Abstract
In the methane production from waste activated sludge (WAS), complex bacterial interactions in WAS have been known as a major contribution to methane production. Therefore, the influence of bacterial community changes toward methane production from WAS was investigated by an application of antibiotics as a simple means for it. In this study, azithromycin (Azm) as an antibiotic was mainly used to observe the effect on microbial changes that influence methane production from WAS. The results showed that at the end of fermentation, Azm enhanced methane production about twofold compared to control. Azm fostered the growth of acid-producing bacterial communities, which synthesized more precursors for methane formation. DGGE result showed that the hydrolysis as well as acetogenesis stage was improved by the dominant of B1, B2 and B3 strains, which are Clostridium species. In the presence of Azm, the total population of archaeal group was increased, resulting in higher methane productivity achievement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The activated sludge process is used worldwide by domestic and industrial wastewater treatment plants (WWTP) because it degrades the organic contaminants in wastewater so that it produces clean water, carbon dioxide, and biomass [1]. However, a drawback of this method is a vast quantity of waste activated sludge (WAS) generated, which accounts for ≤47 % of industrial waste (approximately 2,150,000 tons in dry base) in Japan [2] and 25–60 % of the operating costs of WWTPs [3]. WAS includes contaminants [4], which can harm the environment if it is inappropriately discharged. Practically, WAS is disposed in landfills or by incineration under the strict regulation. The shortage of available land also increased the operation cost of the sanitary landfill sites. To date, many methods and strategies have been developed to minimize WAS generation; for example, chemical treatments using metal cations [5] and oxidizing substances [6, 7]. Individual or combined thermal processes and ultrasound and alkaline treatments have widely been used for sludge solubilization [8–11]. Combined thermal, chemical, and mechanical treatments have also been used as a sludge reduction strategy [12, 13].
Anaerobic digestion is a treatment process, which can convert a majority of organic compounds into valuable energy sources such as biohydrogen [14] and electricity generation based on microbial fuel cells [15]. Among different approaches, methane production is considered to be the most promising for reducing the quantity of sludge generated in parallel of renewable energy recovery [16]. Anaerobic fermentation involves in continuous degradation of organic materials by various microorganisms producing methane [17]. The process generally consists of four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. In general, methane is produced by methanogens from acetate acid, carbon dioxide, and hydrogen generated in the prior reactions [18]. Hence, different microbial communities are involved in each of the four stages of methane production process. However, the activities of these mixed communities and the detail interactions among various bacterial populations during anaerobic fermentation are still not entirely well understood, and the optimum bacterial community for methane production has not been determined. This study aimed to understand the change of cell viability of the bacterial community in the presence of an antibiotic to determine its effects on methane production. The use of antibiotics is a simple approach to alter bacterial viability in anaerobic fermentation toward methane generation. Azithromycin (Azm) is a well-known macrolide antibiotic [19], and its application in medicine was investigated. Azm has been used for the treatment of respiratory tract infections [20] and acute otitis media [21]. Azm is also reported to be an inhibitor regulating Pseudomonas aeruginosa biofilm formation [19, 22]. In this study, Azm was mainly used as an initial concept to modify the viability of the bacterial community in a fermentation culture to understand its effects on anaerobic fermentation during methane production.
Materials and methods
Sludge preparation
Raw sludge was collected from Hiagari WWTP in Kitakyushu City, Japan. Sludge composition has been reported previously [23]. Protein is a major component of the sludge (40–45 % of total solid) and followed by carbohydrate (12–14 % of total solid) and lipid (11–13 % of total solid). Fresh sludge was washed by centrifugation at 8,000×g for 10 min at 4 °C, and the pellet was resuspended in distilled water thrice, before adjusting its concentration to 10 % (wt/wt) by distilled water.
