig. 2) to donate the corresponding monomers for incorporation in the repeating units. Table 2 shows the EPS production and the activity of the involved enzymes leading to the synthesis of UDP-glucose and UDP-galactose in L. helveticus ATCC 15807 when grown on lactose cultures at pH 4.5 and pH 6.2. In general, this microorganism displayed higher enzyme activities at pH 4.5 with respect to the cultures grown at pH 6.2, which in addition was coincident with the highest total (91 mg l−1, 208 mg l−1) and specific (5.3×10−2 mg EPS mg−1 biomass, 7.3×10−2 mg EPS mg−1 biomass) EPS production observed at pH 4.5 after 12 h and 24 h incubation, respectively. The maximum enzyme activities at both pH values were found at 12 h incubation before the highest EPS production was reached. α-PGM displayed the highest activity values independently of the evaluated pH. This enzyme was the only one which showed a direct correlation with the total and specific EPS production by L. helveticus ATCC 15807, being 2.0-fold higher in cultures at pH 4.5, with respect to pH 6.2, either after 12 h incubation (exponential growth phase) or 24 h (stationary growth phase; Table 2). No significant differences were observed with GalT, GalU or GalE. Since α-PGM is a glucose metabolism-related enzyme, the results obtained suggested that the glucose moiety of lactose is greatly involved as a carbon source for EPS production. Using the galactose moiety of lactose, L. helveticus ATCC 15807 showed scarce growth and polysaccharide synthesis at pH 4.5 (data not shown). To confirm the former approach, EPS production and enzyme activities were determined in BM cultures containing glucose as energy source at pH 4.5 (optimal for EPS production). The results are shown in Table 3. The total and specific EPS synthesis and the α-PGM and GalT activities were lower for glucose-grown cultures with respect to the values obtained for lactose (Table 3), the pronounced decrease (4.5-fold) in EPS production being more correlated with a decrease in GalT activity (3.5-fold) than with α-PGM activity (1.3-fold).
Discussion
The thermophilic ropy strain L. helveticus ATCC 15807 produced greater amounts of EPS at pH 4.5 than at pH 6.2, using lactose as carbon source. For L. delbrueckii subsp. bulgaricus, another thermophilic EPS-producing strain, the best culture pH for polymer formation was coincident with the optimum for culture growth (pH 6.0; Petry et al. 2000). In contrast, Mozzi et al. (2003) observed a higher EPS production by L. casei CRL 87 at acidic pH values (5.0) than at pH 6.0 on galactose-grown cultures. These apparent controversial results show that culture conditions should be optimized for each EPS-producing strain for efficient production (Font de Valdez et al. 2003).
The chemical composition of the EPS produced by L. helveticus ATCC 15807 is composed of glucose and galactose (2.0:1.0) either on lactose-grown cultures or in milk fermentations; and the monomeric composition was not modified by the carbon source when lactose, glucose or galactose were used in a chemically defined medium (unpublished data). In general, the carbohydrate source does not influence the monomer composition of the EPS produced by LAB (Degeest and De Vuyst 1999, 2000; Looijesteijn et al. 1999; van Calsteren et al. 2002), although some differences have been observed in polysaccharides produced by certain L. delbrueckii subsp. bulgaricus strains (Grobben et al. 1996; Petry et al. 2000).
Regarding EPS production, a substantial difference can be noticed when diverse sugars are used as energy source. Carbohydrates may enter the cell either in a phosphorylated state or as free sugar, which has to be phosphorylated inside the cell prior to further degradation. A key intermediate linking the anabolic pathways of EPS production and the catabolic pathways of sugar degradation appears to be glucose 6-phosphate, from which the flux of carbon bifurcates between the formation of fructose 6-phosphate toward the biosynthesis of sugar nucleotides, the precursor of EPS (Fig. 2). Phosphoglucomutase, the enzyme responsible for the conversion of glucose 6-phosphate into glucose 1-phosphate, plays an important role in this flux divergence between the catabolic and anabolic pathways (Degeest and De Vuyst 2000; Hugenholtz and Kleerebezem 1999). In order to investigate the sugar fluxes into the direction of sugar nucleotides in L. helveticus ATCC 15807, the activities of the enzymes involved in the synthesis of UDP-glucose and UDP-galactose were determined on lactose cultures at pH 4.5 and pH 6.2 and on glucose cultures at pH 4.5. The α-PGM enzyme was the only one which showed a direct correlation with the EPS production by this microorganism in BM medium under controlled pH conditions, the highest levels being reached at pH 4.5 (Table 1). These results suggested an important role for the glucose moiety of lactose in polymer production. However, when glucose was used as sole carbon source instead of lactose, a marked decrease in EPS production was observed, accompanied by a decrease in the activity of α-PGM and a detrimental reduction in GalT activity. GalU and GalE activities were similar in both lactose- and glucose-grown cells, showing that they were not directly correlated with the behavior of this L. helveticus strain, regarding polymer formation. The role of GalU (together with dTDP-rhamnose and α-PGM) was important for EPS synthesis in S. thermophilus LY03 (Degeest and De Vuyst 2000), while GalE was crucial for EPS production by L. casei CRL 87 (Mozzi et al. 2003) and Lcc. lactis NIZO B40 (Boels et al. 2001).
