Abstract
Agriculture faces serious constraints due to drought and salinity. Soils under these pressures can result in low productivity, decreasing the farmlands area and a negative impact on the food security. In this context, the cyanobacteria (blue-green algae) can be considered important microorganisms. They are commonly found in rice fields and agricultural soils where they perform important ecological functions. Cyanobacteria improve the soil fertility and productivity of crops through the fixation of atmospheric nitrogen, phosphate solubilization and release of nutrients. Several cyanobacteria also secrete biologically active compounds such as phytohormones, amino acids, polysaccharides and vitamins which help in plant growth promotion. Studies have shown the potential of these compounds in the alleviation of abiotic stress in crop plants. Through an array of physiological, biochemical and molecular mechanisms, the cyanobacteria improve plant growth and development. Therefore, mitigation strategies using cyanobacteria are important in combating the drought and salinity stress. This article discussed the possible outcomes of employing cyanobacteria to regulate the growth and development of plants as an effective way to overcome the drought and salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world population is increasing rapidly and is expected to be about 9 billion by 2050 (Zhang et al. 2015). This will be accompanied by an increase in incidences of land degradation, abiotic stresses and global climate change. All these factors make it more difficult to achieve the agricultural productivity required. Drought stress is considered as one of the most serious threats to agriculture as it can adversely affect agricultural production. Lau and Lennon (2012) observed that long duration drought and extremely dry events are occurring more often due to changes in the mean global temperature and pattern of precipitation. The drought stress creates serious plant developmental problems in more than 50% of the arable lands (Vinocur and Altman 2005; Kasim et al. 2013). Similarly, salinity is also one of the major abiotic stresses for agriculture worldwide. Increasing concentration of salts in the soil leads to wide range of perturbations due to osmotic stress and accumulation of Na+ and Cl− ions in plants (Deinlein et al. 2014). Severe reduction in crop production and economic losses due to soil salinization have been reported (Qadir et al. 2014). Drought and salinity also may pose a severe threat to soil microorganisms causing a decline in their population (Lan et al. 2010). This effect is also observed in cyanobacteria that are key components in ecosystems, playing significant role in the biogeochemical cycles involving carbon and nitrogen (Stal 2007). Therefore, the threat due to abiotic stressors such as drought and salinity is a serious concern for modern agriculture due to decline in the population of microorganisms.
Farming is difficult in land suffering from drought and salinity. Therefore, reclamation of drought and salt affected soil is important in restoring the fertility and productivity of soils. Land reclamation can be achieved by different physical, chemical and biological means. For example, Luo et al. (2018) employed vegetation systems to reclaim saline soils and reclamation of saline soil through organic amendments was attempted by Saifullah et al. (2018). Several chemicals have also been used to reclaim desertified soils (Yang et al. 2007; Lee et al. 2011; Li et al. 2011). To cope with drought stress, resource management practices, shifting of crop calendars and development of drought tolerant varieties have been attempted (Venkateswarlu and Shanker 2009). Drought management involving plant breeding and growth systems take time and is expensive (Evseeva et al. 2019). Recent investigations reveal that microorganisms can help plants to overcome drought and salinity stress. The rhizosphere is where rhizosphere-associated microorganisms perform diverse metabolic activities to enhance soil fertility and plant growth (Dennis et al. 2010). Mustafa et al. (2019) observed the role of beneficial microbes that help plants to cope with drought conditions. Recently, Vurukonda et al. (2016) reviewed the enhancement of drought stress tolerance in plants through the application of plant growth promoting bacteria that colonize the rhizosphere and benefit root growth. Therefore, microbe assisted/mediated technologies are considered to be better options to minimize stress as these techniques are less time consuming and cost effective.
Cyanobacteria are important constituents of the soil microbial community. Some cyanobacteria fix atmospheric nitrogen and release it to soil (Issa et al. 2014). In addition to nitrogen fixation, the ability of cyanobacteria to solubilize phosphate has been reported (Latha et al. 1992). Excretion of phytohormones such as auxins and gibberellins, vitamins and amino acids by the cyanobacteria has been shown to have positive consequences on the growth and development of plants (Roger and Reynaud 1982; Rodríguez et al. 2006; Seyed et al. 2012; Shariatmadari et al. 2013). The processes of nitrogen fixation and production and excretion of metabolites play an important role in plant growth and development. Lau and Lennon (2011) observed that the microbial population below the ground level influenced plant traits through mitigation of the effects of abiotic stress. However, most of the work carried out on cyanobacteria is regarding their biofertilizer potential and very little attention has been paid to elucidate the beneficial role of cyanobacteria on crop plants. Therefore, the present review is aimed to highlight cyanobacteria-mediated plant growth and regulation including abiotic stress mitigation in crop plants.
Cyanobacteria and their diversity
Cyanobacteria, also known as blue-green algae are Gram negative prokaryotes that grow under photoautotrophic conditions. They are primary producers of the ecosystem and play a key role in shaping the microbial diversity and community structure (Yang et al. 2016). Their structural organization ranges from simple unicellular forms to complex filamentous forms that possess a variety of highly differentiated cell types, but they reproduce by simple vegetative means. Among the filamentous forms, some are capable of simple branching whereas some are multicellular and truly branching. Several filamentous forms exhibit cellular differentiation into vegetative cells and specialized cells known as heterocysts having the ability to fix atmospheric nitrogen (Waterbury 2006). The vegetative cells perform photosynthesis whereas nitrogen fixation is performed by the heterocysts. The cyanobacteria also can thrive in extreme environments such as deserts (Rossi et al. 2017; Roncero-Ramos et al. 2019). Recently, Gaysina et al. (2019) reviewed the diversity of cyanobacteria from different climatic zones such as temperate, tropical and polar regions. Another interesting aspect of the cyanobacteria is their ability to establish symbiotic associations with a wide range of hosts (Adams et al. 2013). The host in the symbiotic association provides a unique environment and nutrition to the cyanobacteria whereas the cyanobacteria supply fixed nitrogen to the host plants. Symbiotic association between cyanobacteria and fungi (Tschermak-Woess 1988), bryophytes (Meeks 2003), pteridophytes (Plazinski et al. 1990), gymnosperms (Costa and Lindblad 2002) and angiosperms (Bergman 2002) are well known. Epiphytic growth of cyanobacteria also has been observed on the bark of trees and leaves (Rigonato et al. 2012; Ambika and Krishnamurthy 2019). The cyanobacteria are clearly versatile organisms and therefore have been exploited in research for agricultural, medicinal and industrial applications.
Cyanobacteria are divided into five orders (Rippka et al. 1979). The Chroococcales consists of unicellular or colonial forms, pseudo-filaments without trichomes and which reproduce by binary fission. Unicellular or colonial lithophytes, epiphytes or epilithic exhibiting polarity and reproduce by endospores and exospores are placed in the order Chaemosiphonales. Cyanobacterial forms which are heterotrichous, filamentous without heterocyst and which reproduce by endospores belong to the order Pleurocapsales. The Nostocales are characterized by non-heterotrichous and heterocystous filamentous forms often showing false branching and reproduction by hormogonia, hormocysts, planococci and akinetes. The Stigonematales consists of heterotrichous filamentous forms mostly with heterocysts and reproduce by akinites. The rich biodiversity of the cyanobacteria in various environments such as marine waters, brackish waters, soda lakes, deserts, hypersaline environments, polar regions, fresh waters, paddy fields and soil has been described by Thajuddin and Subramaniyan (2005). Dash et al. (2017a) also have reported the dominance of unicellular cyanobacterial forms such as Aphanothece sp. This widespread colonization is promoted by the availability of nutrients, water, high level of carbon and the ideal temperature (Kondo and Yasuda 2003). Diversity studies conducted in the rice fields of Eastern Uttar Pradesh, India revealed the presence of diverse forms of cyanobacteria and the majority belonging to the order Nostocales (Srivastava et al. 2009). Bhatnagar et al. (2008) reported the dominance of heterocystous and non heterocystous cyanobacterial strains such as Phormidium, Oscillatoria, Lyngbya, Nostoc, Scytonema and Calothrix in Thar desert, India. Culture-dependent studies conducted by Patel et al. (2019) on the dry mats collected from the subtropical region of Rann of Kutch suggested the dominance of unicellular forms such as Euthece and Halothece sp. Desert crusts from Utah’s Colorado Plateau showed significant differences in the community structure of cyanobacteria (Garcia-Pichell et al. 2001). Several other studies conducted in Mexico and USA showed the dominance of similar cyanobacterial forms in desert crusts (Fernandes et al. 2018). Cyanobacteria have also been observed in the phyllosphere region of rice plants (Dhankar et al. 2021). Venkatachalam et al. (2016) reported the presence of several diverse forms of unicellular and filamentous non-heterocystous cyanobacteria in the phyllosphere region of rice (Pusa Basmati cv. 1509) having a role in plant growth promotion. They constitute one of the dominant microbial communities in the rice fields and in the tropical paddy field ecosystem, a huge population of diazotrophic cyanobacteria such as Anabaena, Nostoc, Tolypothrix and Aulosira has been reported (Dash et al. 2016; Dash et al. 2017b).
Cyanobacteria exhibit plant promoting traits
In general, compounds produced by cyanobacteria are important for the growth and development of plants. The ubiquitous presence of cyanobacteria in the soil rhizosphere of a number of crops is therefore important in relation to plant growth and development. An array of metabolites produced by the cyanobacteria can also help in the regulation of plant growth under abiotic stress conditions induced by drought and salinity. Since use of conventional technologies alone on the mitigation of abiotic stress has not been successful, the exploitation of beneficial cyanobacteria in the crop rhizosphere can also be considered an important strategy in the alleviation and mitigation of abiotic stresses such as drought and salinity. The cyanobacteria have several unique attributes which confer them the potential to be employed as bio-inoculants for the mitigation of salinity/drought in plants (Table 1).