Methane production
The fermentation process was carried using 20 g of 10 % sludge in 50-mL vials as substrate and inoculum sources. Azm was added at different concentrations (5, 10, and 15 µg/mL), whereas a vial without Azm was used as the control. Kanamycin (Kan) and tetracycline (Tet) were used with the same concentration of 5 µg/mL instead of Azm as positive controls, since both were reported to give similar character and impact [19, 22, 24]. The fermentation vials were sealed by crimped butyl rubber stoppers and sparged with nitrogen for 5 min and then incubated at 37 °C in a shaking incubator at 120 rpm for 5 days (Bio-shaker, BV-180LF, Taitec, Saitama, Japan). Each day, 50 μL of the headspace gas in vials was analyzed using gas chromatography equipped with a thermal conductive detector (GC system, 6890 series, Agilent, Santa Clara, CA, USA).
Microbial community analysis
RNA extraction and cDNA synthesis
A 4-mL fermented sludge sample was mixed with 3 mL of RNAlater solution (Formerly Ambion, Applied Biosystems) in a 15-mL falcon tube before centrifuging at 15,000 rpm for 2 min. The cell pellet was soaked in a dissolved dry ice in ethanol for 30 s and stored at −70 °C prior to RNA extraction. Total RNA was extracted using RNeasy kit (Qiagen, Inc., Valencia, CA, USA) and bead beater (Wakenyaku Co. Ltd, Kyoto, Japan, model 3011b) as described in previous paper [25]. The cDNA was synthesized from the RNA extract using PrimeScript RT reagent kit Perfect Real Time by random oligomers (TAKARA Bio Inc., Shiga, Japan). Agarose gel electrophoresis was conducted to verify the success of RNA isolation as well as cDNA synthesis. The cDNA were later used for denaturing gradient gel electrophoresis (DGGE) analysis and real-time polymerase chain reaction (RT-PCR) quantification.
DGGE analysis
To analyze the change of bacterial community, DGGE was conducted using cDNA samples. The PCR amplification was performed by RoboCycler Gradient 40 (Strategene, La Jolla, CA, USA). The nucleotide sequences of the primers were shown in Table 1. Primer 3 contains the same sequence as primer 1, but has at its 5′ end an additional 40-nucleotide GC-rich sequence (GC-clamp). A combination of primers 1 and 2 or primers 2 and 3 was used to amplify the 16S rDNA regions in the different bacterial species, which correspond to positions 341–534 in Escherichia coli [26]. The PCR step comprised: 94 °C for 5 min, 94 °C for 1 min, 63 °C and 1 min decreasing 1 °C every cycle until 57 °C, 72 °C for 1 min, followed by 18 cycles of: 94 °C for 1 min, 57 °C for 1 min and 72 °C for 1 min, and final at 72 °C for 25 min. DGGE was performed by 8 % (wt/vol) acrylamide gel containing a 30–57 % denaturant gradient. Electrophoresis was conducted in 1 × TBE buffer (Tris/Borate/EDTA) for 7 h at 60 °C and 50 V. The gel was stained with ethidium bromide (0.5 µg/mL) for 45 min, and then the stained gel was visually scanned by Astec Imager software (Gel Scene Imaging system). Visual bands were excised and immersed into sterilized water overnight before being used for PCR amplification. A 2.3 µL of mixture solution was used for PCR amplification with the primer set without GC-clamp. Electrophoresis was performed with the entire PCR product by 0.8 % agarose gel at 100 V for 25 min for confirmation and purification. A DGGE marker used in this study was DGGE Marker II (Nippon Gene, Toyama, Japan). Visual band was extracted by QIAquick Gel extraction kit (Qiagen, Limburg, The Netherlands). The 16S rRNA gene fragments were sent for sequencing by FASMAC Co. (Kanagawa, Japan). Sequenced results were compared with the reference in the National Centre of Biotechnology Information (NCBI), nucleotide sequence database.
