Abstract
With the purpose of generating a microbial strain for l-ornithine production in Corynebacterium glutamicum, genes involved in the central carbon metabolism were inactivated so as to modulate the intracellular level of NADPH, and to evaluate their effects on l-ornithine production in C. glutamicum. Upon inactivation of the 6-phosphoglucoisomerase gene (pgi) in a C. glutamicum strain, the concomitant increase in intracellular NADPH concentrations from 2.55 to 5.75 mmol g−1 (dry cell weight) was accompanied by reduced growth rate and l-ornithine production, suggesting that l-ornithine production is not solely limited by NADPH availability. In contrast, inactivation of the gluconate kinase gene (gntK) led to a 51.8 % increase in intracellular NADPH concentration, which resulted in a 49.9 % increase in l-ornithine production. These results indicate that excess NADPH is not necessarily rate-limiting, but is required for increased l-ornithine production in C. glutamicum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corynebacterium glutamicum has long since been a subject of interest for the industrial production of amino acids, mainly l-glutamate and l-lysine. Various genetic manipulations have been applied to C. glutamicum to successfully develop amino acid producing strains and the metabolic consequences of genetic manipulations have been studied.
In particular, the close connection between l-lysine production and carbon flux through pentose phosphate pathway (PPP) is crucial for improving l-lysine production in C. glutamicum [2, 9, 14, 15, 18]. The relationship between a metabolite production and NADPH regeneration has been well established for l-lysine biosynthesis in C. glutamicum [2, 15, 18, 23]. NADPH is generated predominantly by the oxidative part of the PPP enzymes, glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGD), during l-lysine production in this organism [13, 23]. Diverse metabolic flux studies have revealed a correlation between l-lysine production and the NADPH supplied by carbon flux through the PPP [2, 23]. There is much evidence to indicate that redirection of the carbon flux towards PPP is a general target for biosynthesis of the desired metabolites for which NADPH supply is necessary [2, 15, 16, 18, 20].
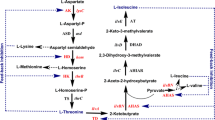
We developed an understanding of the metabolism, and identified rational targets to optimize l-ornithine production in C. glutamicum [10, 11]. l-ornithine is biosynthesized from the precursor l-glutamate by the so-called cyclic pathway in C. glutamicum [6], in which the third step is catalyzed by NADPH-dependent reductase encoded by the argC gene (Fig. 1). Previous work in our laboratory demonstrated that the homologous expression of NCgl1469 ORF exhibiting n-acetyl glutamate synthase activity in a mutant strain, which carries gene-disruptions in citrulline and proline biosynthesis and is blocked in the feedback repression by the arginine repressor (ArgR), resulted in a marginal increase of l-ornithine production [10]. However, a sufficient supply of NADPH may be required to maintain a maximal carbon flux toward the l-ornithine biosynthetic pathway, in addition to overexpressing the NCgl1469 ORF. In the present study, the carbon flux of the oxidative PPP, which is regulated by changes in specific G6PDH and 6PGD activities [22] and is responsible for NADPH regeneration, was genetically engineered so as to investigate the possibility that intracellular availability of NADPH might be necessary for l-ornithine biosynthesis.
Materials and methods
Bacterial strains and growth conditions
The wild-type C. glutamicum strain utilized in this study was C. glutamicum SJ8039 (C. glutamicum ATCC 13032, argFΔ, argRΔ) [10] and was employed as the parent strain for constructing the mutant strains used in this study. Shake flask cultures were prepared for testing the effects of mutagenesis on l-ornithine production. For the l-ornithine production experiments, a seed culture was prepared by inoculating cells into recovery glucose medium (80 g brain heart infusion, 20 g glucose, and 60 g sorbitol l−1) followed by growing the cells overnight. Cells were harvested, washed, and resuspended in 10 ml of CGI medium [0.8 g KH2PO4, 10 g (NH4)2SO4, 1 g MgSO4·7H2O, 1.2 g Na2HPO4, 2 mg MnSO4·H2O, 2 mg FeSO4·7H2O, 1 mg ZnSO4·7H2O, 10 g yeast extract, 20 g CaCO3, and 60 g glucose l−1] in a 100-ml baffled flask to an OD600 of 0.4–0.5, and grown for 20 h. All cultures were grown at 30 °C and 200 rpm on a rotary shaker, and samples were withdrawn at regular intervals to measure l-ornithine and biomass concentrations.