As expected, low GalT levels on glucose-grown cells were detected, since the GalT enzyme is linked to galactose metabolism, where the formation of UDP-galactose is mediated by both GalE and GalT (Frey 1996). Considering this, a low flux in the pathway leading to UDP-galactose synthesis occurs on glucose, resulting in lower EPS production by L. helveticus ATCC 15807. In lactose-grown cells, the higher GalT activity detected (compared with that obtained on glucose) showed that this enzyme played an important role either supplying UDP-galactose to incorporate the monomer into the EPS repeating unit or following the flux to the formation of glucose 1-phosphate (Fig. 2). The results obtained in our research suggest that the enzymes α-PGM and GalT are the ones which should be targeted to in order to increase EPS yields by L. helveticus ATCC 15807. In this respect, Levander et al. (2002) have seen that the overexpression of the pgm and galU genes (coding for the α-PGM and GalU enzymes, respectively) enhanced the EPS synthesis in S. thermophilus LY03, while the overexpression of each gene separately did not influence EPS production.
For the first time, the enzymes involved in EPS biosynthesis by a L. helveticus strain were studied. The crucial role of α-PGM for achieving high polymer formation by L. helveticus ATCC 15807 was observed, although it was associated with high EPS production if GalT was also increased.
References
Bettenbrock K, Alpert CA (1998) The gal genes for the Leloir pathway of Lactobacillus casei 64H. Appl Environ Microbiol 64:2013–2019
Boels IC, Ramos A, Kleerebezem M, Vos WM de (2001) Functional analysis of the Lactococcus lactis galU and galE genes and their impact on sugar nucleotide and exopolysaccharide biosynthesis. Appl Environ Microbiol 67:3033–3040
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Broadbent JR, McMahon DJ, Oberg CJ, Welker DL (2001) Use of exopolysaccharide-producing cultures to improve the functionality of low fat cheese. Int Dairy J 11:433–439
De Vuyst L, Degeest B (1999) Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev 23:153–177
De Vuyst L, Vanderveken F, Ven S van de, Deggest B (1998) Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth-associated biosynthesis. J Appl Microbiol 84:1059–1068
Degeest B, De Vuyst L (2000) Correlation of activities of the enzymes α-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl Environ Microbiol 66:3519–3527
Degeest B, Janssens B, De Vuyst L (2001a) Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0-1: production kinetics, enzyme activities, and EPS yields. J Appl Microbiol 67:470–477
Degeest B, Vaningelgem F, Laws A, De Vuyst L (2001b) UDP-N-acetylglucosamine-4-epimerase activity indicates the presence of N-acetylgalactosamine in exopolysaccharides of Streptococcus thermophilus strains. Appl Environ Microbiol 67:3976–3984
Dubois M, Gilles KA, Hamilton JK, Roberts PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Duboc P, Mollet B (2001) Applications of exopolysaccharides in the dairy industry. Int Dairy J 11:759–768
Escalante A, Wacher-Rodarte C, García-Garibay M, Farrés A (1998) Enzymes involved in carbohydrate metabolism and their role on exopolysaccharide production in Streptococcus thermophilus. J Appl Microbiol 84:108–114
Escalante A, Villegas J, Wacher C, García-Garibay M, Farrés A (2002) Activity of enzymes involved in the synthesis of exopolysaccharide precursors in an overproducing mutant ropy strain of Streptococcus thermophilus. FEMS Microbiol Lett 209:289–293
Faber EJ, van den Haak MJ, Kamerling JP, Vliegenthart JFG (1998) The exopolyasccharides produced by Lactobacillus delbrueckii subsp. bulgaricus 291. Carbohydr Res 331:183–194
Fortina MG, Nicastro G, Carminati D, Neviani E, Manachini PL (1998) Lactobacillus helveticus heterogeneity in natural cheese starters: the diversity in phenotypic characteristics. J Appl Microbiol 84:72–80
Font de Valdez G, Torino MI, de Vuyst L, Mozzi F (2003) Food-grade heteropolyaccharides: ongoing research and future trends of biopolymers from lactic acid bacteria. Appl Biotechnol Food Sci Pol 4:223–233
Frey PA (1996) The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. Fed Am Soc Exp Biol J 10:461–470