Katoh et al. (2012) observed that the enhancement in growth and rooting of several vegetable plants was due to germination promoting compounds released by the cyanobacteria. Apart from enriching the nutrient content of the soil, cyanobacteria have been reported to release various biologically active compounds extracellularly which act as signaling molecules in plant growth promotion. These compounds consist of phytohormones, proteins, vitamins, amino acids carbohydrates and polysaccharides (Rodgers et al. 1979; Selykh and Semenova 2000; Sergeeva et al. 2002; Hussain et al. 2013; Delattre et al. 2016; Singh et al. 2017). Cyanobacteria have shown the ability to accumulate and secrete phytohormones. Endogenous indole-3-acetic acid (IAA) production has been detected in cyanobacteria (Mazhar et al. 2013). In rice, inoculation of the cyanobacteria enhanced the IAA levels and increased the salinity tolerance (Singh et al. 2011). Phytohormones such as indole-3 acetic acid (IAA), indole-3 butyric acid (IBA) along with free volatile fatty acids from the wet cyanobacterial biomass promoted the plant growth in cucumber (Gayathri et al. 2017). In general, phytohormone production has been reported mostly from cyanobacteria isolated from crop fields. However, Arthrospira platensis, which is not a common inhabitant of agricultural fields exhibit the potential of IAA production and enhanced the root and plant growth (Mehboob et al. 2010). Through the excretion of phytohormones, the microbes can change the endogenous hormone balance of the host plants and promote plant cell division, growth and nutrient release (Glick et al. 1999). High performance liquid chromatography (HPLC) of the extracts of cyanobacterial species such as Nostoc carneum, Nostoc punctiforme and Wollea vaginicola showed their ability to produce Indole-3-acetic acid, Indole-3-propionic acid and Indole-3-butyric acid and a significant and positive correlation was observed between these hormones and growth of Matricaria chamomilla plants (Zarezadeh et al. 2020). In addition to auxins, cyanobacteria also are known to produce cytokinins. Osman et al. (2010) reported the occurrence of cytokinin production from several cyanobacterial strains. Cyanobacterial species such as Oscillatoria angustissima, Cylindrospermum sp. and Anabaenopsis sp. have been reported to produce gibberellin-like substances (Tsavkelova et al. 2006). Yang et al. (2009) observed that the phytohormones are able to enhance the resistance of the plants to environmental stresses. Further, the role of phytohormones in the induction of genes for the synthesis of enzymes, pigments and metabolites have been reported (Bari and Jones 2009; Shan et al. 2012). Singh (2014) highlighted the positive role of signalling molecules produced by the cyanobacteria in bringing about qualitative and quantitative changes in the phytochemical composition of plants. The phytohormone, abscisic acid (ABA) acts as a signalling molecule and plays an important role in plant response to abiotic stresses. Hartung (2010) reported the production of abscisic acid (ABA) in cyanobacteria exposed to stress conditions induced by salinity. Signalling molecules such as jasmonates and their derivatives are also important in the regulation of several physiological processes related to growth, development and abiotic stress tolerance in plants (Kazan 2015). Jasmonic acid production has been reported in several cyanobacteria such as Anabaena, Calothrix, Cylindrospermum, Nostoc, Scytonema, Spirulina and Synechococcus sp. (Ueda et al. 1991; Tsavkelova et al. 2006). However, Han (2017) reported that further studies are needed to confirm the presence of jasmonates in cyanobacteria. Excretion of phytohormones such as auxins and gibberellins by the cyanobacteria has been reported to have positive consequences on the growth and development of plants (Rodríguez et al. 2006; Hussain and Hasnain 2011; Gayathri et al. 2015). The role of signaling molecules produced by cyanobacteria is therefore crucial in the management of abiotic stresses.
The cyanobacterial exudates have also been reported to contain amino acids (Karthikeyan et al. 2007), vitamins (Borowitzka 1988) and antibiotics (Gromov et al. 1991) which aid in plant growth promotion. The cellular as well as extracellular products of Hapalosiphon fontinalis and Nostoc muscorum increased the length of coleoptiles and radicle of rice seedlings due to the presence of amino acids (Misra and Kaushik 1989a, b). Another important secondary metabolite of cyanobacterial origin are the peptides and their constituent amino acids (Sivonen et al. 2010). These peptides are synthesized by non-ribosomal peptide synthetase (NRPs), polyketides (PK) or hybrid non-ribosomal peptide synthetase/polyketide synthase pathways (Kehr et al. 2011). The structural elucidation of a number of cyanobacterial peptides has been carried out (Welker and Dӧhren 2006; Fidor et al. 2019). Filtrates obtained from N. muscorum enhanced the growth and development of crops probably due to amino acids and peptides (Adam 1999). Foliar application of cyanobacteria as biofertilizer enhanced the photosynthetic performance and growth of Salix viminalis plants (Grzesik et al. 2017).
Vitamins also play a significant role in the growth and development of plants and the cyanobacteria are known to be a rich source of vitamins (Abed et al. 2009). In rice seedlings, enhancement in the root growth obtained with the application of extract of Calothrix muscicola was found to be at par with the results obtained with pure cyanocobalamin (Vitamin B12) and folic acid (Venkataraman and Neelakantan 1967). Several vitamins play an important role in the antioxidant defence. The antioxidant defence system of the plants consists of superoxide dismutase, catalase, peroxidase, glutathione reductase and low molecular weight compounds such as ascorbate, glutamine and tocopherols and the cyanobacterial peptides have been reported to stimulate the antioxidant defense system in plants (Chen et al. 2004; Pflugmacher et al. 2007). Under stress conditions, the vitamins such as A, C, E (α-tocopherol) and carotenoids play an important role in scavenging the reactive oxygen species (ROS) and protecting the cells against lipid peroxidation (Havaux et al. 2009; Latifi et al. 2009). Cyanobacteria are rich in pigments such as chlorophyll a, carotenoids and phycobiliproteins such as phycocyanin, phycoerythrin and allophycocyanin. Phycocyanin isolated from Arthrospira (Spirulina) platensis enhanced the accumulation of anthocyanin pigment in plants (Rao et al. 1996). Anthocyanin is important in protecting the plants against various abiotic stresses partially due to their powerful antioxidant properties (Ahmed et al. 2014).
Cyanobacterial polysaccharides are present as a mucilaginous external layer around the cell. Ultrastructure and chemical composition of the extracellular sheath in Phormidium uncinatum has been studied by Hoiczyk (1998). A number of cyanobacterial species, ranging from unicellular to filamentous and hetrocystous to non-heterocystous forms are known to release soluble polysaccharides into the environment during their growth. Nisha et al. (2007) reported the production of exopolysaccharides (EPS) by several strains of cyanobacteria. The sheath is composed of exopolysaccharide fractions with complex monosaccharide composition and is comprised of proteins, lipids, nucleic acids and secondary metabolites (De Philippis and Vincenzini 1998; Delattre et al. 2016). Although, glucose is the most common monosaccharide, in some cyanobacteria, rhamnose, xylose, arabinose, mannose, fructose and uronic acids are the most dominant monosaccharides (Rossi and De Philippis 2016). At the same time, these compounds are important in soil aggregation and maintaining water availability under desiccated environments (Malamlssa et al. 2001; Arora et al. 2010; Mugnai et al. 2018a). The dominance of cyanobacteria in extreme environments is attributed to the presence the sheath made up of extrapolymeric substances. These attributes are used for their own advantage, allowing them to survive and dominate extreme environments (Di Pippo et al. 2013). Peat and Potts (1987) observed that N. commune UTEX 584 subjected to extreme desiccation was able to survive due to the cellular organization. The extracellular sheath in Gleocapsa sp. contains photoprotective pigments which help them to survive high rates of desiccation (Garcia-Pichel and Castenholz 1993). Availability of adequate soil moisture and organic content in turn support the growth and survival of plant growth producing rhizobacteria in the plant rhizosphere. Flaibani et al. (1989) reported that exopolysaccharides from cyanobacteria also contribute to reclamation of the desert soils. EPS producing cyanobacteria along with other plant growth producing rhizobacteria may thus help in the reclamation of non-productive soils (Paul and Nair 2008). Importance of extracellular polysaccharides in Nostoc sp. in alleviating the salinity stress has been highlighted by Yoshimura et al. (2012). The extracellular polysaccharides released by the cyanobacteria chelates the dissociated sodium ions and thereby reduce the abundance and toxicity of these ions in the rhizosphere, making the soil suitable for the proliferation of root growth and development (Arora et al. 2010). Production of extracellular matrix and compatible solutes by cyanobacterial species such as Anabaena and Nostoc sp. was found to reduce the problem of soil salinization to a great extent (Li et al. 2019).
The supply and utilization of nitrogen is key to the successful production of rice. Venkataraman (1972) suggested algalization of rice fields in the improvement and maintenance of soil fertility. Most of the beneficial effects of cyanobacteria have been reported in rice plants due to their natural presence in rice field ecosystems (Rodgers et al. 1979; Singh et al. 1985; Fernández Valiente et al. 2000; Whitton 2000; Pereira et al. 2009; Dash et al. 2018). The flooded rice fields provide cyanobacteria a unique niche to grow due to the availability of light, water, nutrients and temperature to maintain/enhance the productivity of the rice fields (Roger et al. 1993). Seasonal and crop stage specific diversity of the cyanobacterial flora was also observed in flooded rice fields (Roger and Kulasooriya 1980; Nayak et al. 2001). Besides organic matter, the cyanobacteria can contribute about 20–30 kg N ha−1 to the paddy fields (Issa et al. 2014). Further, the excretion of organic acids and extracellular phosphatases by the cyanobacteria also contribute to the mobilization of inorganic phosphates (Rai and Sharma 2006). Death and decay of cyanobacteria leads to the generation of humus and improvement in the soil structure and fertility (Abdel-Raouf et al. 2012). Microbial biomass inclusive of cyanobacteria acts as labile sink and source of nutrients in the submerged rice field ecosystems (De Datta 1987; Mandal et al. 1998). Therefore, Choudhury and Kennedy (2004) observed that even in the absence of any fertilizer inputs, wetland rice field systems can produce a grain yield of 2–4 t ha−1. Application of the cyanobacterial biofertilizers Aulosira fertilissima (12.5 kg ha−1) and urea (90 kg ha−1) significantly increased the biomass and yield of rice (Dubey and Rai 1995). Jha and Prasad (2006) also observed increase in grain and straw yield in rice through the application of cyanobacterial biofertilizers. Kaushik et al. (2019) reported that higher yields can be ensured due to judicious application of nitrogenous fertilizers and cyanobacteria. The production cost involved in rice cultivation reduced by half when the recommended dose of chemical nitrogen was replaced by 50% with cyanobacterial biofertilizers (Chittapun et al. 2018).