Site-specific gene disruption
The bacterial strains and plasmids constructed for this study are listed in Table 1. The oligonucleotide sequences utilized in this study are also provided in Table 1. Site-specific gene disruption was conducted using the nonreplicable integration vector, pK18mobsacB, which allows for the marker-free deletion of the target gene [21]. pK18mobSacB integration vectors, harboring the internally deleted pgi and gntK genes, were constructed to create the gene-disrupted mutant strains (Table 1). These recombinant plasmids were introduced into the wild-type C. glutamicum strain via electroporation, and the gene-disrupted mutant strains were created via a method described previously by Yoon and Cho [24]. The locus tag numbers of the DNA sequences reported in this study are NCgl0817, NCgl2399, and NCgl2905.
Enzyme assays
Corynebacterium glutamicum cells were grown in CGI media, harvested by centrifugation during the exponential phase, and washed in 100 mM Tris/HCl buffer (pH 7.5). The cells were disrupted using glass beads, and the resulting homogenate was centrifuged to obtain a crude extract. All treatments were performed at 4 °C, and the supernatant was used immediately for enzyme assay. Protein quantity was determined by the Bradford method [4]. Activities of G6PDH, 6PGD, and glucose dehydrogenase (GD) in crude cell extracts were measured by spectrophotometric determination of NADPH formation at 340 nm, as described previously [1, 8]. Gluconate kinase (GntK) activity was measured in crude cell extracts using the coupled enzymatic assay of 6PGD as described previously [8].
Analytical methods
Cell growth in the CGI broth was estimated at OD600 using spectrophotometry, and l-ornithine concentrations (g l−1 culture medium) were determined using an Agilent 1100 series HPLC (Agilent Technologies, Palo Alto, CA, USA) and a Zorbax Eclipse C18 column. Dry cell weight (DCW) was estimated by correlating OD600 values with 0.3 g DCW l−1 [3]. NADPH concentrations were determined by the enzymatic cycling reaction using the EnzyChrom NADP+/NADPH Assay kit (BioAssay Systems, Hayward, CA, USA).
Results and discussion
Evaluation of the pgi mutant strain
Redirecting carbon flux to the PPP by disrupting the pgi gene might be successful in increasing the formation of metabolites for which intracellular NADPH supply is of critical importance [15, 16]. Therefore, a simple metabolic engineering strategy to redirect all carbon flux through the oxidative PPP by blocking the entry of carbon into the Embden–Meyerhof–Parnas (EMP) pathway was tested by disrupting the pgi gene in C. glutamicum SJ8039. When glucose was used as the carbon source, the pgi-disrupted mutant strain showed an increased intracellular NADPH concentration in comparison with that in the parent strain (Table 2). It can be assumed that the pgi-disrupted mutant strain overproduced NADPH, as all carbon flux had to be channeled through the PPP. However, a significantly reduced growth rate was observed in the pgi-disrupted mutant strain, a phenomenon that has been reported previously for pgi-disrupted mutants of other microbial strains of C. glutamicum [15], Escherichia coli [5], and Saccharomyces cerevisiae [7].
We next examined how this mutation influences G6PDH and 6PGD activity, as the reduced growth rate in the pgi-disrupted mutant strain may have been limited by the activities of these enzymes (Table 3). Interestingly, higher 6PGD activity was observed for the pgi mutant strain grown on glucose, as compared with that of the parent strain, indicating that blocking the EMP pathway affected carbon metabolism at the level of enzyme expression. However, no discernible change in G6PDH activity was observed. This finding agreed with previous results showing that regulation of the oxidative part of the PPP of C. glutamicum mainly occurs by 6PGD [17]. C. glutamicum may have another route for biosynthesizing 6-phosphogluconate (Fig. 2), which is an intermediate of the oxidative PPP, i.e., the gluconate bypass that is typically found in Pseudomonas [12] and Bacillus [19, 25]. GD and GntK enzyme activities were determined in cell-free extracts of the parent and mutant strains to identify the physiological function of the enzymes in the gluconate bypass. The parent strain used in this study exhibited both GD and GntK activities (Table 3). More interestingly, a significant increase in GD and GntK activities was observed in the pgi-disrupted mutant strain, suggesting that glucose can also be catabolized, at least partly, through the gluconate bypass to produce a substantial excess of 6-phosphogluconate and NADPH. This pattern of induction is consistent with a key role of the 6-phosphogluconate active with NADP+ to produce the reducing power required for biosynthetic reactions.
Metabolic schematic of glucose and gluconate metabolism: a PEP:glucose phosphotransferase system; b phosphoglucose isomerase (pgi); c glucose dehydrogenase (gdh); d glucose 6-phosphophate dehydrogenase (zwf); e gluconate kinase (gntK); f 6-phosphogluconate dehydrogenase (gndA). Dotted arrows (① and ②) represent possible routes for gluconolactonase and 6-phosphogluconolactonase, respectively, but no genes are known to date
The growth rate of the pgi mutant strain on glucose was shown to be decreased significantly. The simplest interpretation might be that increased formation of gluconate from glucose, due to the increased GD activity in the pgi mutant strain, derepressed gntK expression and repressed glucose transport, as reported previously [8], resulting in the reduced growth rate of the mutant strain on glucose. Consistent with this speculation, we found that the pgi mutant strain showed a reduced growth rate on glucose but not on sucrose/fructose (data not shown). The differences between the carbon sources are most likely a consequence of different cellular activities of central carbon metabolism. However, the production of excess NADPH in the pgi mutant strain did not result in an increase in l-ornithine production. These results indicate that redirecting carbon flux through the PPP and gluconate bypass led to a dramatic increase in intracellular NADPH supply, which was not necessarily rate-limiting for l-ornithine production.
