Abstract
In this study fusaricidin, a cyclic depsipeptide isolated from Paenibacillus polymyxa E681 (E681), was demonstrated to control Phytophthora blight infection caused by Phytophthora capsici in red-pepper. The minimal inhibitory concentration (MIC) of fusaricidin was found to be 16 ppm against P. capsici. The disease severity of P. capsici was 40% at 0.1 ppm of fusaricidin when compared with water-treated control (81.7%) on post-treatment, whereas the disease severities on pre-treatment were 45% and 83.3% in fusaricidin (0.1 ppm) and water-treated control, respectively, in red-pepper plants. Significant (P < 0.05) disease suppression was observed on treatment with fusaricidin (0.1 ppm) by foliar spray and soil drench. The disease severity was drastically reduced to 3.3% by soil drench of fusaricidin (1.0 ppm), whereas in water-treated control, the disease severity was 83.3% in the first experiment. Fusaricidin at 0.1 ppm reduced disease severity of P. capsici to 27.5% when compared with positive control (43.1%) and water-treated control (66.2%) in the second experiment. Soft rot disease in tobacco was suppressed upon treatment with fusaricidin at 1.0 ppm by leaf infiltration. RT-PCR analyses of Arabidopsis thaliana revealed that there was an up-regulation of pathogenesis-related (PR) gene expression in wild type A. thaliana (Col-0), while there was no accumulation of PR genes, which implies that the mechanism of protection might be based on a salicylic acid-dependent pathway. This is the first report that fusaricidin exhibits protection against plant pathogens in addition to activity as an antibiotic agent. Hence, E681 can play a role in plant protection by secretion of ISR elicitors including fusaricidin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological control using microorganisms to suppress soilborne plant pathogens has been successfully carried out in several crops (Park et al. 1988; Weller 1988). The mechanisms how these diseases were controlled by the microorganisms remained unclear until Homma & Suzui (1989) elaborated the role of antibiotics by suppression of the pathogens. Among the myriads of bacteria thriving in the plant rhizosphere, some spore forming plant growth-promoting rhizobacteria (PGPR), in particular gram-positive Bacilli and Streptomycetes, attracted special attention due to their advantages over non-spore-forming bacteria in product formulation and stable maintenance in soil (Emmert & Handelsman 1999). Among these, the genus Paenibacillus comprises more than 89 species (Ash et al. 1993; Truper 2005). Strains of Paenibacillus polymyxa suppress several plant diseases and promote plant growth (Ryu & Park 1997).
Paenibacillus polymyxa strains are capable of producing several hydrolytic enzymes, including proteases, β-1,3-glucanases, cellulases, xylanase, lipase, amylase and chitinases which play an important role in the biocontrol of plant pathogens (Beatty & Jensen 2002; Raza et al. 2008). These strains have been isolated from the rhizosphere of various crops (Choi et al. 2008; Petersen et al. 1996). P. polymyxa has been successful in the control of gray mold in strawberries caused by Botrytis cinerea (Helbig 2001); Fusarium oxysporum and Pythium spp.– causal agents of seedling blight and wilt, root rot of cucumber and water melon (Dijksterhuis et al. 1999; Yang et al. 2004); sesame damping off (Ryu et al. 2006); and to control diseases caused by Phytophthora palmivora and Pythium aphanidermatum (Timmusk &Wagner 1999). P. polymyxa is known to produce two types of peptide antibiotics: one is active against bacteria and the other one is active against fungi and actinomycetes (Beatty & Jensen 2002). This species also synthesizes plant hormones such as auxin and cytokinin (Timmusk & Wagner 1999), solubilizes soil phosphorus, enhances soil porosity (Gouzou et al. 1993) and has been used for flocculation and flotation of various minerals (Deo & Natarajan 1998).
Generally, it is difficult to manage diseases caused by Phytophthora because of their high aggressiveness and increase in resistance to metalaxyl, an effective systemic compound against oomycetes (Gavino et al. 2000); and 95 Phytophthora and 100 Pythium species which have been previously reported (Erwin & Ribeiro 1996; Kamoun et al. 1999). The emergence of insensitive strains, and their persistence, makes the application of a chemical ineffective and rather exacerbates the problem. These issues necessitated us to look for alternative methods for disease control in order to reduce pesticide application to food crops and the concern regarding environmental pollution. Phytophthora blight caused by Phytophthora capsici is one of the most devastating soilborne diseases of red-pepper worldwide (Hausbeck & Lamour 2004; Hwang & Kim 1995). Intensive studies have been concentrated on the biology of P. capsici, such as evaluation of pepper germplasm for disease resistance, yield-loss assessment, and testing of chemical, biological, and cultural measures of control (Hwang & Kim 1995).