Beneficial effects of cyanobacteria on crops that do not require flooded conditions have been reported (Dadhich et al. 1969; Kaushik and Venkataramanan 1979). Therefore, the ambit of cyanobacterial application has now been expanded to crops other than rice (Nain et al. 2010; Manjunath et al. 2011). Application of cyanobacteria per se as biofertilizer was found to be beneficial in wheat (Obreht et al. 1993; Gantar et al. 1995a, b). In vitro positive results were also found with cyanobacterial inoculation resulting in improvement of the plant shoot/root length, dry weight and yield (Spiller and Gunasekaran 1990; Karthikeyan et al. 2007, 2009). Similarly, their inoculation along with plant growth producing rhizobacteria resulted in the biofortification of wheat (Rana et al. 2012). Cyanobacteria-mediated plant growth promotion was observed in tomato (Prasanna et al. 2013). Recently, Prasanna et al. (2015) developed a formulation based on cyanobacteria for integrated nutrient management in maize crops. Evaluation of the interaction among maize hybrids and cyanobacterial inoculants showed positive influence due to cyanobacterial application (Prasanna et al. 2016). Because of the properties of the cyanobacteria mentioned above, attempts have been made to develop carrier based biofertilizers for effective delivery and physiological stability/effects (Venkataraman 1972; Kaushik 2004; Jha and Prasad 2006; Prasanna et al. 2013).
Maintenance of ion homeostasis is an integral component of the drought and salinity stress management. Ionic adjustments through restricted entry of sodium and its active efflux enhance the salt tolerance in cyanobacteria (Reed et al. 1984b; Apte et al. 1987). Operation of Na+/H+ antiporter mechanism helps in the efflux of sodium and maintenance of low intracellular sodium content in cyanobacteria (Elanskaya et al. 2002; Berry et al. 2003; Tsunekawa et al. 2009). Accumulation of potassium ions is required for various cellular processes and the biosynthesis of organic osmolytes is important in salinity and desiccation tolerance of cyanobacteria (Apte and Alahari 1994; Matsuda et al. 2004). Nanatani et al. (2015) observed that in Synechocystis PCC 6803, high affinity K+ uptake system helps in maintaining turgour and K+/Na+ ratio. Protection of the cell membrane system and cellular enzymes is important in response to drought and salinity. Several cyanobacteria accumulate low molecular weight organic solutes which are synthesized either de novo or accumulated from the medium. Accumulation of compatible solutes such as quarternary ammonium compounds, sugars and glycine betaine lead to osmoprotection in cyanobacteria (Erdmann 1983; Reed and Stewart 1983; Reed et al. 1984a, 1986; Borowitzka 1986; Waditee et al. 2002). Synthesis and accumulation of glucosyl glycerol as compatible solute was reported in the cyanobacterium Synechocystis PCC 6803 due to salinity (Hagemann 2011; Kirsch et al. 2019). In cyanobacteria such as Anabaena and Nostoc sp. synthesis of osmotic stress/water stress proteins conferred osmotic protection and modification of osmotic tolerance (Fulda et al. 1999). Production of reactive oxygen species (ROS) is deleterious to the cells in response to drought and salinity stress. However, increase in the antioxidant activity confers stress tolerance to the cyanobacteria. Increase in the antioxidant defense due to upregulation of level of proline, superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and glutathione reductase (GR) in Anabaena sp. resulted in enhanced halotolerance (Yadav et al. 2016). Therefore, the salt tolerance mechanism(s) employed by the cyanobacteria is important in their exploitation for the efficient management of drought and salinity stress in crop plants.
Plant growth promoting rhizobacteria and stress alleviation
The zone surrounding the plant root where the soil biological and chemical properties are influenced by the root is known as rhizosphere. Rhizospheric region of the soil is rich in nutrients due to the accumulation of plant/root exudates such as sugars and amino acids which is a source of energy for bacteria (Gray and Smith 2005). Bacteria that colonize the rhizosphere are known as rhizobacteria (Schroth and Hancock 1982). The interaction between rhizosphere and the microbes is considered to be positive, negative or neutral (Pii et al. 2015). Positive effects are observed when bacteria associated with roots are able to induce positive effects on the plant growth and fitness. Pathogenic bacteria producing metabolites with toxic effects can have detrimental effects on plant growth leading to negative interactions. However, in neutral interactions, rhizobacteria can take up the root exudates as nourishment without affecting the growth of plants (Lynch 1990; Dobbelaere et al. 2003). Bacteria belonging to diverse genera which aids in plant growth have been identified as plant growth promoting rhizobacteria (PGPR) and the most predominant being Pseudomonas, Bacillus, Enterobacter, Penibacillus, Arthrobacter and Acinetobacter etc. (Zhang et al. 2017). Plant growth promoting rhizobacteria exert beneficial effects on the growth and development of many crop plants and a number of studies have been conducted to understand the beneficial effects of these rhizobacteria (Wang et al. 2012; Mendes et al. 2013; Willis et al. 2013; Govindasamy et al. 2020). The surface of above‐ground organs of plants, especially the phyllosphere is densely populated by bacteria (Remus-Emsermann et al. 2014). Ali et al. (2009) observed that the plant growth promoting compounds produced by phyllospheric bacteria influence the growth and development of host plants. Rhizospheric bacteria capable of altering the physiological response of plants under water stress have been reported (Naylor and Coleman-Derr 2018). Complex microorganisms and microbial communities colonize the plant endosphere (Compant et al. 2021). Certain bacteria found in the plant endosphere stimulate plant growth through the production of hormones and increasing the nutrient availability resulting in resilience to abiotic stresses (Bulgarelli et al. 2013). Thus, the natural associations of microorganisms with plants residing in the phyllosphere, rhizosphere and the endosphere lead to beneficial effects such as production of hormones, increasing the nutrient availability and enhancing the water availability etc. (Oleńska et al. 2020). Root-tissue colonizing bacteria such as Ochrobactrum sp. EB-165, Microbacterium sp. EB-65, Enterobacter sp. EB-14 and Enterobacter cloacae strain EB-48 isolated from Sorghum plants showed plant growth promotion as well as induced stress tolerance (Govindasamy et al. 2020).
Bacteria belonging to different phylogenetic groups colonize the intercellular spaces and vascular tissues (Compant et al. 2010; Reinhold-Hurek and Hurek 2011). Such bacteria are known as endophytes and can have beneficial interactions with the plants and contribute significantly to the nutrient supply of their host plant (George et al. 2013). The endophytic bacterial diversity showed richness of the species and helps in the solubilization of phosphate and assimilation of nitrogen (Rosenblueth and Martínez-Romero 2006). On the other hand, in an endosymbiotic bacterial interaction, beneficial endophytic microorganisms are accommodated within a host membrane intracellularly through the development of an organized symbiotic structure and contribute significantly to the nutrient supply of their host plant and can help the plant to overcome a variety of abiotic stresses (Govindasamy et al. 2018).
Plant growth promoting rhizobacteria improve plant growth either directly or indirectly through various mechanisms. Several reports are available on the ability of these organisms to produce signalling molecules known as elicitors. The elicitors can bind to the receptor sites on plasma membrane and trigger signalling cascades leading to activation of genes that produce enzymes and proteins or the secondary metabolites (Nürnberger et al. 1994; Zhao and Sakai 2003a, b, Zhao et al. 2004, 2005). Some of these compounds produced by the plant growth producing rhizobacteria also help the plants to develop tolerance to abiotic stress such as salinity (Dodd and Perez-Alfocea 2012). Rhizobacterial and bacterial endophytes induced physical and chemical changes in plants leading to enhanced tolerance against various abiotic stresses such as drought and salinity has been observed (Dimkpa et al. 2009; Yang et al. 2009). Inoculation of rhizobacteria played a significant role in enhancing osmotic stress tolerance in Capsicum annuum (Sziderics et al. 2007). Induced systemic tolerance (IST) through rhizobacteria mediated production of elicitors such as enzymes, volatiles, phytohormones, antioxidants and exopolysaccharides have been reported (Govindasamy et al. 2011). Studies conducted by Sziderics et al. (2007) and Govindasamy et al. (2011) revealed that the application of plant growth promoting rhizobacteria induce tolerance through expression of host plant stress-responsive genes.
As highlighted before, plant growth and productivity are greatly affected by environmental stresses such as drought, high salinity and high/low temperature (Zheng et al. 2010). Therefore, the microorganisms offer an opportunity to mitigate the abiotic stress induced challenges in crop production. Only a few phylogenetic groups of rhizobia and mycorrhizal fungi had their plant beneficial effects targeted under stressed and unstressed environments. Enebe and Babalola (2018) have described the influence of plant growth promoting rhizobacteria in relation to abiotic stress tolerance and plant growth. Induction of induced systemic resistance to drought and salinity by plant growth promoting rhizobacteria was reported (Yang et al. 2009). Willis et al. (2013) highlighted the role of arbuscular mycorrhizal fungi and roots of higher plants in stressed environment. Lugtenberg et al. (2013) observed that inoculation of crop plants with beneficial microbes improved salinity tolerance and yield. A strain of Pseudomonas fluorescens isolated from the rhizosphere of the date palm from the desert region promoted root growth in Maize seedlings under salinity stress (Zerrouk et al. 2016). It was observed that the halophilic bacterium, Serratia sp. Sl-12 isolated from a salt lake improved salinity tolerance and increased the shoot biomass in wheat (Singh and Jha 2016). Thus, the application of PGPR inoculants in the agricultural fields was found to be a promising strategy to combat salinity and increase food production (Ilangumaran and Smith 2017).
Possibility of employing cyanobacteria for the management of abiotic stress in crop plants
The role of cyanobacteria in the management of abiotic stress is gaining importance. In this context, various mechanisms such as production of hormones, amino acids, vitamins, exopolysaccharides, compatible solutes and antioxidants would be important in cyanobacteria mediated drought and salinity stress tolerance in plants. Furthermore, the potential of nitrogen fixation and the ability to regulate the ion metabolism is also important in efficient stress management. The mechanisms operating in cyanobacteria and their possible role in alleviation of drought and salinity stress are important for further exploiting them in sustainable agriculture.