Effects of GntK mutation on l-ornithine production
An alternative attempt to maximize carbon flux through the oxidative PPP was targeted to increase intracellular NADPH supply with balanced growth by disrupting the putative GntK genes (NCgl2399 and Ncgl2905 ORFs) in the parent strain, resulting in the creation of an in-frame double deletion mutant strain of the NCgl2399 and NCgl2905 ORFs. GntK enzyme activities in cell-free extracts of the parent and double deletion mutant strains were determined. However, the specific enzyme activity assay showed residual GntK activity of 22 % by the parent strain in the double deletion mutant strain. The low residual activity in the specific GntK enzyme activity assay with the double deletion mutant strain might be due to a sugar kinase activity, which retains the ability to catalyze identical biochemical reactions on numerous substrates in vitro.
A significant increase in intracellular NADPH concentration and l-ornithine production was observed for the double-deletion mutant strain, with a slower growth rate when the double-deletion mutant and parent strains were compared. We determined the specific activities of G6PDH and 6PGD in cell-free extracts of the double-deletion mutant and parent strain to elucidate the underlying mechanism for the increased NADPH and l-ornithine production by the double-deletion mutant strain. As shown in Table 3, the specific activity of 6PGD increased significantly in the double-deletion mutant strain, as compared with that in the parent strain. The double-deletion mutant strain appeared to compensate for loss of operation of the gluconate bypass, by expanding the PPP to provide the 6-phosphogluconate required for formation of the reducing power. The observation that the parent strain, but not the mutant strain, was able to grow normally on gluconate (data not shown), confirmed that the GntK activity encoded by NCgl2399 and NCgl2905 ORFs represented the active route for 6-phosphogluconate formation. The apparent increase in l-ornithine production, resulting from blocking GntK activity, can be interpreted by an effect of increased NADPH regeneration by the stimulated 6-phosphogluconate formation via the oxidative PPP with a concomitant reduction of 6-phosphogluconate formation through GntK activity. The reduced growth rate of the double deletion mutant strain could be due to increased gluconate accumulation in the cell that repressed glucose transport, as described previously [8].
Based on these results, the mechanisms of increased l-ornithine production from glucose in the double-deletion mutant strain can be proposed. The increased capacity to regenerate NADPH with balanced growth by the increased level of 6PGD was responsible for the increase in l-ornithine production from C. glutamicum. The finding that l-ornithine production was improved by redirecting the carbon flux through the PPP only when the NADPH supply was adequate in the double-deletion mutant strain, but not in the pgi mutant strain, suggests that the whole metabolome is influenced in such a way that the pools of redox cofactors are more favorable for those biosynthetic pathways that depend on NADPH as a cofactor. The importance of reducing power in l-lysine production from C. glutamicum was also observed through the over-expression of the zwf gene [2]. G6PDH, along with 6PGD, are allosterically regulated in C. glutamicum [17]. Thus, it is likely that deregulation of this enzyme in the GntK-deficient mutant strain might lead to a larger effect on l-ornithine production. The present results have clearly demonstrated the potential of targeted inactivation of the gluconate bypass to significantly extend the performance of existing production strains, including l-arginine, l-lysine and l-threonine, as redirecting the carbon flux towards the PPP is desirable for a sufficient NADPH supply to produce those metabolites. In future studies, metabolic flux analysis methods will be used to quantitatively investigate the complex responses of the metabolic network to disruption of the pgi or gntK genes in C. glutamicum.