RT-PCR quantification
RT-PCR was performed to quantify the 16S rRNA gene abundance of total Archaea and Bacteria groups. An RT-PCR system (Step one, Applied Biosystem, Tokyo, Japan) was used to conduct the amplification and fluorescence detection. The used primer and probe set are shown in Table 1. The reaction mixture was prepared as follows: 10 μL of TaqMan fast advanced master mix (2×), 0.72 μL of each primer (final concentration 900 nM), 0.34 μL of TaqMan probe (final concentration 200 nM), 6.22 μL of nuclease free water and 2 μL of cDNA template. The running protocol was designed as follows: UNG incubation at 50 °C for 2 min, polymerase activation at 95 °C for 20 s and 40 cycles of: PCR at 95 °C for 1 s and 60 °C for 10 s. Each template cDNA, prepared for the reaction, was analyzed in triplicate. The standard curve was calculated as described in previous paper [27]. Representative strains were used to construct each standard curve (Table 1). The tenfold serial dilution was generated and amplified in triplicate using real-time PCR with the corresponding primer and probe sets. The C T values were plotted against the logarithm of their initial template copy numbers.
Analytical methods
Sludge reduction and organic acid analysis
Sludge reduction ratio was measured as described previously by Maeda et al. [28]. Sludge samples (before and after fermentation) were collected (10 g wet weight) and centrifuged at 18,000×g for 10 min. The pellets were transferred to porcelain dishes and dried in an oven (D-300, Iuchi, Japan) at 105 °C for 2 days to determine the dry weight. For the organic acid analysis, sludge samples were centrifuged at 13,000 rpm for 7 min and the supernatant was filtered using a 0.2-µm syringe filter, and subsequently analyzed using high-performance liquid chromatography (Shim-pack SCR-102H, Shimadzu, Tokyo, Japan) [25]. The same samples were used to measure protease activity, soluble carbohydrate, and protein concentrations. All the presented data were at least obtained from triplicate independent experiments.
Analysis of soluble carbohydrate, protein, and pH
Soluble carbohydrate concentration was measured using the phenol–sulfuric acid method in which d-glucose was used to generate a standard curve. Soluble protein concentration was analyzed using the Lowry method in which bovine serum albumin was used as the standard substance [4]. A UV/Vis Spectrophotometer V-530 (Jasco, Tokyo, Japan) was used to measure the absorbance of samples. The pH of the culture solution was measured using a compact pH meter (AS-211, AS ONE, Japan).
Protease activity assay
The protease activity was measured as reported in a previous study [28]. 2 % casein solution was used as the substrate. Protease activity was measured by a UV/Vis spectrophotometer (Jasco, V-530, Tokyo, Japan) at 660 nm. One unit of protease activity was calculated as the quantity of tyrosine (µmol) produced from casein by 1 mg of enzyme per min.
Viable cells counting
Serial dilutions of cultured samples during the fermentation process were spread onto Luria–Bertani agar plates (LB plate) containing 10 g of Bacto tryptone, 5 g of Bacto yeast extract, 5 g of NaCl, and 15 g of agar in 1 L for the colony counting. An MRS plate (Oxoid, Hampshire, England) containing 0.5 % of calcium carbonate was prepared for counting the number of organic acid-producing bacteria, and the modified MRS plates were anaerobically incubated at 37 °C for 2 days for colony counting [23]. All the presented data were at least obtained from triplicate independent experiments.
Results and discussion
Initial research concept using antibiotics
Our initial research interest was to investigate how bacterial cell-to-cell communication influences methane fermentation from WAS, which is triggered by a complex bacterial interaction. Therefore, as an initial experiment, Azm, ceftazidime, and ciprofloxacin were added to WAS samples at a low concentration (0.02, 0.01 and 0.6 μg/mL, respectively) to see the impact on methane fermentation because these three antibiotics have been reported as a quorum sensing inhibitor for Pseudomonas aeruginosa [22]. As a result, there is no big impact for methane fermentation under the low concentration of each antibiotic. On the other hand, only when a higher concentration of Azm is used (5, 10 and 15 μg/mL, respectively), methane fermentation was enhanced whereas no impact was observed in the other antibiotics (data not shown).