Drought
Diminishing availability of water poses a threat to the proliferation of cyanobacteria and in the fields, they are constantly exposed to continuous dehydration, desiccation and re-wetting. Drought tolerant cyanobacteria can be used as biofertilizer for crops such as wheat, sugar cane, cotton, sorghum, and vegetables (Abd-Alla et al. 1994). Reports are available on the desiccation tolerance of a number of cyanobacteria (Potts 1994). Cyanobacteria exhibit considerable resilience to osmotic/water stress due to their ability to regulate ion fluxes, accumulation of compatible solutes and through the expression of tolerant genes and protein synthesis (Borowitzka 1986; Fernandes et al. 1993; Apte 2001). However, it has also been observed that vital metabolic activities of cyanobacteria such as photosynthesis, respiration and nitrogen fixation revive within minutes of re-wetting (Sherer et al. 1984). In the cyanobacterium Anabaena PCC 7120, the genes involved in photosynthesis, nitrogen metabolism and protein synthesis are down-regulated during dehydration (Katoh et al. 2004). On the other hand, a remarkable capacity to repair the highly disintegrated genome and proteome was also observed in response to desiccation (Singh et al. 2013). The role of cyanobacteria is important in relation to plant growth and nutrient management under drought stress conditions. It was observed that the production of exopolysaccharides by cyanobacteria helps in enhanced retention of water, nutrients and ensure the survival of microorganisms from desiccation and nutrient limitation (Zhang 2005; Colica et al. 2014; Adessi et al. 2018). Stress priming is an adaptive strategy to enhance the stress tolerance of plants. Priming of the seeds of Senna notabilis and Acacia hilliana with cyanobacterial strains Nostoc sp. and Microcoleus sp. resulted in better germination and establishment of the plants in drought affected ecosystem (Muñoz-Rojas et al. 2018b). Chua et al. (2019) reported that bio-priming of the seeds with different cyanobacterial strains improved growth of Eucalyptus gamophylla and Grevillea wickhamii plants used in the restoration of drylands.
Biological soil crusts or biocrusts are commonly found in dry lands and it consists of several microorganisms in varying proportions (Maestre et al. 2011). These biocrusts are often found at the top soil of the terrestrial ecosystem. Lin and Wu (2014) observed that the biological soil crusts are dominated by cyanobacteria. In biocrusts, the cyanobacteria are pioneer organisms among biological communities that improve soil conditions and promote the colonization and succession of other species such as lichens and mosses (Belnap and Gardner 1993; Lan et al. 2013). Further, it has been observed that the biological crusts ensure soil water availability (Chamizo et al. 2016), soil stability (Rodríguez-Caballero et al. 2012) and nutrient cycling (Delgado-Baquerizo et al. 2013). Recently, cyanobacteria have been employed to develop biological soil crusts even under laboratory conditions (Park et al. 2017). Chamizo et al. (2018) also observed that the formation of biocrusts improves stability and fertility of different soils due to inoculation with nitrogen fixing and non-nitrogen fixing cyanobacterial species. The cyanobacterium Leptolyngbya ohadi found in the biocrust produces complex exopolysaccharides that maintain hydration, chelates nutrients and aggregates sand (Mugnai et al. 2018b). Muñoz-Rojas et al. (2018a) demonstrated the capability of cyanobacteria in the formation of biocrust and substantial increase in the total organic carbon content of the soil. Becerra-Absalón et al. (2019) reported that the common cyanobacterial strains found in the biocrusts of dry lands of Central Mexico consists of species of Scytonema hyalinum, Scytonema crispum, Nostoc sp., N. commune and Calothrix parietina. All these studies have showed the potential of cyanobacteria to survive and grow in extremely desiccated conditions. Therefore, the drought tolerant cyanobacterial strains can be employed to develop strategies for the management of drought stress. However, very little information is available regarding the cyanobacteria mediated enhancement in growth of plants from extreme environmental conditions such as drought.
Salinity
Increase in the salinity level of the soil leads to changes in the water potential and osmotic equilibrium and create “physiological dryness” in the cells. However, the cyanobacteria exhibit considerable salinity tolerance and have been found to grow in salt affected soils (Thomas and Apte 1984). Reports are available on the reclamation of salt affected soils using cyanobacteria (Roman et al. 2018). It was observed that the cyanobacteria form a thick stratum on the soil surface and conserve the organic C, N and P as well as moisture and convert the Na+ clay to Ca2+ clay and hence they are considered ideal candidates for the reclamation of usar soils which are impermeable and unproductive due to the presence of undesirable salts (Singh 1961). Significant level of salinity tolerance in the range of 7 to 15 g L−1 was observed in species such as Anabaena oscillarioides, Anabaena aphanizomenoides and Microcystis aeruginosa (Coutinho and Seeliger 1984; Moisander et al. 2002). Salinity tolerance in many cyanobacteria can be due to the operation of different types of Na+/H+ antiporters that play a key role in maintaining low sodium concentrations in the cell (Waditee et al. 2002; Wutipraditkul et al. 2005) or even, to increase the photosynthetic activity and the accumulation of compatible solutes (Joset et al. 1996; Hagemann 2011).
Inoculation of cyanobacteria was found to be beneficial in providing abiotic stress tolerance in various crop plants (Table 2). Anabaena torulosa, a brackish-water nitrogen-fixing cyanobacterium grew successfully and fixes nitrogen on moderately saline “Kharland” soils (Apte and Thomas 1997). de Caire et al. (1997) observed considerable enhancement in soluble carbon and aggregation of soils inoculated with N. muscorum. In rice seedlings, most of the salinity induced effects on growth and biochemical alterations were either partially or completely reversed by the extracellular products secreted by Scytonema hofmanni (Rodríguez et al. 2006). Cyanobacterial inoculation in rice plants stressed with heavy metals improved the growth and yield due to improvement in the mineral nutrition and reduction in metal uptake (Tripathi et al. 2008). Application of cyanobacteria not only enhanced the soil biomass but also reduced the soil salinity and suppressed the weed growth in paddy fields (Saadatnia and Riahi 2009). Singh and Singh (2015) employed the halotolerant cyanobacterium Nostoc calcicola in combination with gypsum and observed it as a better option to reclaim saline-alkaline soils. To reclaim the salt affected soils, various strategies have been used which involve chemical, physical and biological methods (Luo et al. 2018; Saifullah et al. 2018; Zhao et al. 2018). Apte and Thomas (1997) identified the potential of Anabaena torulosa in reducing the electrical conductivity (EC) of highly saline soil. Nisha et al. (2018) observed that inoculation of cyanobacteria such as Nostoc ellipsosporum HH205 and Nostoc punctiforme HH-206 improved the physico-chemical and biological properties of the soil besides promoting the yield of pearl millet and wheat. They have attributed the improvement in the organic carbon, nitrogen and other nutrients in the soil due to the autotrophic nature and nitrogen fixing potential of cyanobacteria. Furthermore, the application of cyanobacteria decreased the bulk density and enhanced the water holding capacity of the soil. Roman et al. (2018) observed that the properties of sodic soils were improved through the application of cyanobacteria such as N. commune, Scytonema hyalinum and Tolypothrix distorta. The results obtained from these studies are important in the exploitation and field application of cyanobacteria for the remediation of salt affected soils. Further, it was suggested that restoration of soil fertility and removal of salts could be achieved using combinations of salinity tolerant plants and cyanobacteria (Jesus et al. 2015). Sustained nitrogen fixation will be maintained under saline growth conditions by the application of cyanobacteria. However, the prevailing soil conditions in the stressed environments may lead to reduction in the potential of biological nitrogen fixation (Dash et al. 2018). Wang et al. (2015) observed that cyanobacterial biological nitrogen fixation is critical for the remediation of salt affected soils.
Biological methods involving cyanobacteria as potential candidates have been employed in the bioremediation of salt-affected soils (Rady et al. 2018; Roman et al. 2018). Rice plants were grown on salt stressed soil (pH 8.8, EC 5.2 dS/m) and inoculated with different strains of cyanobacteria namely Anabaena oryzae, Anabaena doliolum, Tolypothrix tenuis, Aulosira fertilissima, Calothrix geitonos, Plectonema boryanum, Phormidium fragile and Oscillatoria acuta (Singh et al. 2011). Inoculation of cyanobacteria has resulted in significant enhancement in the accumulation of phytohormones and plant growth promotion. Significant enhancement in seed germination, vigour index and nutrient mobilization efficiency was observed in rice, wheat and maize plants subjected to salinity stress and inoculated with cyanobacteria (Arora et al. 2010). Chittapun et al. (2018) also showed improvement in growth and yield in salinity stressed rice plants inoculated with cyanobacteria. Improvement in the structural characteristics and growth was observed in wheat plants due to cyanobacterial application under salinity stress conditions (Gheda and Ahmed 2015). Shariatmadari et al. (2015) observed that application of cyanobacteria in Mentha piperita exposed to salinity stress enhanced plant growth and oil content. Thus, the salinity tolerant cyanobacteria could be exploited for plant growth promotion under salt stressed environments. Evaluation of salt tolerant strains under pot experimentation and field based trials is therefore critical for their exploitation in the management of salinity stress.