Fusaricidin comprises a group of cyclic depsipeptides with an unusual 15-guanidino-3-hydroxypentadecanoic acid moiety bound to a free amino group (Kajimura & Kaneda 1997). Fusaricidin is produced by P. polymyxa PKB1 at the beginning of sporulation (Beatty & Jensen 2002) and gatavalin is produced by Bacillus polymyxa spp. and Colistinus koyama ATCC 21830 (Nakajima et al. 1972). Both products belong to the same or a similar family of peptide antibiotics with very similar HPLC profiles. Two further antibacterial substances were isolated and purified from culture broth of P. polymyxa, namely, gavaserin and saltavalin (Pichard et al. 1995). Kajimura & Kaneda (1995) reported the taxonomy, fermentation, isolation, structure elucidation and biological activity of Fusaricidin A, an antibiotic produced by Bacillus polymyxa KT-8. However, there is only limited information on the activity of fusaricidin against oomycetes, and there is no report available on disease suppression through ISR. Thus, in the present investigation, we report for the first time the protection exerted by fusaricidin, an antibiotic compound active against Phytophthora blight in red-pepper plants.
Materials and methods
Culturing of microorganisms and the media used
The strain P. polymyxa E681 (E681) was obtained from the Korean Research Institute of Bioscience and Biotechnology (KRIBB), South Korea. The strain was identified and deposited at Microbial Resources Data Base, KRIBB, South Korea, under lyophilized conditions, and maintained at 4°C until use for mass cultivation. Media used for cultivation and bioassay of the strains were potato dextrose agar (PDA) and potato dextrose broth (PDB) purchased from Difco (Detroit, MI, USA). pH of the medium was adjusted to 7.2 before sterilization. Bennett’s agar and Bennett’s broth for preservation and liquid culture of the strain contained (per liter) glucose 10 g, yeast extract 1 g, bacto peptone 2 g, beef extract 1 g, agar 20 g (or without agar), 5 g each of thiamine-HCl, riboflavin, niacin, pyridoxine-HCl, inositol, Ca-pantothenate and amino benzoic acid, biotin 25 mg, cycloheximide 50 mg and nalidixic acid 10 mg. Fungi P. capsici was grown on V8 juice agar plate at 28°C for 7 days.
Screening of cyclic depsipeptide producer E681 and culturing
Fusaricidin producing strain E681 was screened and identified as a potential biocontrol agent (BCA) according to the method followed by Lee et al. (2005). For the preparation of culture broth, E681 was first inoculated in 200 ml erlenmeyer flasks containing 60 ml of the screening medium consisting of (per liter) soluble starch 10 g, glucose 20 g, soybean meal 25 g, beef extract 1 g, yeast extract 4 g, NaCl 2 g, K2HPO4 25 g and CaCO3 2 g. pH of the medium was adjusted to 7.2 before sterilization and cultured on a rotary shaker at 150 rpm and 28°C for 5 days. For scale-up fermentation, the strain was precultured in a 500 ml erlenmeyer flask for 3 days, and a stock solution (3%) was used to re-inoculate in a 5 l jar fermentor (Korea Fermentor Co., Daejeon, Korea) to produce fusaricidin. The purified fusaricidin was stored at 4°C until further use.
In vitro antifungal activity of fusaricidin
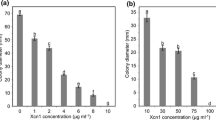
The antifungal activity of cyclic depsipeptide, fusaricidin-A, was examined on PDA plates containing fusaricidin at various concentrations (0 to 512 ppm) against major fungal plant pathogens (Rhizoctonia solani, Fusarium oxysporum, Phytophthora capsici, Colletotrichum acutatum, Pythium ultimum, Alternaria alternata, Botrytis cinerea and Sclerotinia sclerotiorum) for in vitro primary screening. The mycelial discs of each pathogen, prepared with a sterile cork borer (5 mm), were placed on PDA plates. The plates were incubated at 25°C for 48 h, and then observed for mycelial growth. Antifungal activity is expressed as the minimal inhibitory concentration (MIC) at which no mycelial growth was observed.