Methane production in the presence of Azm
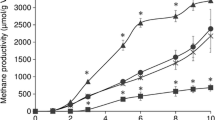
The optimum concentration affecting methane production at different concentrations of Azm (5, 10, and 15 µg/mL) were investigated and the obtained results showed that 5 µg/mL of Azm gave the highest methane production of 8046 ± 84 µmol/g dry-weight sludge compared to 0, 10, and 15 µg/mL which were 4,068 ± 94, 7,148 ± 56, and 6,339 ± 79 µmol/g dry-weight sludge after 5-day incubation, respectively. Furthermore, the effect of higher concentration of Azm (50 and 100 µg/mL) on the fermentation was also tested. The result shows that under these two concentrations, methane production was certainly inhibited. After 5 day of incubation, methane productivities in the presence of 50 and 100 µg/mL of Azm were 1,162 ± 92 and 473 ± 56 µmol/g dry-weight sludge, respectively. Probably, under a high concentration of Azm, bacterial community is totally inactivated [29]. Methane production in the presence of Azm (5 µg/mL) was about twofold higher than the control (Fig. 1a). Fermentation in the presence of Azm increased methane production from the first day and persisted throughout the fermentation process. Therefore, the concentration of 5 µg/mL of Azm was applied throughout the experiments. In fact, the positive effect of Azm on methane production was observed until the 10th day of the fermentation. At this day, methane productivities in the presence and absence of Azm were 11,077 ± 72 and 10,566 ± 63 µmol/g dry-weight sludge, respectively. Hence, we could conclude that Azm facilitates methane fermentation at an early stage.
a Methane production in the presence of Azm (squares), Kan (diamonds), Tet (triangles) at the same concentration of 5 µg/mL and the antibiotic-free control (circles). b Methane production at the 5th day of the fermentation from different types of sludge: Hiagari sludge, sludge A, sludge B and sludge C in the presence of Azm at the concentration of 5 µg/mL and the antibiotic-free control (Ctrl). The data represented as ±SD from triplicate independent experiments
Next, in order to investigate the effect of other antibiotics for methane production, Kan and Tet were used as another candidate of antibiotics. Results showed that Tet had less impact than Azm and there was no impact in the presence of Kan (Fig. 1a).
To confirm the effect of Azm on methane production, seven different types of WAS obtained from the different sources were used as a substrate to verify the impact of Azm on methane production. Figure 1b showed the methane productivity of three highest methane levels from three different sludge sources together with the Hiagari sludge used mainly in this study. In the presence of Azm, higher methane was observed for all types of sludge, and Azm had a certain positive impact on the methane fermentation processes. The variable quantities of methane production from the different types of sludge may have been caused by the specific composition of each type of sludge. However, when added to the anaerobic sludge fermentation process, Azm always improved the methane production level.
Volatile fatty acids (VFAs), which are the essential precursors for methane production [30], were analyzed to understand the correlation between degradation and methane production. Five types of VFA were detected in the fermentation broth at the 5th day of the fermentation including acetic, propionic, butyric, succinic, and formic acids. Acetic acid showed the highest proportion in all the samples, followed by propionic acid and butyric acid (Table 2). These results were in agreement with another study in which these short chain fatty acids (C2–C4) are the main products of acidogenesis stage in anaerobic sludge digestion [31]. The results showed that Azm affected the VFA synthesis, particularly acetic acid production, since the concentration was the highest (31 ± 3 mM) at the end of the incubation. Meanwhile, in the presence of Kan and Tet, the concentration of accumulated acetic acid was only 18.1 ± 0.1 mM and 23 ± 2 mM whereas 18.0 ± 0.2 mM in the absence of antibiotic (control), (Fig. 2a). On the other hand, when used higher concentration of Azm (50 and 100 µg/mL), acetic acid concentrations were lower than in that of 5 µg/mL of Azm (data not shown).