Conclusions and Future perspectives
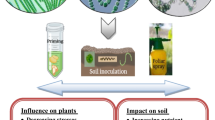
Food security is directly related to agriculture, which is a highly vulnerable sector to climate change. Drought and salinity stress conditions restrict crop growth and yield and therefore optimal growth of plants is not possible in such stressed environments. Soil health is important in the sustenance of agriculture, but climate change induced drought, salinity and other accelerated edaphic stressors pose a serious threat to the agriculture. Microbe based interventions for the reclamation of salt and drought affected soil is an important strategy in maintaining the agricultural sustainability. In the soil, large populations of microbes exist, which form a dynamic and complex ecological community and these microbial populations influence the plant growth, development and productivity through their synergistic interaction. The roots maintain its associated beneficial microbiome which can influence the plant traits positively. Such associations are further important due to their ability to promote plant growth especially under drought and salinity stress conditions. One of the most important constituents of soil microbial community is the cyanobacteria. It has been observed that inoculation of cyanobacteria improved the soil properties, microbial community dynamics that leads to further availability and uptake of nutrients by plants. Several cyanobacteria produce extracellular polysaccharides which play an important role in withholding of salt and water and help in the improvement of soil nutrient dynamics. Inoculation of cyanobacteria in the rhizosphere of drought/salinity stressed ecosystem may lead to enhancement in various soil properties and trigger plant growth through various direct and indirect mechanisms as illustrated in Fig. 1. The cyanobacteria can help plants from the adverse consequences of the stress through a range of mechanisms such as nitrogen fixation, improved microbial biomass, mobilization of nutrients, induced systemic tolerance, production of phytohormones, exopolysaccharides. vitamins, peptides, antioxidants and osmolytes. Apart from improving the soil structure, the exopolysaccharides also protect the cyanobacteria from adverse conditions and help them to survive under drought and salinity. Cyanobacteria can therefore play an important role in improving plant adaptation to drought and salinity stress. Hence, the application of cyanobacteria in the management of drought and salinity stress is gaining considerable importance. However, systematic efforts have not been undertaken to assess their drought and salinity tolerance imparting potential. In this context, it is important to isolate, screen and identify drought and salinity tolerant cyanobacterial strains from drought and salinity affected areas as potential inoculants. Several difficulties and constraints have been encountered in the mass multiplication of cyanobacteria, like poor growth and contamination during their large scale multiplication is a matter of concern which needs to be addressed. In the absence of proper delivery mechanisms, it will be difficult to enhance their field efficacy. Therefore, proper delivery mechanisms using appropriate carrier may be developed to improve the viability and efficacy of cyanobacteria based bioinoculants. Based on the information already available, the research focus must be directed towards the identification and evaluation of potential cyanobacterial strains for plant growth promotion attributes under abiotic stress environments. Besides, a detailed understanding of induced systemic tolerance mechanisms by cyanobacteria against abiotic stress factors will further drive their utility and application in agriculture to enhance the survival of plants under drought and salinity stress. Therefore, development of efficient formulations based on cyanobacteria is important in improving the soil health and plant growth in stressed environments.
Agricultural productivity is adversely affected by increasing incidences of drought and salinity stress. Cyanobacteria play an important role in plant growth promotion and furthermore the cyanobacteria found in drought and salt affected soils exhibit considerable stress tolerance and display plant growth promoting attributes. Therefore, the progress made in cyanobacterial research on the physiological, biochemical, and molecular aspects of drought and salinity tolerance will have broader applications in selecting potential strains for the mitigation of stress and management. Characterization of drought and salinity tolerant cyanobacteria is thus important in the mitigation and protection of plants from these stresses. However, their effectiveness will be further significant if they also exhibit multiple plant growth promoting traits such as phosphate solubilization, nitrogen fixation, indole acetic acid production, and siderophore production etc. Cyanobacteria mediated alleviation of drought and salinity stress in plants needs further research and field trials.
References
Abd-Alla MH, Mahmoud ALE, Issa AA (1994) Cyanobacterial biofertilizer improves growth of wheat. Phyton 34:11–18
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IB (2012) Agricultural importance of algae. Afr J Biotechnol 11:11648–11658
Abed RM, Dobretsov S, Sudesh K (2009) Applications of cyanobacteria in biotechnology. J Appl Microbiol 106:1–12
Adam MS (1999) The promotive effect of the cyanobacterium Nostoc muscorum on the growth of some crop plants. Acta Microbiol Pol 48:163–171
Adams DG, Bergman B, Nierzwicki-Bauer SA, Duggan PS, Rai AN, Schüßler A (2013) Cyanobacterial-plant symbioses. In: Falkow S, Rosenberg E, Schleifer K, Stackebrandt E (eds) Dworkin M. The Prokaryotes Springer, Berlin, pp 359–400
Adessi A, Cruz de Carvalho R, De Philippis R, Branquinho C, Marques da Silva J (2018) Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol Biochem 116:67–69
Ahmed NU, Park JI, Jung HJ, Yang TJ, Hur Y, Nou IS (2014) Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene 550:46–55
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Lett Appl Microbiol 48:542–547
Ambika HD, Krishnamurthy SR (2019) Diversity of subaerial algae and cyanobacteria on tree bark in tropical mountain habitats. Algol Stud 155:15–27
Apte SK (2001) Coping with salinity/water stress: cyanobacteria show the way. Proc Ind Natl Sci Acad B67:285–310
Apte SK, Alahari A (1994) Role of alkali cations (K+ and Na+) in cyanobacterial nitrogen fixation and adaptation to salinity and osmotic stress. Indian J Biochem Biol 31:267
Apte SK, Reddy BR, Thomas J (1987) Relationship between sodium influx and salt tolerance of nitrogen-fixing cyanobacteria. Appl Environ Microbiol 53:1934–1939
Apte SK, Thomas J (1997) Possible amelioration of coastal soil salinity using halotolerant nitrogen-fixing cyanobacteria. Plant Soil 189:205–211
Arora M, Kaushik A, Rani N, Kaushik CP (2010) Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination. J Environ Biol 31:701–704
Bari R, Jones JD (2009) Role of plant hormones in plant defense responses. Plant Mol Biol 69:473–488
Becerra-Absalón I, Muñoz-Martín MÁ, Montejano G, Mateo P (2019) Differences in the cyanobacterial community composition of biocrusts from the drylands of central Mexico. Are there endemic species? Front Microbiol 10:937
Belnap J, Gardner JS (1993) Soil microstructure in soils of the Colorado Plateau: the role of the cyanobacterium Microcoleus vaginatus. Great Basin Nat 53:40–47
Bergman B (2002) Nostoc-Gunnera symbiosis. In: Rai AN, Bergman B, Rasmussen U (eds) Cyanobacteria in Symbiosis. Kluwer, Dordrecht, pp 207–232
Berry S, Esper B, Karandashova I, Teuber M, Elanskaya I, Rogner M, Hagemann M (2003) Potassium uptake in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 mainly depends on a Ktr-like system encoded by slr1509 (ntpJ). FEBS Lett 548:53–58
Bhatnagar A, Makandar M, Garg M, Bhatnagar M (2008) Community structure and diversity of cyanobacteria and green algae in the soils of Thar Desert (India). J Arid Environ 72:73–83
Borowitzka LJ (1986) Osmoregulation in blue-green algae. Prog Phycol Res 4:243–256
Borowitzka MA (1988) Vitamins and fine chemicals from micro-algae. In: Borowitzka MA, Borowitzka LJ (eds) Micro-Algal Biotechnology. Cambridge University Press, Cambridge, pp 153–196
Bulgarelli D, Schlaeppi K, Spaepen S, Themaat VLV, EVL, Schulze-Lefert P, (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Chamizo S, Cantón Y, Rodríguez-Caballero E, Domingo F (2016) Biocrusts positively affect the soil water balance in semiarid ecosystems. Ecohydrology 9:1208–1221
Chamizo S, Mugnai G, Rossi F, Certini G, De Philippis R (2018) Cyanobacteria inoculation improves soil stability and fertility on different textured soils: gaining insights for applicability in soil restoration. Front Environ Sci 6:49
Chen J, Song L, Da J, Gan N, Liu Z (2004) Effects of microcystins on the growth and the activity of the superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43:393–400
Chittapun S, Limbipichai S, Amnuaysin N, Boonkerd R, Charoensook M (2018) Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: a pot experiment. J Appl Phycol 30:79–85
Choudhury ATMA, Kennedy IR (2004) Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol Fertil Soils 39:219–227
Chua M, Erickson TE, Merritt DJ, Ooi MKJ, Muñoz-Rojas M (2019) Bio-priming seeds with cyanobacteria: effect on native plant growth and soil properties. Restor Ecol 28:S168–S176
Colica G, Li H, Rossi F, Li D, Liu Y, De Philippis R (2014) Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol Biochem 68:62–70
Compant S, Cambon MC, Vacher C, Mitter B, Samad A, Sessitsch A (2021) The plant endosphere world–bacterial life within plants. Environ Microbiol 23:1812–1829
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Costa JL, Lindblad P (2002) Cyanobacteria in symbiosis with cycads. In: Rai AN, Bergman B, Rasmussen U (eds) Cyanobacteria in symbiosis. Kluwer, Dordrecht, pp 195–205
Coutinho R, Seeliger U (1984) The horizontal distribution of benthic algal flora in the Patos Lagoon estuary, Brazil, in relation to salinity, substratum and wave. J Exp Marine Biol Ecol 80:247–257
Dadhich KS, Varma AK, Venkataraman GS (1969) The effect of Calothrix inoculation on vegetable crops. Plant Soil 31:377–379
Dash NP, Kaushik MS, Kumar A, Abraham G, Singh PK (2018) Toxicity of biocides to native cyanobacteria at different rice crop stages in wetland paddy field. J Appl Phycol 30:483–493
Dash NP, Kumar A, Kaushik MS, Abraham G, Singh PK (2017a) Nitrogenous agrochemicals inhibiting native diazotrophic cyanobacterial contribution in wetland rice ecosystem. J Appl Phycol 29:929–939
Dash NP, Kumar A, Kaushik MS, Abraham G, Singh PK (2017b) Agrochemicals influencing nitrogenase, biomass of N2-fixing cyanobacteria and yield of rice in wetland cultivation. Biocat Agri Biotechnol 9:28–34
Dash NP, Kumar A, Kaushik MS, Singh PK (2016) Cyanobacterial (unicellular and heterocystous) biofertilization to wetland rice as influenced by nitrogenous agrochemical. J Appl Phycol 28:3343–3351
de Caire GZ, De Cano MS, De Mule MZ, Palma RM, Colombo K (1997) Exopolysaccharides of Nostoc muscorum (Cyanobacteria) in the aggregation of soil particles. J Appl Phycol 9:249–253
De Datta SK (1987) Nitrogen transformation processes in relation to improved cultural practices for lowland rice. Plant Soil 100:47–69
De Philippis R, Vincenzini M (1998) Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–175
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379
Delattre C, Pierre G, Laroche C, Michaud P (2016) Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotech Adv 34:1159–1179
Delgado-Baquerizo M, Maestre FT, Gallardo A (2013) Biological soil crusts increase the resistance of soil nitrogen dynamics to changes in temperatures in a semi-arid ecosystem. Plant Soil 366:35–47
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities. FEMS Microbiol Ecol 72:313–327
Di Pippo F, Ellwood NTW, Gismondi A, Bruno L, Rossi F, Magni P, De Philippis R (2013) Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J Appl Phycol 25:1697–1708
Dimkpa C, Weinand T, Asch F (2009) Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. CRC Crit Rev Plant Sci 22:107–149
Dodd C, Perez-Alfocea F (2012) Microbial amelioration of crop salinity stress. J Exp Bot 63:3415–3428
Dubey AK, Rai AK (1995) Application of algal biofertilizers (Aulosira fertilissima Tenuis and Anabaena doliolum Bhardwaja) for sustained paddy cultivation in Northern India. Isr J Plant Sci 43:41–51
Dhankhar R, Mohanty A, Gulati P (2021) Microbial diversity of phyllosphere: Exploring the unexplored, In: Phytomicrobiome Interactions and Sustainable Agriculture, Amit Verma et al., (eds), John Wiley and Sons Ltd, USA, pp. 66–85.