Preparation of spore suspension of P. capsici and bacterial pathogen P. carotovorum SCC1 inocula
- P. capsici inocula were prepared as described by Ploetz et al. (2002). A 5-mm-diam mycelial plug of an isolate was transferred to a V8 agar plate. After 1 week of incubation at 25°C, V8 agar plugs with mycelia were placed into a petri dish containing V8 broth, and allowed to grow for another week at 25°C. The V8 broth was then drained and each plate was washed twice with sterile distilled water (SDW). SDW was added to cover the mycelia on each plate, and then the plates were placed under wide-spectrum light at room temperature for 48 h to induce sporangial development. The sporangia were chilled at 4°C for 45 min to induce the release of zoospores. The zoospores were adjusted to a final concentration of 1 × 105 spores ml−1 to use for challenge inoculation under greenhouse conditions. For the preparation of P. carotovorum SCC1 (SCC1) inoculum, the bacterial cell suspensions were prepared from 24-h-old culture at 28°C. Ten ml of SDW was poured on tryptic soy agar (TSA) culture plate and scraped with sterile plastic loop and adjusted to a final concentration of 1 × 108 cfu ml−1 (OD600 = 0.8) before challenge inoculation.
Pre- or post-treatment of fusaricidin on red-pepper plants against Phytophthora blight
Three-week-old red-pepper (Capsicum annuum L.) cv. Hanbyul seedlings were used in this study. The seedlings were treated with fusaricidin by foliar spray at various concentrations (0.1, 1.0, 10, 100 and 1,000 ppm) in pre- or post-treatments. In pre-treatment, the red-pepper plants were foliar sprayed with fusaricidin 7 days before challenge inoculation with pathogen, whereas in post-treatment, 7 days after pathogen challenge red-pepper plants were treated with fusaricidin by foliar spray. Dimethomorph, a common systemic fungicide, was used as positive control at 1.0 ppm. It is a cinnamic acid derivative and a member of the morpholine chemical family. One week later, the disease severity of Phytophthora blight on red-pepper was assessed during pre-and post-treatments according to the modified method by Sunwoo et al. (1996) based on a 0–5 scale: where 0 = no visible disease symptoms; 1 = leaves slightly wilted with brownish lesions beginning to appear on stems; 2 = 30–50% of entire plant diseased; 3 = 50–70% of entire plant diseased; 4 = 70–90% of entire plant diseased; 5 = completely wilted or plant dead. Based on these ratings, percentage of disease severity was calculated.
Evaluation of fusaricidin for ISR activity against P. capsici and SCC1
To induce the protection exerted by fusaricidin, 3-week-old red-pepper seedlings were treated with fusaricidin by soil drench at various concentrations (0.1, 1.0, 10 and 100 ppm). During 3 weeks of time, a second and third dose of fusaricidin was applied to seedlings by soil drench at 7-day intervals. For the induced protection of red-pepper from P. capsici, fusaricidin-treated plants after challenge inoculation with zoospores by soil drench were placed on a greenhouse bench after incubating them in a humidity chamber at 28°C for 24 h. The greenhouse conditions were 22–26°C, with a natural photoperiod. One week later, the percentage of disease severity was recorded as above. For the induced protection of tobacco plants from SCC1, 3-week-old plants were treated with fusaricidin by infiltration, and were challenge inoculated with the suspensions of SCC1. The plants were kept on a greenhouse bench after incubating them in a humidity chamber for 24 h. Three days later, the percentage of disease incidence was recorded.
Defense gene expression in Arabidopsis thaliana through RT-PCR
- Arabidopsis thaliana wild type, Columbia (Col-0) and salicylic acid (SA) insensitive transgenic line (Nah-G) were obtained from the Ohio State University Stock Centre, Columbus OH, USA. Two-week-old Arabidopsis seedlings were soil drenched with fusaricidin at various concentrations (0.1, 1.0 and 10 ppm), and then the leaf tissues were collected for RNA isolation 7 days after treatment. Total RNA was isolated using easy-spinTM IIP Total RNA Extraction Kit (iNtRON Biotechnology, South Korea). Reverse transcriptase (RT)-PCR was performed according to Kishimoto et al. (2005) with Ex Taq polymerase (Takara Biomedicals, Otsu, Japan). The reaction mixture contained 0.1 μg of cDNA, 10 pMol each of forward and reverse primers, 250 nM dNTPs, and 0.5 U of Ex Taq polymerase in 20 μl of buffer solution. The PCR was carried out in a MJ Research thermal cycler (PTC-100, USA) with the following conditions: 94°C for 5 min followed by 94°C for 1 min, 57°C for 1 min for 25 cycles, followed by 72°C for 10 min for final extension. Primers for defense gene are F-5′-AACCGCCAAAAGCAAACGCA-3′ (PR-1a-F), R-5′-TCACGGAGGCACAACCAAGTC-3′ (PR-1a-R). Amplified PCR products were analyzed with 1% agarose gel electrophoresis and the gels were documented (LAS-3000, Fuji Photo Film Co. Ltd., Tokyo, Japan).