a Acetic acid concentration in the presence of Azm (filled squares), Kan (filled diamonds), and Tet (filled triangles) and in the antibiotic-free control (filled circles). b pH of cultures in the presence of Azm (open squares), Kan (open diamonds), and Tet (open triangles) and the antibiotic-free control (open circles). The data represented as ±SD from triplicate independent experiments
It has been reported that the conversion of long-chain fatty acids to acetate contributes to methane formation during the methanogenesis stage [32]. In the presence of Azm, the acetic acid concentration was the highest compared with the other samples, which made a large amount of substrate available for the methanogens, thereby yielding the highest methane production. Therefore, a higher concentration of VFAs especially acetic acid was believed to be triggered by an addition of Azm. As shown in the result that a high concentration of acetic acid was produced in the presence of Azm in agreement with pH decrease from the initial 6.8 to 5 during the fermentation (Fig. 2b), and finally methane generation was facilitated. The decline in the pH had a direct proportion to the quantity of acetic acid produced.
Effects of Azm on sludge reduction
The highest sludge reduction ratio was observed 2 days after fermentation in all samples. In the presence of Azm, the sludge degradation ratio was approximately 37 ± 4 %, which was 13 % higher than that of the control (24 ± 1 %). In the presence of Kan and Tet, the sludge reduction ratios were 29.6 ± 0.4 and 25 ± 2 %, respectively. Thus, Azm probably promoted the hydrolysis stage, which is known to be the rate-limiting step during anaerobic digestion [18] and helped to release more soluble material for later stages.
Changes in different components during fermentation
In this study, protein was a major component of sludge as previously described [23]. Soluble protein and carbohydrate concentrations were the products of hydrolysis step in the methane fermentation and hence monitored during the anaerobic digestion in the presence of Azm (Fig. 3a). Protein concentration greatly increased after 1 day of incubation and gradually accumulated throughout the process. Protein concentration was highest in the presence of Azm (5 µg/mL) (reaching to 162 ± 5 µg/mL) compared with the control and the cultures that contained Kan and Tet (Fig. 3a). The accumulation of protein during the fermentation was not observed in the presence of 50 and 100 µg/mL of Azm (data not shown).
a Soluble protein (straight line) and carbohydrate concentrations (dash line), respectively, in the presence of Azm (open squares with straight line and open square with dash line), Kan (open diamonds with straight line and open diamonds with dash line), Tet (open triangles with straight line and open triangles with dash line) and the antibiotic-free control (open circles with straight line and open circles with dash line). b Protease activity in the presence of Azm (filled squares with straight line), Kan (filled diamonds with straight line), Tet (filled triangles with straight line) and the antibiotic-free control (filled circles with straight line). The data represented as ±SD from triplicate independent experiments
The highest protease activity (52 ± 2 mU) was also detected in the presence of Azm (Fig. 3b). The higher protease activity helped to solubilize the protein into the liquid phase. Throughout the fermentation, the protein components in WAS were hydrolyzed by proteases, but protein accumulation may have occurred because the rate of protein utilization was lower than its rate of release [30].
The carbohydrate concentration (Fig. 3a) in the liquid phase increased with the rate of protein hydrolysis after 1 day of incubation. However, the carbohydrate concentration was slightly lower on day 2, before increasing on day 3, and slightly changing on day 5. The soluble carbohydrate concentration was higher in the presence of Azm and Tet than that in the presence of Kan and in the control, although the differences were not substantial. The carbohydrate concentration trend was different from that of the protein concentration because carbohydrate was only a minor constituent of the sludge compared with the protein.