Elanskaya IV, Karandashova IV, Bogachev AV, Hagemann M (2002) Functional analysis of the Na+/H+ antiporter encoding genes of the cyanobacterium Synechocystis PCC 6803. Biochemistry 67:432–440
Enebe MC, Babalola OO (2018) The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: a survival strategy. Appl Microbiol Biotechnol 102:7821–7835
Erdmann N (1983) Organic osmoregulatory solutes in blue-green algae. Z Pflanzenphysiol 110:147–155
Evseeva NV, Kachenko OV, Denisoa AY, Burygin GL, Veselov DS, Matora LY, Shchyogolev SY (2019) Functioning of plant-bacterial associations under osmotic stress in vitro. World J Microbiol Biotechnol 35:195
Fernandes TA, Iyer V, Apte SK (1993) Differential responses of nitrogen-fixing cyanobacteria to salinity and osmotic stresses. Appl Environ Microbiol 59:899–904
Fernandes VMC, Machado de Lima NM, Roush D, Rudgers J, Collins SL, Garcia-Pichel F (2018) Exposure to predicted precipitation patterns decreases population size and alters community structure of cyanobacteria in biological soil crusts from the Chihuahuan Desert. Environ Microbiol 20:259–269
Fernández Valiente E, Ucha A, Quesada A, Leganés F, Carreres R (2000) Contribution of N2 fixing cyanobacteria to rice production: availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 221:107–112
Fidor A, Konkel R, Mazur-Marzec H (2019) Bioactive peptides produced by cyanobacteria of the genus Nostoc: a review. Mar Drugs 17:561
Flaibani A, Olsen Y, Painter TJ (1989) Polysaccharides in desert reclamation: compositions of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr Res 190:235–248
Fulda S, Huckauf J, Schoor A, Hagemann M (1999) Analysis of stress responses in the cyanobacterial strains Synechococcus sp. PCC 7942, Synechocystis sp. PCC 6803, and Synechococcus sp. PCC 7418: osmolyte accumulation and stress proteins synthesis. J Plant Physiol 154:240–249
Gantar M, Kerby NW, Rowell P, Obreht Z, Scrimgeour R (1995a) Colonization of wheat (Triticumvulgare L.) by N2-fixing cyanobacteria. IV. Dark nitrogenase activity and effects of cyanobacteria on natural 15N abundance on plants. New Phytol 129:337–343
Gantar M, Rowell P, Kerby NW, Sutherland IW (1995b) Role of extracellular polysaccharide in the colonization of wheat (Triticum vulgare L.) roots by N2-fixing cyanobacteria. Biol Fertil Soils 19:41–48
Garcia-Pichel F, Castenholz RW (1993) Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl Environ Microbiol 59:163–169
Garcia-Pichell F, López-Cortés A, Nübel U (2001) Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado Plateau. Appl Environ Microbiol 67:1902–1910
Gayathri M, Kumar PS, Prabha AML, Muralitharan G (2015) In vitro regeneration of Arachis hypogaea L., and Moringa oleifera Lam. using extracellular phytohormones from Aphanothece sp. MBDU 515. Algal Res 7:100–105
Gayathri M, Shunmugum S, Thajuddin N, Muralitharan G (2017) Phytohormones and free volatile fatty acids from cyanobacterial biomass wet extract (BWE) elicit plant growth promotion. Algal Res 26:56–64
Gaysina LA, Saraf A, Singh P (2019) Cyanobacteria in diverse habitats. In: Mishra AK, Tiwari DN (eds) Cyanobacteria: from basic science applications. Academic Press, London, pp 1–28
George P, Gupta A, Gopal M, Thomas L, Thomas GV (2013) Multifarious beneficial traits and plant growth promoting potential of Serratia marcescens KiSII and Enterobacter sp. RNF 267 isolated from the rhizosphere of coconut palms (Cocos nucifera L.). World J Microbiol Biotechnol 29:109–117
Gheda SF, Ahmed DA (2015) Improved soil characteristics and wheat germination as influenced by inoculation of Nostoc kihlmani and Anabaena cylindrica. Rend Fis Accad Lincei 26:121–131
Glick BR, Patten CL, Holguim G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. Imperial College Press, London
Govindasamy V, George P, Kumar M, Aher L, Raina SK, Rane J, Annapurna K, Minhas PS (2020) Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench]. 3 Biotech 10:13
Govindasamy V, George P, Raina SK, Kumar M, Rane J, Annapurna K (2018) Plant-associated microbial interactions in the soil environment: role of endophytes in imparting abiotic stress tolerance to crops. In: Bal SK, Mukherjee J, Choudhury BU, Dhawan AK (eds) Advances in crop environment interaction. Springer, Heidelberg, pp 245–284
Govindasamy V, Senthilkumar M, Magheshwaran V, Kumar U, Bose P, Sharma V, Annapurna K (2011) Bacillus and Paenibacillus spp.: potential PGPR for sustainable agriculture. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin, pp 333–364
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412
Gromov BV, Vepritskii AA, Titota NN, Mamkayeva KA, Alexandrova OV (1991) Production of the antibiotic cyanobacterin LU-1 by Nostoc linckia CALU 892. J Appl Phycol 3:55–59
Grzesik M, Romanowska-Duda Z, Kalaji HM (2017) Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica 55:510–521
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35:87–123
Han GZ (2017) Evolution of jasmonate biosynthesis and signaling mechanisms. J Exp Bot 68:1323–1331
Hartung W (2010) The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct Plant Biol 37:806–812
Havaux M, Ksas B, Szewczyk A, Rumeau D, Franck F, Caffarri S, Triantaphylidès C (2009) Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol 9:130
Hoiczyk E (1998) Structural and biochemical analysis of the sheath of Phormidium uncinatum. J Bacteriol 180:3923–3932
Hussain A, Hamayun M, Shah ST (2013) Root colonization and phytostimulation by phytohormones producing entophytic Nostoc sp. AH-12. Curr Microbiol 67:624–630
Hussain A, Hasnain S (2011) Phytostimulation and biofertilization in wheat by cyanobacteria. J Ind Microbiol Biotechnol 38:85–92
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768
Issa AA, Abd-Alla MH, Ohyama T (2014) Nitrogen fixing cyanobacteria: future prospects. In: Ohyama T (ed) Advances in biology and ecology of nitrogen fixation. IntechOpen. https://doi.org/10.5772/56995
Jesus JM, Danko AS, Fiuza A, Borges MT (2015) Phytoremediation of salt-affected soils: a review of processes, applicability, and the impact of climate change. Environ Sci Pollut Res 22:6511–6525
Jha MN, Prasad AN (2006) Efficacy of new inexpensive cyanobacterial biofertilizer including its shelf-life. World J Microbiol Biotech 22:73–79
Joset F, Jeanjean R, Hagemann M (1996) Dynamics of the response of cyanobacteria to salt stress: deciphering the molecular events. Physiol Plant 96:738–744
Karthikeyan N, Prasanna R, Lata DP, Kaushik BD (2007) Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur J Soil Biol 43:23–30
Karthikeyan N, Prasanna R, Sood A, Jaiswal P, Nayak S, Kaushik BD (2009) Physiological characterization and electron microscopic investigations of cyanobacteria associated with wheat rhizosphere. Folia Microbiol 54:43–51
Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant growth promoting bacteria. J Plant Growth Regul 32:122–130
Katoh H, Asthana RK, Ohmori M (2004) Gene expression in the cyanobacterium Anabaena sp. PCC 7120 under desiccation. Microb Ecol 47:164–174
Katoh H, Furukawa J, Tomita-Yokotani K, Nishi Y (2012) Isolation and purification of an axenic diazotrophic drought-tolerant cyanobacterium, Nostoc commune, from natural cyanobacterial crusts and its utilization for field research on soils polluted with radioisotopes. Biochim Biophys Acta 1817:1499–1505
Kaushik BD (2004) Use of blue-green algae and Azolla biofertilizers in rice cultivation and their influence on soil properties. In: Jain PC (ed) Microbiology and biotechnology for sustainable development. CBS Publishers & Distributors, New Delhi, pp 166–184
Kaushik BD, Venkataramanan GS (1979) Effect of algal inoculation on the yield and vitamin C content of two varieties of tomato. Plant Soil 52:135–137
Kaushik MS, Kumar A, Abraham G, Dash NP, Singh PK (2019) Field evaluations of agrochemical toxicity to cyanobacteria in rice field ecosystem: a review. J Appl Phycol 31:471–489
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20:219–229
Kehr J, Picchi DG, Dittmann E (2011) Natural product biosyntheses in cyanobacteria: a treasure trove of unique enzymes. Beilstein J Org Chem 7:1622–1635
Kirsch F, Klahn S, Hagemann M (2019) Salt-regulated accumulation of the compatible solutes sucrose and glucosylglycerol in cyanobacteria and its biotechnological potential. Front Microbiol 10:2139
Kondo M, Yasuda M (2003) Seasonal changes in N2 fixation activity and N enrichment in paddy soils as affected by soil management in the northern area of Japan. Japan Agri Res Quarterly 37:105–111
Lan S, Wu L, Zhang D, Hu C (2013) Assessing the level of development and successional stages in biological soil crusts with biological indicators. Microb Ecol 66:394–403
Lan S, Wu L, Zhang D, Hu C, Liu Y (2010) Effects of drought and salt stresses on man-made cyanobacterial crusts. Europ J Soil Biol 46:381–386
Latha A, Shanthi K, Kannan N (1992) Rock phosphate solubilization by free-living nitrogen fixing blue-green algae. In: Proceedings of National Symposium on Cyanobacterial Nitrogen Fixation. Associated Publishing Co., New Delhi, pp 415–421
Latifi A, Ruiz M, Zhang CC (2009) Oxidative stress in cyanobacteria. FEMS Microbiol Rev 33:258–278