Statistical analysis
Data were analyzed (mean ± SE) with SAS JMP software. Significant differences in treatment means on each sample data were determined using LSD at P = 0.05. All experiments were repeated at least once. For each experiment, data were analyzed separately. Results of one representative experiment are shown.
Results
In vitro antifungal activity
In vitro antifungal activity of the cyclic depsipeptide, fuaricidin-A, was assayed against various phytopathogenic fungi that are a common major cause of diseases in agricultural crops. The minimal inhibitory concentration (MIC) values are listed in Table 1. These data reveal that the tested antibiotic agent, fusaricdin, has potent antifungal activity against the fungal pathogens: in particular, the oomycetes of P. capsici and P. ultimum were inhibited by treatment with fusaricidin at the lowest (16 ppm) concentration.
Effect of fusaricidin on suppression of Phytophthora leaf blight infection
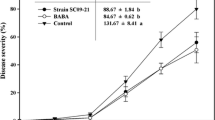
The present study identified the potential for fusaricidin as one of the ISR elicitors for protecting red-pepper plants against Phytophthora blight under greenhouse conditions. Fusaricidin treatment significantly (P < 0.05) reduced Phytophthora leaf blight infection when compared with water-treated control plants. This disease suppression was found to be at the lowest concentration (0.1 ppm of fusaricidin) by foliar spray (Fig. 1). Suppression of Phytophthora blight infection on red-pepper revealed that fusaricidin treatment by foliar spray at 0.1 ppm has brought down the leaf blight infection to 40% in post-treatment, and 45% in pre-treatment when compared with 81.7% and 83.3% disease severities in post-treatment and pre-treatments, respectively, in water-treated control. In the case of soil drench with fusaricidin, there was a significant (P < 0.05) reduction of disease severity to 3.3% by treatment with fusaricidin at 1.0 ppm concentration when compared to other concentrations of fusaricidin, dimethomorph and water-treated control (Fig. 2). The results demonstrated the role of fusaricidin as a potential ISR agent against Phytophthora infections in red-pepper plants. It is interesting to note that the percentage of disease severity was increased at higher concentrations (10 ppm to 1,000 ppm) of fusaricidin. This indicates that lower concentrations of fusaricidin elicit systemic resistance for protecting the plants from disease. In the second experiment, the study identified 0.1 ppm as an optimum dosage of fusaricidin in reducing disease severity to 27.50% when compared with other, higher, concentrations as well as dimethomorph at 1.0 ppm (43.13%) and 66.25% in water-treated control. Figure 3 shows that there was a reduced disease severity at 1.0 ppm concentration of fusaricidin and is on a par with the chemical fungicide dimethomorph (1.0 ppm) when compared with higher concentrations and water-treated control.
Suppression of Phytophthora blight in red-pepper plants on pre- or post-treatments of fusaricidin (Fusa) or dimethomorph (D.M) by foliar spray. Distilled water served as untreated control. Red-pepper plants were pre- and post-treated with fusaricidin at various concentrations before and after challenge inoculation of Phytophthora capsici spores by soil drench. The plants were kept under greenhouse conditions 24 h after incubating them in a humidity chamber, after which the percentage of disease severity was measured. The experiment was conducted at least twice with 12 replications per treatment with similar results. Bars with the same letter do not differ significantly from each other according to Fischer’s least significant difference test (P = 0.05)
Suppression of Phytophthora blight on red-pepper plants by soil drench of fusaricidin (Fusa). Dimethomorph (D.M) and distilled water served as positive and negative controls, respectively. Red-pepper plants were treated with fusaricidin at various concentrations, and 3 days later the plants were challenge-inoculated with Phytophthora capsici zoospores by soil drench; after 24 h of incubation in a humidity chamber, the plants were kept under greenhouse conditions for 1 week. The percentage of disease severity was measured. Bars with the same letter do not differ significantly from each other according to Fischer’s least significant difference test (P = 0.05)
Induced suppression of disease development in red-pepper plants against Phytophthora capsici by soil drench with fusaricidin (F) at various concentrations under greenhouse conditions. Percentage of disease severity was recorded 7 days after pathogen challenge with P. capsici zoospore suspensions by soil drench. Fusaricidin at lower concentrations (1.0 and 10 ppm) induced the suppression of disease development. Dimethomorph (D.M) and distilled water served as positive and negative controls, respectively. The experiment was repeated at least once with 12 replications per treatment with similar results
Effect of fusaricidin on disease suppression of soft rot in tobacco
Further, the disease suppression development in transgenic tobacco against soft rot disease was assessed by leaf infiltration of fusaricidin. Infiltration of fusaricidin at 1.0 ppm recorded reduced disease severity (22%) when compared with the chemical elicitor BTH at 0.1 ppm (18.3%) and by 85% in water-treated control (Fig. 4). On the other hand, with fusaricidin at 0.1 ppm there was also significant reduced disease severity of 30%. There was greater disease severity at higher concentrations (100 and 1,000 ppm).