Effects of Azm on culturable bacteria and organic acid-producing bacteria
Colony counts were conducted to determine the effects of Azm on the bacterial viability during anaerobic digestion. Throughout the fermentation process, samples were collected from the fermentation cultures in the presence of Azm (Azm culture) and absence of Azm (control) and spread on LB and modified MRS agar plates. On the LB plates, the colony count trends were similar to the Azm culture and the control; however, the number of colonies detected from the Azm culture was always higher than that of the control throughout the fermentation process (Table 3). At the end of the fermentation, the count of organic acid-producing bacteria, which formed a halo zone, was 0.1 × 103 colony-forming unit/mL (cfu/mL) while with Azm culture, the counts was higher than that of the control five times (0.5 × 103 cfu/mL). These results show that Azm addition increased the bacterial viability in terms of the total viable bacteria and organic acid-producing bacteria. Thus, introduction of Azm promoted the growth of organic acid-producing bacterial community, which is correlated with the higher levels of acetic acid production (the end-product of the acid formation stage). This effect generated greater quantities of precursors for the methane formation stage resulting in higher methane production.
Bacterial community analysis by DGGE
Denaturing gradient gel electrophoresis analysis was performed using cDNA templates, which were synthesized from RNA extract to evaluate only active bacteria which are actually vital in the presence or absence of Azm. Figure 4 shows the several visual bands which were detected from DGGE analysis. Thereby, three dominant bands (B1, B2 and B3) from Azm culture, which showed higher intensity compared to the control, were excised and used for sequencing. According to phylogenetic affiliation, B1, B2 and B3 were mostly closed to the firmicutes phylum. B1 sequence was closely (97 %) matched with Clostridium cellulovorans (Accession No. NR102875), which is an anaerobic mesophilic bacterium at the optimum growth temperature of 37 °C. This organism produces extracellular enzyme complex known as cellulosomes, which can degrade cellulolytic components [33]. B2 band showed a strong similarity (94 %) to anaerobic Clostridium carboxidivorans (Accession No. NR104768). This strain is primarily acetogenic which can grow on glucose, cellulose, and starch; and acetate and butyrate are the end-products of metabolism [34]. B3 band showed a high intensity in the presence of Azm and almost could not be detected in the control culture. The sequenced result of B3 was closed (94 %) to Clostridium putrefaciens (Accession No. NR024995), which is a strict anaerobe and is markedly by proteolytic activity [35]. This organism has ability to break down a major composition of the sludge (protein components) into smaller polypeptides or amino acids resulting in higher protease activity and soluble protein concentration in the Azm culture than those in the control (Fig. 4). Our result is in agreement with a literature which mentions that Clostridium species are resistant to Azm [29]. This literature supports our results that Clostridium strains are still active and vital in the presence of Azm (5 μg/mL). Thereby, in the presence of Azm, hydrolysis and acidogenesis process were facilitated by activated dominant bacterial species such as B1, B2 and B3 strains. Subsequence soluble materials are abundant precursors for methane-forming stage resulting in higher methane production achievement.
Quantification of archaeal and bacterial groups
The total archaeal and bacteria communities were quantified by RT-PCR. Variation in the 16S rRNA gene concentration of archaea and bacteria in the presence or absence of Azm was quantified. At the end of the fermentation, the total concentration of archaea in the presence of Azm was (5.5 ± 0.1) × 106 copies/mL which was great higher than that of the control which was (1.7 ± 0.3) × 106 copies/mL. This result indicates that in the presence of Azm, archaeal community, which has a key role in methane production, certainly increased, thereby resulting in about twofold higher methane productivity than the control (Fig. 1a). The total bacterial concentration of Azm culture was correlated with DGGE analysis in which higher intensity bands were detectable in the presence of Azm. The total bacterial population in the presence or absence of Azm was (3.5 ± 0.2) × 1012 and (2.39 ± 0.04) × 1012 copies/mL, respectively. Therefore, in terms of either archaeal or bacterial groups, Azm introduction has shown positive effect on enhancement of the community population.