Lau JA, Lennon JT (2011) Evolutionary ecology of plant–microbe interactions: soil microbial structure alters selection on plant traits. New Phytol 192:215–224
Lau JA, Lennon JT (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci USA 109:14058–14062
Lee SS, Gantzer CJ, Thompson AL, Anderson SH (2011) Polyacrylamide efficacy for reducing soil erosion and runoff as influenced by slope. J Soil Water Conserv 66:172–177
Li H, Zhao Q, Huang H (2019) Current status and challenges of salt-affected soil remediation by cyanobacteria. Sci Total Environ 669:258–272
Li Y, Shao M, Horton R (2011) Effect of polyacrylamide applications on soil hydraulic characteristics and sediment yield of sloping land. Procedia Environ Sci 11:763–773
Lin C, Wu J (2014) Tolerance of soil algae and cyanobacteria to drought stress. J Phycol 50:131–139
Lugtenberg BJ, Malfanova N, Kamilova F, Berg G (2013) Plant growth promotion by microbes. In: de Bruijn FJ (ed) Molecular microbial ecology of the rhizosphere. Wiley Blackwell, Hoboken pp 559–573
Luo S, Tian L, Chang C, Wang S, Zhang J, Zhou X, Li X, Tran LSP, Tian C (2018) Grass and maize vegetation systems restore saline-sodic soils in the Songnen Plain of northeast China. Land Degrad Dev 29:1107–1119
Lynch JM (1990) The rhizosphere. Wiley-Interscience, Chichester
Maestre FT, Bowker MA, Cantón Y, Castillo-Monroy AP, Cortina J, Escolar C, Escuderoa A, LázarodR MI (2011) Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. J Arid Environ 75:1282–1291
Malamlssa OL, Bissonnais Y, Defarge C, Trichet J (2001) Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma 101:15–30
Mandal B, Vlek PLG, Mandal LN (1998) Beneficial effect of blue green algae and Azolla excluding supplying nitrogen, on wetland rice fields: a review. Biol Fertil Soils 27:329–342
Manjunath M, Prasanna R, Sharma P, Nain L, Singh R (2011) Developing PGPR consortia using novel genera Providencia and Alcaligenes along with cyanobacteria for wheat. Arch Agron Soil Sci 57:873–887
Matsuda N, Kobayashi H, Katoh H, Ogawa T, Futatsugi L, Nakamura T, Bakker EP, Uozumi N (2004) Na1-dependent K1 uptake Ktr system from the cyanobacterium Synechocystis sp. PCC 6803 and its role in the early phases of cell adaptation to hyperosmotic shock. J Biol Chem 279:54952–54962
Mazhar S, Cohen JD, Hasnain S (2013) Auxin producing non-heterocystous cyanobacteria and their impact on the growth and endogenous auxin homeostasis of wheat. J Basic Microbiol 53:996–1003
Meeks JC (2003) Symbiotic interactions between Nostoc punctiforme, a multicellular cyanobacterium and the hornwort Anthoceros punctatus. Symbiosis 35(1–3):55–71
Mehboob A, Stal LJ, Hasnain S (2010) Production of indole-3-acetic acid by the cyanobacterium Arthrospira platensis strain MMG-9. J Microbiol Biotechnol 20:1259–1265
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663
Misra S, Kaushik BD (1989a) Growth promoting substances of cyanobacteria. II. Detection of amino acids, sugars and auxins. Proc Indian Natl Sci Acad B 55:499–504
Misra S, Kaushik BD (1989b) Growth promoting substances of cyanobacteria. I. Vitamin and their influence on rice plant. Proc Indian Natl Sci Acad B 55:295–300
Mohsen A, Dowidar S, Abo-Hamad S, Khalaf B (2013) Role of cyanobacteria in amelioration of toxic effects of copper in Trigonella foenumgracum. Aust J Crop Sci 7:1488–1493
Moisander PH, McClinton E, Paerl HW (2002) Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb Ecol 43:432–442
Mugnai G, Rossi F, Felde VJMNL, Colesie C, Büdel B, Peth S, Kaplan A, De Philippis R (2018a) Development of the polysaccharidic matrix in biocrusts induced by a cyanobacterium inoculated in sand microcosms. Biol Fert Soils 54:27–40
Mugnai G, Rossi F, Felde VJMNL, Colesie C, Büdel B, Peth S, Kaplan A, De Philippis R (2018b) The potential of the cyanobacterium Leptolyngbya ohadii as inoculum for stabilizing bare sandy substrates. Soil Biol Biochem 127:318–328
Muñoz-Rojas M, Chilton A, Liyanage GS, Erickson TE, Merritt DJ, Neilan BA, Ooi MKJ (2018a) Effects of indigenous soil cyanobacteria on seed germination and seedling growth of arid species used in restoration. Plant Soil 429:91–100
Muñoz-Rojas M, Román JR, Roncero-Ramos B, Erickson TE, Merritt DJ, Aguila-Carricondo P, Cantón Y (2018b) Cyanobacteria inoculation enhances carbon sequestration in soil substrates used in dryland restoration. Sci Total Environ 636:1149–1154
Mustafa S, Kabir S, Shabbir U, Batool R (2019) Plant growth promoting rhizobacteria in sustainable agriculture: from theoretical to pragmatic approach. Symbiosis 78:115–123
Nain L, Prasanna R, Joshi M, Jadhav SD, Kumar D, Shivay YS, Paul S (2010) Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant Soil 331:217–230
Nanatani K, Shijuku T, Takano Y, Zulkifli L, Yamazaki T, Tominaga A, Souma S, Onai K, Morishita M, Ishiura M, Hagemann M, Suzuki I, Maruyama H, Arai F, Uozumi N (2015) Comparative analysis of kdp and ktr mutants reveals distinct roles of the potassium transporters in the model cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 197:676
Nayak S, Prasanna R, Dominic TK, Singh PK (2001) Floristic abundance and relative distribution of different cyanobacterial genera in rice field soil at different crop growth stages. Phykos 40:14–21
Naylor D, Coleman-Derr D (2018) Drought stress and root-associated bacterial communities. Front Plant Sci 8:2223
Nisha R, Kaushik A, Kaushik CP (2007) Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geoderma 138:49–56
Nisha R, Kiran B, Kaushik A, Kaushik CP (2018) Bioremediation of salt affected soils using cyanobacteria in terms of physical structure, nutrient status and microbial activity. Int J Environ Sci Technol 15:571–580
Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D (1994) High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78:449–460
Obreht Z, Kerby NW, Gantar M, Rowell P (1993) Effects of root associated N2-fixing cyanobacteria on the growth and nitrogen content of wheat (Triticum vulgare L.) seedlings. Biol Fertil Soils 15:68–72
Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J (2020) Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ 743:140682
Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol Fertil Soils 46:861–875
Park CH, Li H, Jia R, Js H (2017) Combined application of cyanobacteria with soil fixing chemicals for rapid induction of biological soil crust formation. Arid Land Res Manage 31:81–93
Patel HM, Rastogi RP, Trivedi U, Madamwar D (2019) Cyanobacterial diversity in mat sample obtained from hypersaline desert, Rann of Kachchh. 3 Biotech 9:304
Paul D, Nair S (2008) Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol 48:378–384
Peat A, Potts M (1987) The ultrastructure of immobilised desiccated cells of the cyanobacterium Nostoc commune UTEX 584. FEMS Microbiol Lett 43:223–227
Pereira I, Ortega R, Barrientos L, Moya M, Reyes G, Kramm V (2009) Development of a biofertilizer based on filamentous nitrogen-fixing cyanobacteria for rice crops in Chile. J Appl Phycol 21:135–144
Pflugmacher S, Aulhorn M, Grimm B (2007) Influence of a cyanobacterial crude extract containing microcystin LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol 175:482–489
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growthpromoting rhizobacteria on nutrient acquisition process. A Review Biol Fertil Soils 51:403–415
Plazinski J, Zheng Q, Taylor R, Croft L, Rolfe BG, Gunning BES (1990) DNA probes show genetic variation in cyanobacterial symbionts of the Azolla fern and a closer relationship to free-living Nostoc strains than to free living Anabaena strains. Appl Environ Microbiol 56:1263–1270
Potts M (1994) Desiccation tolerance of prokaryotes. Microbiol Storage Soil Biol Biochem 23:313–322
Prasanna R, Chaudhary V, Gupta V, Babu S, Kumar A, Shivay YS, Nain L (2013) Cyanobacteria mediated plant growth promotion and bioprotection against Fusarium wilt in tomato. Eur J Plant Pathol 136:337–353
Prasanna R, Hossain F, Babu S, Bidyarani N, Adak A, Verma S, Shivay YS, Nain L (2015) Prospecting cyanobacterial formulations as plant-growth-promoting agents for maize hybrids. S Afr J Plant Soil 32:199–207
Prasanna R, Kanchan A, Ramakrishnan B, Ranjan K, Venkatachalam S, Hossain F, Shivay YS, Krishnan P, Nain L (2016) Cyanobacteria based inoculants influence growth and yield by modulating the microbial communities favourably in the rhizospheres of maize hybrids. Eur J Soil Biol 75:15–23
Qadir M, Quillérou E, Nangia V, Murtaza G, Singh M, Thomas RJ, Noble AD (2014) Economics of salt-induced land degradation and restoration. Nat Resour Forum 38:282–295
Rady MM, Taha SS, Kusvuran S (2018) Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Sci Hortic 233:61–69
Rai AK, Sharma NK (2006) Phosphate metabolism in the cyanobacterium Anabaena doliolum under salt stress. Curr Microbiol 52:6–12
Rana A, Joshi M, Prasanna R, Shivay YS, Nain L (2012) Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur J Soil Biol 50:118–126
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B-and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Reed RH, Borowitzka LJ, Mackay MA, Chudek JA, Foster R, Warr SRC, Moore DJ, Stewart WDP (1986) Organic solute accumulation in osmotically stressed cyanobacteria. FEMS Microbiol Lett 39:51–56