Suppression of disease development in tobacco plants against soft rot disease caused by Pectobacterium carotovorum SCC1 by treatment with fusaricidin (Fusa). Benzothiadiazole (BTH) and distilled water served as positive and negative controls, respectively. Tobacco plants were leaf infiltrated with fusaricidin at various concentrations. Three days after challenge inoculation with P. carotovorum SCC1 suspensions, the plants were observed for disease incidence and percentage was calculated. The experiment was repeated at least once with 12 replications per treatment with similar results. Bars with the same letter do not differ significantly from each other according to Fischer’s least significant difference test (P = 0.05)
Effect of fusaricidin on defense-related gene expression in A. thaliana through RT-PCR
In order to ascertain the enhancement of ISR activity of fusaricidin, RT-PCR analysis for defense gene transcript in Arabidopsis was carried out to confirm the activation of the defense gene PR-1a by mRNA accumulation in the leaves of A. thaliana wild type (Col-0). Gene expression studies were performed with Arabidopsis gene-specific primers for the defense-related genes at 12 or 24 or 48 h after challenge inoculation with SCC1 (Fig. 5). The PR-1a gene was up-regulated in wild type (Col-0) by treatment with fusaricidin at 1.0 ppm concentration at 24 h, while there was minor expression in the transgenic line (nah-G) at the same time, and no expression at 12 and 48 h after pathogen challenge, which implies that the salicylic acid (SA)-dependent pathway was elicited by fusaricidin treatment in Arabidopsis plants. This molecular evidence of fusaricidin (1.0 ppm)-mediated ISR was supported by Fig. 4, where the same level of treatment induced the suppression of disease development of SCC1 in tobacco plants. Collectively, the disease suppression by treatment with fusaricidin derived from E681 might be due to systemic resistance rather than to direct antagonism.
Elicitation of defense-related gene expression in Arabidopsis thaliana wild type, Col-1 (Lane 1), and transgenic line, nahG (Lane 2), by treatment with fusaricidin at different concentrations (0.1, 1.0 and 10 ppm). The expression of pathogenesis-related (PR) gene PR-1a analyzed by RT-PCR was examined at 12 or 24 or 48 h after challenging with pathogen. Amplified products were separated by gel electrophoresis and visualized by ethidium bromide staining. The experiment was conducted two times with similar results. M = 100 bp DNA ladder
Discussion
In the present study, we demonstrated the potential of fusaricidin for suppressing the disease development of Phytophthora blight caused by P. capsici in red-pepper plants in vitro and under greenhouse conditions as well. Importantly, the MICs of the cyclic depsipeptide fusaricidin has been found similar to or better than those of previous antifungal proteins and peptides (De Lucca et al. 2005; Diz et al. 2006). Plant protection by the elicitation of ISR has been successfully demonstrated in many crops by treatment with various biocontrol agents against major pathogens. A research effort all over the world in this direction has led to successful development of biotic and abiotic agents that can protect the plant either directly by antagonism mechanisms or indirectly through induced systemic resistance in host plants against invading pathogens. Some of the classical examples of chemical inducers for innate improvement in host defense are 2,6-dichloroisonicotinic acid, benzothiadiazole, methyl jasmonate and probenazole (Von Rad et al. 2004). The results demonstrated that the fusaricidin tested in the present investigation effectively inhibited P. capsici and SCC1 infections in red-pepper and tobacco, respectively. This is the first report on the cyclic depsipeptide antibiotic fusaricidin derived from Paenibacillus polymyxa E681 (E681) in suppression of disease development through ISR in plants. Previously, aminoglycoside antibiotic compounds had been reported to reduce the intensity of disease caused by oomycetes (Xiao et al. 2002), including P. infestans (Lee et al. 2005), The commercial aminoglycoside antibiotics such as neomycin, ribostamycin, streptomycin, including fusaricidin, were tested against P. infestans. In addition, recently the role of hyaluronic acid from Streptomyces spp. as a potential ISR agent in cucumber and tomato plants against major economically important diseases has been established by Park et al. (2007). Previously Kajimura & Kaneda (1995) also discussed the isolation, structure and biological activity of fusaricidin from P. polymyxa. The present study demonstrated the potential of biochemical agent fusaricidin for the induced protection of red-pepper from P. casici. The fact that there was greater disease suppression of Phytophthora blight on treatment with fusaricidin by soil drench compared with foliar spray implies that the mechanism of disease suppression is through systemic rather than direct antagonism. This also suggests that soil application of fusaricidin at 0.1 ppm is an ideal dosage for triggering the host defense in red-pepper plants.