References
Rocher M, Goma G, Begue AP, Louvel L, Rols JL (1999) Towards a reduction in excess sludge production in activated sludge processes: biomass physicochemical treatment and biodegradation. Appl Microbiol Biotechnol 51:883–890
Yoshida H, Tokumoto H, Ishii K, Ishii R (2009) Efficient, high-speed methane fermentation for sewage sludge using subcritical water hydrolysis as pretreatment. Bioresour Technol 100:2933–2939
Yan S, Miyanaga K, Xing X-H, Tanji Y (2008) Succession of bacterial community and enzymatic activities of activated sludge by heat-treatment for reduction of excess sludge. Biochem Eng J 39:598–603
Li X, Ma H, Wang Q, Matsumoto S, Maeda T, Ogawa HI (2009) Isolation, identification of sludge-lysing strain and its utilization in thermophilic aerobic digestion for waste activated sludge. Bioresour Technol 100:2475–2481
Kim Y-K, Bae J-H, Oh B-K, Hong Lee W, Choi J-W (2002) Enhancement of proteolytic enzyme activity excreted from Bacillus stearothermophilus for a thermophilic aerobic digestion process. Bioresour Technol 82:157–164
Neyens E, Baeyens J, Weemaes M, De Heyder B (2003) Pilot-scale peroxidation (H2O2) of sewage sludge. J Hazard Mater 98:91–106
Weemaes M, Grootaerd H, Simoens F, Verstraete W (2000) Anaerobic digestion of ozonized biosolids. Water Res 34:2330–2336
Bougrier C, Carrère H, Delgenès JP (2005) Solubilisation of waste-activated sludge by ultrasonic treatment. Chem Eng J 106:163–169
Kepp U, Machenbach I, Weisz N, Solheim OE (2000) Enhanced stabilisation of sewage sludge through thermal hydrolysis—three years of experience with full scale plant. Water Sci Technol 42:89–96
Li H, Jin Y, Mahar R, Wang Z, Nie Y (2008) Effects and model of alkaline waste activated sludge treatment. Bioresour Technol 99:5140–5144
Vlyssides AG, Karlis PK (2004) Thermal-alkaline solubilization of waste activated sludge as a pre-treatment stage for anaerobic digestion. Bioresour Technol 91:201–206
Nah IW, Kang YW, Hwang K-Y, Song W-K (2000) Mechanical pretreatment of waste activated sludge for anaerobic digestion process. Water Res 34:2362–2368
Valo A, Carrère H, Delgenès JP (2004) Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J Chem Technol Biotechnol 79:1197–1203
Mohd Yasin NH, Fukuzaki M, Maeda T, Miyazaki T, Maail C, Ariffin H, Wood TK (2013) Biohydrogen production from oil palm frond juice and sewage sludge by a metabolically engineered Escherichia coli strain. Int J Hydrogen Energy 38:10277–10283
Mohd Yusoff MZ, Hu A, Feng C, Maeda T, Shirai Y, Hassan MA, Yu C-P (2013) Influence of pretreated activated sludge for electricity generation in microbial fuel cell application. Bioresour Technol 145:90–96
Kanai M, Ferre V, Wakahara S, Yamamoto T, Moro M (2010) A novel combination of methane fermentation and MBR—Kubota submerged anaerobic membrane bioreactor process. Desalination 250:964–967
Buczkowska A, Witkowska E, Górski Ł, Zamojska A, Szewczyk KW, Wróblewski W, Ciosek P (2010) The monitoring of methane fermentation in sequencing batch bioreactor with flow-through array of miniaturized solid state electrodes. Talanta 81:1387–1392
Miyamoto K (1997) Renewable biological systems for alternative sustainable energy production. Food and Agriculture Organization of the United Nations Chapter 4. http://www.fao.org/docrep/W7241E/W7241E00.htm. Accessed 6 Jan 2014
Tateda K, Comte R, Pechere JC, Kohler T, Yamaguchi K, Van Delden C (2001) Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 45:1930–1933
Ferrara A, Dos Santos C, Cimbro M, Grassi GG (1996) Comparative antimicrobial activity and post-antibiotic effect of azithromycin, clarithromycin and roxithromycin against some respiratory pathogens. Int J Antimicrob Agents 7:181–186