References
Anderson WB, Nordlie RC (1968) Glucose dehydrogenase activity of yeast glucose 6-phosphate dehydrogenase. I. Selective stimulation by bicarbonate, phosphate, and sulfate. Biochemistry 7:1479–1485
Becker J, Klopproggr C, Herold A, Zelder O, Bolten CJ, Wittmann C (2007) Metabolic flux engineering of l-lysine production in Corynebacterium glutamicum over expression and modification of G6P dehydrogenase. J Biotechnol 132:99–109
Blombach B, Schreiner ME, Moch M, Oldiges M, Eikmanns BJ (2007) Effect of pyruvate dehydrogenase complex deficiency on l-lysine production with Corynebacterium glutamicum. Appl Microbiol Biotechnol 76:615–623
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Canonaco F, Hess TA, Heri S, Wang T, Szyperski T, Sauer U (2001) Metabolic flux response to phosphoglucose isomerase knock-out in Escherichia coli and impact of overexpression of the soluble transhydrogenase UdhA. FEMS Microbiol Lett 204:247–252
Cunin R, Glansdorff N, Pierard A, Stalon V (1986) Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev 50:314–352
Fiaux J, Çakar P, Sonderegger M, Wüthrich K, Szyperski T, Sauer U (2003) Metabolic-flux profiling of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Eukaryot Cell 2:170–180
Frunzke J, Engels V, Hasenbein S, Gätgens C, Bott M (2008) Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol Microbiol 67:305–322
Georgi T, Rittmann D, Wendisch VF (2005) Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphate. Metab Eng 7:291–301
Hwang J-H, Hwang G-H, Cho J-Y (2008) Effect of increased glutamate availability on l-ornithine production in Corynebacterium glutamicum. J Microbiol Biotechnol 18:704–710
Hwang G-H, Cho J-Y (2010) Identification of a suppressor gene for the arginine-auxotrophic argJ mutation in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 37:1131–1136
Lessie TG, Phibbs PV Jr (1984) Alternative pathways of carbohydrate utilization in pseudomonads. Ann Rev Microbiol 38:359–387
Marx A, Striegel K, de Graaf AA, Sahm H, Eggeling L (1997) Response of the central metabolism of Corynebacterium glutamicum to different flux burdens. Biotechnol Bioeng 56:168–180
Marx A, Eikmanns BJ, Sahm H, de Graaf AA, Eggeling L (1999) Response of the central metabolism in Corynebacterium glutamicum to the use of an NADH-dependent glutamate dehydrogenase. Metab Eng 1:35–48
Marx A, Hans S, Möckel B, Bathe B, de Graaf AA, McCormack AC, Stapleton C, Burke K, O’Donohue M, Dunican LK (2003) Metabolic phenotype of phosphoglucose isomerase mutants of Corynebacterium glutamicum. J Biotechnol 104:185–197
Mascarenhas D, Ashworth DJ, Chen CS (1991) Deletion of pgi alters tryptophan biosynthesis in a genetically engineered strain of Escherichia coli. Appl Environ Microbiol 57:2995–2999
Mortiz B, Striegel K, de Graaf AA, Sahm H (2000) Kinetic properties of the glucose 6-phosphate and 6-phosphogluconate dehydrogenases from Corynebacterium glutamicum and their application for predicting pentose phosphate pathway flux in vivo. Eur J Biochem 267:3442–3452
Ohnishi J, Katahira R, Mitsuhashi S, Kakita S, Ikeda M (2005) A novel gnd mutation leading to increased l-lysine production in Corynebacterium glutamicum. FEMS Microbiol Lett 242:265–274
Otani M, Ihara N, Umezawa C, Sano K (1986) Predominance of gluconate formation from glucose during germination of Bacillus megaterium QM B1551 spores. J Bacteriol 167:148–152
Sauer U, Hatzimanikatis V, Hohmann HP, Manneberg M, van Loon APGM, Bailey JE (1996) Physiology and metabolic fluxes of wild-type and riboflavin-producing Bacillus subtilis. Appl Environ Microbiol 62:3687–3696
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined selections in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Vallino J, Stephanopoulos G (1994) Carbon flux distributions at the glucose 6-phosphate branch point in Corynebacterium glutamicum during lysine production. Biotechnol Prog 10:327–334
Wittmann C, Heinzle E (2002) Genealogy profiling through strain improvement by using metabolic network analysis: metabolic flux genealogy of several generations of lysine producing corynebacteria. Appl Environ Microbiol 68:5843–5849
Yoon K-H, Cho J-Y (2007) Transcriptional analysis of the gum gene cluster from Xanthomonas oryzae pathovar oryzae. Biotechnol Lett 29:95–103
Zamboni N, Fischer E, Laudert D, Aymerich S, Hohmann H-P, Sauer U (2004) The Bacillus subtilis yqjI gene encodes the NADP+-dependent 6-P-gluconate dehydrogenase in the pentose phosphate pathway. J Bacteriol 186:4528–4534
Acknowledgments
This study was supported by the Advanced R&D supporting business between industry and University funded by the Small and Medium Business Administration, Republic of Korea, a grant from the Next-Generation BioGreen 21 Program (no. PJ0080992011), Rural Development Administration, Republic of Korea, and, in part, by the Sangji University Research Fund 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, GH., Cho, JY. Implication of gluconate kinase activity in l-ornithine biosynthesis in Corynebacterium glutamicum . J Ind Microbiol Biotechnol 39, 1869–1874 (2012). https://doi.org/10.1007/s10295-012-1197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1197-7