A naturally soilborne bacterium, P. polymyxa, possesses several desirable properties as a biocontrol active agent against plant-pathogenic fungi, and exhibits resistance to several fungicides and herbicides approved for use on canola crops in Canada (Ramarathnam & Fernando 2006). Strains of P. polymyxa have also been shown to produce a wide range of antibiotic peptides, which may give them a growth advantage in the competitive soil environment. Their antagonistic activity against fungi and gram-positive bacteria, and the fact that they have no effect on gram-negative bacteria, distinguishes this second group of peptides from the first group (Katz & Demain 1977). The fusaricidin group of antibiotics seems to be less diverse as compared with the Polymyxin-Colistin-Circulin group. However, both groups still lack complete structural and molecular characterization. The occurrence of Phytophthora isolates resistant to metalaxyls and oxadixyls in field crops has posed new challenges to manage this important group of plant pathogens causing significant yield loss in several crop species (Parra & Ristaino 2001). The extensive and indiscriminate use of metalaxyl has led to the rapid development and widespread distribution of metalaxyl-resistant strains of Phytophthora spp. throughout the world (Deahl et al. 1993; Goodwin et al. 1996). The occurrence of metalaxyl resistance to Phytophthora species in potato and pepper fields has resulted in devastating late blight problems due to the failure of disease control in most of the production areas (Parra & Ristaino 2001; Ristaino & Johnston 1999).
Thus, this study brought out the importance of antifungal and anti-oomycete activity of fusaricidin, exhibiting induced protection from Phytophthora leaf blight of red-pepper and soft rot of tobacco through systemic resistance which can be used in large-scale field application in the future. Thus, fusaricidin plays a significant role as an effective ISR elicitor against Phytophthora blight of red-pepper. Earlier reports documented only the antifungal activity of fusaricidin against this major group of plant pathogens (Choi et al. 2004; Kajimura & Kaneda 1995), but this study identified the role of fusaricidin as one of the potential ISR elicitors against Phytophthora leaf blight pathogen. Molecular evidence of PR gene expression demonstrated that the soil drench of fusaricidin was effective in establishing the systemic resistance in leaves of activation of the defense gene in transgenic Arabidopsis treated with fusaricidin, and proved that fusaricidin activated the defense genes to protect the plants from P. capsici through ISR. Based on our findings, the E681 derivative, cyclic depsipeptide fusaricidin, can play a role in inducing resistance for protecting plants against invading plant pathogens in red-pepper plants. Thus, it might serve as an alternative approach to chemical fungicides, and for investigating the biochemical changes in plant responsiveness to pathogen challenge.
References
Ash, C., Priest, F. G., & Collins, M. D. (1993). Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek, 64, 253–260.
Beatty, P. H., & Jensen, S. E. (2002). Paenibacillus polymyxa produces fusaricidin-type antifungal antibiotics active against Leptosphaeria maculans, the causative agent of blackleg disease of canola. Canadian Journal of Microbiology, 48, 159–169.
Choi, O., Kim, J., Ryu, C. M., & Park, C. S. (2004). Colonization and population changes of a biocontrol agent, Paenibacillus polymyxa E681, in seeds and roots. Plant Pathology Journal, 20, 97–102.