Grobben GJ, Smith MR, Sikkema J, de Bont JAM (1996) Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. Appl Microbiol Biotechnol 46:279–284
Hugenholtz J, Kleerebezem M (1999) Metabolic engineering of lactic acid bacteria: overview of the approaches and results of pathway rerouting involved in food fermentations. Curr Opin Biotechnol 10:492–497
Kuruhashi K, Anderson EP (1958) Galactose-1-phosphate uridyl transferase, its purification and application. Biochem Biophys Acta 29:498–502
Levander F, Radström P (2001) Requirement for phosphoglucomutase in exopolysaccharide biosynthesis in glucose- and lactose-utilizing Streptococcus thermophilus. Appl Environ Microbiol 67:2734–2738
Levander F, Svensson M, Radström P (2002) Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl Environ Microbiol 68:784–790
Looijesteijn PJ, Boels IC, Kleerebezem M, Hugenholtz J (1999) Regulation of exopolysaccharide production by Lactococcus lactis subsp. cremoris by the sugar source. Appl Environ Microbiol 65:5003–5008
McMahon DJ, Oberg CJ (1998) Influence of fat, moisture and salt on functional properties of Mozzarella cheese. Aust J Dairy Technol 53:98–101
Merrill RK, Oberg CJ, McMahon DJ (1994) A method for manufacturing reduced fat Mozzarella cheese. J Dairy Sci 77:1783–1789
Mozzi F, Savoy de Giori G, Oliver G, Font de Valdez G (1996) Exopolysaccharide production by Lactobacillus casei in milk under different growth conditions. Milchwissenschaft 51:670–673
Mozzi F, Rollán G, Savoy de Giori G, Font de Valdez G (2001) Effect of galactose and glucose on the exopolysaccharide production and the activities of biosynthetic enzymes in Lactobacillus casei CRL 87. J Appl Microbiol 91:160–167
Mozzi F, Savoy de Giori G, Font de Valdez G (2003) UDP-galactose 4-epimerase: a key enzyme in exopolysaccharide formation by Lactobacillus casei CRL 87 in controlled pH batch cultures. J Appl Microbiol 94:175–183
Perry DB, McMahon DJ, Oberg CJ (1997) Effect of exopolysaccharide producing cultures on moisture retention in low-fat mozzarella cheese. J Dairy Sci 80:799–805
Petersen BL, Dave RI, McMahon DJ, Oberg CJ, Broadbent JR (2000) Influence of capsular and ropy exopolysaccharide-producing Streptococcus thermophilus on Mozzarella cheese and cheese whey. J Dairy Sci 83:1952–1956
Petry S, Furlan S, Crepeau M-J, Cerning J, Desmazeaud M (2000) Factors affecting exocellular polysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus grown in a chemically defined medium. Appl Environ Microbiol 66: 3427–3431
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12:163–171
Torino MI, Mozzi F, Sesma F, Font de Valdez G (2000) Effect of stirring on growth and phosphopolysaccharide production by Lactobacillus helveticus ATCC15807 in milk. Milchwissenschaft 55:204–206
Torino MI, Taranto MP, Sesma F, Font de Valdez G (2001) Heterofermentative pattern and exopolysaccharide production by Lactobacillus helveticus ATCC 15807 in response to environmental pH. J Appl Microbiol 91:1–7
Tuinier R, Casteren WHM van, Looijesteijn PJ, Schols HA, Voragen AGJ, Zoon P (2001) Effect of structural modifications on some physical characteristics of exopolysaccharides from Lactococcus lactis. Biopolymers 59:160–166
Van Calsteren MR, Pau-Roblot C, Begin A, Roy D (2002) Structure determination of the exopolysaccharide produced by Lactobacillus rhamnosus strains RW-9595M and R. Biochem J 363:7–17
Vaningelgem F, Zamfir M, Mozzi F, Adriany T, Vancanneyt M, Swings J, De Vuyst L (2004) Biodiversity of exopolysaccharides produced by Streptococcus thermophilus strains is reflected in their production and their molecular and functional characteristics. Appl Environ Microbiol 70:900–912
Acknowledgements
The authors acknowledge the financial support of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and CIUNT in Argentina. The authors are very grateful to Prof. Dr. ir. Luc de Vuyst and Dr. ir. Frederik Vaningelgem (IMDO, VUB, Brussels, Belgium) for their help in the EPS monomeric composition analysis
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torino, M.I., Mozzi, F. & Font de Valdez, G. Exopolysaccharide biosynthesis by Lactobacillus helveticus ATCC 15807. Appl Microbiol Biotechnol 68, 259–265 (2005). https://doi.org/10.1007/s00253-004-1865-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1865-2