Babl FE, Pelton SI, Li Z (2002) Experimental acute otitis media due to nontypeable Haemophilus influenzae: comparison of high and low azithromycin doses with placebo. Antimicrob Agents Chemother 46:2194–2199
Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, Tolker-Nielsen T, Høiby N, Givskov M (2008) Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:3648–3663
Maeda T, Yoshimura T, Shimazu T, Shirai Y, Ogawa HI (2009) Enhanced production of lactic acid with reducing excess sludge by lactate fermentation. J Hazard Mater 168:656–663
Evers S, Di Padova K, Meyer M, Langen H, Fountoulakis M, Keck W, Gray CP (2001) Mechanism-related changes in the gene transcription and protein synthesis patterns of Haemophilus influenzae after treatment with transcriptional and translational inhibitors. Proteomics 1:522–544
Mohd Yusoff MZ, Maeda T, Sanchez-Torres V, Ogawa HI, Shirai Y, Hassan MA, Wood TK (2012) Uncharacterized Escherichia coli proteins YdjA and YhjY are related to biohydrogen production. Int J Hydrogen Energy 37:17778–17787
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel-electrophoresis analysis of polymerase chain reaction-amplified genes-coding for 16S ribosomal-RNA. Appl Environ Microbiol 59:695–700
Lee C, Kim J, Shin SG, Hwang S (2008) Monitoring bacterial and archaeal community shifts in a mesophilic anaerobic batch reactor treating a high-strength organic wastewater. FEMS Microbiol Ecol 65:544–554
Maeda T, Yoshimura T, García-Contreras R, Ogawa HI (2011) Purification and characterization of a serine protease secreted by Brevibacillus sp. KH3 for reducing waste activated sludge and biofilm formation. Bioresour Technol 102:10650–10656
Spangler SK, Jacobs MR, Appelbaum PC (1994) Effect of CO2 on susceptibilities of anaerobes to erythromycin, azithromycin, clarithromycin and roxithromycin. Antimicrob Agents Chemother 38:211–216
Jiang S, Chen Y, Zhou Q (2007) Effect of sodium dodecyl sulfate on waste activated sludge hydrolysis and acidification. Chem Eng J 132:311–317
Chen Y, Jiang S, Yuan H, Zhou Q, Gu G (2007) Hydrolysis and acidification of waste activated sludge at different pHs. Water Res 41:683–689
McInerney MJ, Bryant MP, Hespell RB, Costerton JW (1981) Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl Environ Microbiol 41:1029–1039
Arai T, Kosugi A, Chan H, Koukiekolo R, Yukawa H, Inui M, Doi RH (2006) Properties of cellulosomal family 9 cellulases from Clostridium cellulovorans. Appl Microbiol Biotechnol 71:654–660
Liou JSC, Balkwill DL, Drake GR, Tanner RS (2005) Clostridium carboxidivorans sp nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp nov. Int J Syst Evol Microbiol 55:2085–2091
Sturges WS, Drake ET (1927) A complete description of Clostridium putrefaciens (McBryde). J Bacteriol 14:175–179
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology (UK) 148:257–266
Acknowledgments
The authors would like to thank the Japan Student Services Organization for the scholarship of M. T. Nguyen during this study. This research was supported by the Research Center for Advanced Eco-fitting Technology of Kyushu Institute of Technology and the City of Kitakyushu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, M.T., Maeda, T., Mohd Yusoff, M.Z. et al. Effect of azithromycin on enhancement of methane production from waste activated sludge. J Ind Microbiol Biotechnol 41, 1051–1059 (2014). https://doi.org/10.1007/s10295-014-1446-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1446-z