Choi, S. K., Park, S. Y., Kim, R., Lee, C. H., Kim, J. F., & Park, S. H. (2008). Identification and functional analysis of the fusaricidin biosynthetic gene of Paenibacillus polymyxa E681. Biochemical and Biophysical Research Communications, 365, 89–95.
De Lucca, A. J., Cleveland, T. E., & Wedge, D. E. (2005). Plant-derived antifungal proteins and peptides. Canadian Journal of Microbiology, 51, 1001–1014.
Deahl, K. L., DeMuth, S. P., Pelter, G., & Ormrod, D. (1993). First report of resistance of Phytophthora infestans to metalaxyl in eastern Washington and southwestern British Columbia. Plant Disease, 77, 429.
Deo, N., & Natarajan, K. A. (1998). Studies on interaction of Paenibacillus polymyxa with iron ore minerals in relation to beneficiation. International Journal of Mineral Processing, 55, 41–60.
Dijksterhuis, J., Sanders, M., Gorris, L. G. M., & Smid, E. J. (1999). Antibiosis plays a role in the context of direct interaction during antagonism of Paenibacillus polymyxa towards Fusarium oxysporum. Journal of Applied Microbiology, 86, 13–21.
Diz, M. S., Carvalho, A. O., Rodrigues, R., Neves-Ferreira, A. G., Da Cunha, M., Alves, E. W., et al. (2006). Antimicrobial peptides from chilli pepper seeds causes yeast plasma membrane permeabilization and inhibits the acidification of the medium by yeast cells. Biochimica et Biophysica Acta, 1760, 1323–1332.
Emmert, E. A. B., & Handelsman, J. (1999). Biocontrol of plant disease: a (Gram-) positive perspective. FEMS Microbiology Letters, 171, 1–9.
Erwin, D. C., & Ribeiro, O. K. (1996). Phytophthora diseases worldwide. St Paul, MN, USA: American Phytopathological Society Press.
Gavino, P. D., Smart, C. D., Sandrock, R. W., Miller, J. S., Hamm, P. B., Lee, T. Y., et al. (2000). Implications of sexual reproduction for Phytophthora infestans in the United States: generation of an aggressive lineage. Plant Disease, 84, 731–735.
Goodwin, S. B., Sujkowski, L. S., & Fry, W. E. (1996). Widespread distribution and probable origin of resistance to metalaxyl in clonal genotypes of Phytophthora infestans in the United States and Western Canada. Phytopathology, 86, 793–800.
Gouzou, L., Burtin, G., Philippy, R., Bartoli, F., & Heulin, T. (1993). Effect of inoculation with Bacillus polymyxa on soil aggregation in the wheat rhizosphere: preliminary examination. Geoderma, 56, 479–491.
Hausbeck, M. K., & Lamour, K. H. (2004). Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Disease, 88, 1292–1303.
Helbig, J. (2001). Biological control of Botrytis cinerea Pers. ex Fr. in strawberry by Paenibacillus polymyxa (Isolate 18191). Journal of Phytopathology, 149, 265–273.
Homma, Y., & Suzui, T. (1989). Role of antibiotic production in suppression of radish damping-off by seed bacterization with Pseudomonas cepacia. Annual Phytopathological Society of Japan, 55, 643–652.
Hwang, B. K., & Kim, C. H. (1995). Phytophthora blight of pepper and its control in Korea. Plant Disease, 79, 221–227.
Kajimura, Y., & Kaneda, M. (1995). Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8, taxonomy, fermentation, isolation, structure elucidation and biological activity. The Journal of Antibiotics, 49, 129–135.
Kajimura, Y., & Kaneda, M. (1997). Fusaricidins B, C and D, new depsipeptide antibiotics produced by Bacillus polymyxa KT-8, isolation, structure elucidation and biological activity. The Journal of Antibiotics, 50, 220–228.
Kamoun, S., Huitema, E., & Vleeshouwers, V. G. A. A. (1999). Resistance to oomycetes: a general role for the hypersensitive response? Trends in Plant Science, 4, 1360–1385.
Katz, E., & Demain, A. L. (1977). The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriological Reviews, 41, 449–474.
Kishimoto, K., Matsui, K., Ozawa, R., & Takabayashi, J. (2005). Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant & Cell Physiology, 46, 1093–1102.
Lee, H. B., Kim, Y., Kim, J. C., Choi, G. J., Park, S. H., Kim, C. J., et al. (2005). Activity of some aminoglycoside antibiotics against true fungi, Phytophthora and Pythium species. Journal of Applied Microbiology, 99, 836–843.
Nakajima, N., Chihara, S., & Koyama, Y. (1972). A new antibiotic, gatavalin. I. Isolation and characterization. The Journal of Antibiotics, 25, 243–247.
Park, C., Paulitz, C. T., & Baker, R. (1988). Biocontrol of Fusarium wilt of cucumber resulting from interactions between Pseudomonas putida and nonpathogenic isolates of Fusarium oxysporum. Phytopathology, 78, 190–194.
Park, K. S., Diby, P., Kim, E., & Kloepper, J. W. (2007). Hyaluronic acid of Streptomyces sp. as a potent elicitor for induction of systemic resistance against plant diseases. World Journal of Microbial Biotechnology. doi:10.1007/s11274-007-9587-0.
Parra, G., & Ristaino, J. B. (2001). Resistance to mefonoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Disease, 85, 1069–1075.
Petersen, D. J., Srinivasan, M., & Chanway, C. P. (1996). Bacillus polymyxa stimulates increased Rhizobium etli populations and nodulation when co-resident in the rhizosphere of Phaseolus vulgaris. FEMS Microbiology Letters, 142, 271–276.
Pichard, B., Larue, J. P., & Thouvenot, D. (1995). Gavaserin and saltavalin, new peptide antibiotics produced by Bacillus polymyxa. FEMS Microbiology Letters, 133, 215–218.
Ploetz, R., Schnell, R. J., & Haynes, J. (2002). Variable response of open-pollinated seedling progeny of avocado to Phytophthora root rot. Phytoparasitica, 30, 262–268.
Ramarathnam, R., & Fernando, W. G. D. (2006). Preliminary phenotypic and molecular screening for potential bacterial biocontrol agents of Leptosphaeria maculans, the blackleg pathogen of canola. Biocontrol Science and Technology, 16, 567–582.
Raza, W., Yang, W., & Shen, Q. R. (2008). Paenibacillus polymyxa: antibiotics, hydrolytic enzymes and hazard assessment. Journal of Plant Pathology, 90, 403–414.
Ristaino, J. B., & Johnston, S. A. (1999). Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Disease, 83, 1080–1089.
Ryu, C. M., & Park, C. S. (1997). Enhancement of plant growth induced by endospore forming PGPR strain, Bacillus polymyxa E681. Proceedings of the Fourth International Workshop on Plant Growth-Promoting Rhizobacteria, Japan-OECD Joint Workshop (Sapporo, Japan) pp. 209–211.
Ryu, C. M., Kim, J., Choi, O., Kim, S. H., & Park, C. S. (2006). Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame. Biological Control, 39, 282–289.
Sunwoo, J. Y., Lee, K. Y., & Hwang, B. K. (1996). Induced resistance against Phytophthora capsici in pepper plants in response to DL-β-amino-n-butyric acid. European Journal of Plant Pathology, 102, 663–670.
Timmusk, S., & Wagner, E. G. H. (1999). The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Molecular Plant-Microbe Interaction, 12, 951–959.
Truper, H. G. (2005). The type species of the genus Paenibacillus polymyxa. Opinion 77 judicial commission of the international committee on systematics of prokaryotes correspondence. International Journal of Systematic and Evolutionary Microbiology, 55, 513.
Von Rad, U., Mueller, J. M., & Durner, J. (2004). Evaluation of natural and synthetic stimulants of plant immunity by microarray technology. New Phytologist, 165, 191–202.
Weller, D. M. (1988). Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annual Review of Phytopathology, 26, 379–407.
Xiao, K., Kinkel, L. L., & Samac, D. A. (2002). Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biological Control, 23, 285–295.
Yang, K. Y., Blee, K. A., Zang, S., & Anderson, A. J. (2004). Oxycom™ treatment suppresses Pseudomonas syringae infection and activates a mitogen-activated protein kinase pathway in tobacco. Physiological and Molecular Plant Pathology, 61, 249–256.
Acknowledgments
The authors are grateful to the National Academy of Agricultural Sciences (NAAS), South Korea, for providing financial assistance with the support of Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ0069010222011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.H., Cho, Y.E., Park, SH. et al. An antibiotic fusaricidin: a cyclic depsipeptide from Paenibacillus polymyxa E681 induces systemic resistance against Phytophthora blight of red-pepper. Phytoparasitica 41, 49–58 (2013). https://doi.org/10.1007/s12600-012-0263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-012-0263-z