Reed RH, Chudek JA, Foster R, Stewart WDP (1984a) Osmotic adjustments in cyanobacteria from hypersaline environments. Arch Microbiol 138:333–337
Reed RH, Stewart WDP (1983) Physiological responses of Rivularia atra to salinity: osmotic adjustment in hyposaline media. New Phytol 95: 595– 603
Reed RH, Richardson DL, Warr SRC, Stewart WDP (1984b) Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol 130:1–4
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Remus-Emsermann MNP, Lücker S, Müller DB, Potthoff E, Daims H, Vorholt JA (2014) Spatial distribution analyses of natural phyllosphere-colonizing bacteria on Arabidopsis thaliana revealed by fluorescence in situ hybridization. Environ Microbiol 16:2329–2340
Rigonato J, Alvarenga DO, Andreote FD, Franco Dias AC, Melo IS, Kent A, Fiori MF (2012) Cyanobacterial diversity in the phyllosphere of a mangrove forest. FEMS Microbiol Ecol 80:312–322
Rippka RJ, Deurelles JB, Waterburry M, Herdman Stanier RY (1979) Genetic assignments, strain histories and properties of pure culture of cyanobacteria. J Gen Microbiol 111:1–61
Rodgers GA, Bergman B, Henriksson E, Udris M (1979) Utilization of blue-green algae as bio-fertilizers. Plant Soil 52:99–107
Rodríguez AA, Stella AM, Storni MM, Zulpa G, Zaccaro MC (2006) Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst 2:7
Rodríguez-Caballero E, Cantón Y, Chamizo S, Afana A, SoléBenet A (2012) Effects of biological soil crusts on surface roughness and implications for runoff and erosion. Geomorphology 145–146:81–89
Roger PA, Kulasooriya SA (1980) Blue green algae and rice. International Rice Research Institute, Manila
Roger PA, Zimmerman WJ, Lumpkin TA (1993) Microbiological management of wetland rice fields. In: Metting FB (ed) Soil microbial ecology-applications in agricultural and environmental management. Dekker, New York, pp 417–455
Roger PA, Reynaud PA (1982) Free living blue green algae in tropical soils. In: Dommergues YR, Diem HG (eds) Microbiology of tropical soils and plant productivity. Martinus Nijhoff, The Hague, pp 147–168
Roman JR, Roncero-Ramos B, Chamizo S, Rodriguez-Caballero E, Canton Y (2018) Restoring soil functions by means of cyanobacteria inoculation: importance of soil conditions and species selection. Land Degrad Dev 29:3184–3193
Roncero-Ramos B, Román JR, Gómez-Serrano C, Cantón Y, Acién FG (2019) Production of a biocrust-cyanobacteria strain (Nostoc commune) for large-scale restoration of dryland soils. J Appl Phycol 31:2217–2230
Rosenblueth M, Martínez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 19:827–837
Rossi F, De Philippis R (2016) Exocellular polysaccharides in microalgae and cyanobacteria: chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka MA, Beardall J, Raven JA (eds) The Physiology of Microalgae. Springer, Cham, pp 565–590
Rossi F, Li H, Liu Y, De Philippis R (2017) Cyanobacterial inoculation (cyanobacterisation): perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth Sci Rev 171:28–43
Saadatnia H, Riahi H (2009) Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ 55:207–212
Saifullah DS, Naeem A, Rengel Z, Naidu R (2018) Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ 625:320–335
Sherer S, Ernst A, Chen TW, Boger P (1984) Rewetting of drought resistant blue-green algae: time course of water uptake and reappearance of respiration, photosynthesis and nitrogen fixation. Oecologia 62:418–423
Schroth MN, Hancock JG (1982) Disease-suppressive soil and root-colonizing bacteria. Science 216:1376–1381
Selykh IO, Semenova LR (2000) Problems of ecology and physiology of microorganisms. Dialog-MGU, Moscow, p 94
Sergeeva E, Liaimer A, Bergman B (2002) Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215:229–238
Seyed HM, Ghassempour AR, Riahi H, Shariatmadari Z, Khanjir M (2012) Endogenous auxins in plant growth promoting cyanobacteria–Anabaena vaginicola and Nostoc calcicola. J Appl Phycol 25:379–386
Shan X, Yan J, Xie D (2012) Comparison of phytohormone signaling mechanisms. Curr Opin Plant Biol 15:84–91
Shariatmadari Z, Riahi H, Abdi M, Hashtroudi MS, Ghassempour AR (2015) Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L. J Appl Phycol 27:2279–2287
Shariatmadari Z, Riahi H, Hashtroudi MS, Ghassempour AR, Aghashariatmadary Z (2013) Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran. Soil Sci Plant Nut 59:535–547
Singh DP, Prabha R, Yandigeri MS, Arora DK (2011) Cyanobacteria-mediated phenylpropanoids and phytohormones in rice (Oryza sativa) enhance plant growth and stress tolerance. Antonie Van Leeuwenhoek 100:557–568
Singh DT, Rai AN, Singh HN (1985) Methylammonium (ammonium) uptake in a glutamine auxotroph of the cyanobacterium Anabaena cycadeae. FEBS Lett 186:51–53
Singh H, Anurag K, Apte SK (2013) High radiation and desiccation tolerance of nitrogen-fixing cultures of the cyanobacterium Anabaena sp. strain PCC 7120 emanates from genome/proteome repair capabilities. Photosynth Res 118:71–81
Singh R, Parihar P, Singh M, Bajguz A, Kumar J, Singh S, Singh VP, Prasad SM (2017) Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front Microbiol 8:515
Singh RN (1961) Role of blue-green algae in nitrogen economy of Indian agriculture. Indian Council of Agricultural Research, New Delhi. pp.175,19631602458
Singh S (2014) A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J Appl Microbiol 117(5): 1221–1244
Singh RP, Jha PN (2016) Alleviation of salinity-induced damage on wheat plant by an ACC deaminase-producing halophilic bacterium Serratia sp SL-12 isolated from a salt lake. Symbiosis 69:101–111
Singh V, Singh DV (2015) Cyanobacteria modulated changes and its impact on bioremediation of saline-alkaline soils. Bangladesh J Bot 44:653–658
Sivonen K, Leikoski N, Fewer DP, Joukela J (2010) Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria. Appl Microbiol Biotechnol 86:1213–1225
Schrader M, Drews G, GoleckiJR, Weckesser J (1982) Isolation and characterization of the sheath from the cyanobacterium Chlorogloeopsis PCC 6912. J Gen Microbiol 128:267–272
Spiller H, Gunasekaran M (1990) Ammonia-excreting mutant strain of the cyanobacterium Anabaena variabilis supports growth of wheat. Appl Microbiol Biotechnol 33:477–480
Srivastava AK, Bhargava P, Kumar A, Rai LC, Neilan B (2009) Molecular characterization and the effect of salinity on cyanobacterial diversity in the rice fields of Eastern Uttar Pradesh. India Saline Systems 5:4
Stal LJ (2007) Cyanobacteria. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments. Springer, Dordrecht, pp 659–680
Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol 53:1195–1202
Thajuddin N, Subramaniyan G (2005) Cyanobacterial diversity and potential applications in biotechnology. Special Section: Microbial Diversity; Curr Sci 89(1):47–57
Thomas J, Apte SK (1984) Sodium requirement and metabolism in nitrogen-fixing cyanobacteria. J Biosci 6:771–794
Tripathi RD, Dwivedi S, Shukla MK, Mishra S, Srivastava S, Singh R, Rai UN, Gupta DK (2008) Role of blue green algae biofertilizer in ameliorating the nitrogen demand and fly-ash stress to the growth and yield of rice (Oryza sativa L.) plants. Chemosphere 70:1919–1929
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Hormones and hormone-like substances of microorganisms: a review. Appl Biochem Microbiol 42:229–235
Tschermak-Woess E (1988) The algal partner. In: Galun M (ed) CRC handbook of lichenology. CRC Press, Boca Raton, pp 39–92
Tsunekawa K, Shijuku T, Hayashimoto M, Kojima Y, Onai K, Morishita M, Ishiura M, Kuroda T, Nakamura T, Kobayashi H, Sato M, Toyooka K, Matsuoka K, Omata T, Uozumi N (2009) Identification and characterization of the Na+/H+ antiporter Nhas3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J Biol Chem 284:16513–16521
Ueda J, Miyamoto K, Aoki M, Hirata T, Sato T, Momotani Y (1991) Identification of jasmonic acid in Chlorella and Spirulina. Bull Osaka Prefect Ser B 43:103–108
Venkatachalam S, Rajan K, Prasanna R, Ramakrishnan B, Thapa S, Kanchan A (2016) Diversity and functional traits of culturable microbiome members, including cyanobacteria in the rice phyllosphere. Plant Biol 8:627–637
Venkataraman GS (1972) Algal biofertilizers and rice cultivation. Today and Tomorrow Printers and Publishers, New Delhi
Venkataraman GS, Neelakantan S (1967) Effect of the cellular constituents of the nitrogen-fixing blue-green alga, Cylindrospermum muscicola, on the root growth of rice plants. J Gen Appl Microbiol 13:53–61
Venkateswarlu B, Shanker AK (2009) Climate change and agriculture: adaptation and mitigation stategies. Ind J Agron 54:226–230
Vinocur B, Altman A (2005) Recent advances in engineering plant tolerance toabiotic stress: achievements and limitations. Curr Opin Biotechnol 16:123–132
Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiolo Res 184:13–24
Waditee R, Hibino T, Nakamura T, Incharoensakdi A, Takabe T (2002) Over expression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proc Natl Acad Sci USA 99:4109–4114
Wang D, Yang S, Tang F, Zhu H (2012) Symbiosis specificity in the legume: rhizobial mutualism. Cell Microbiol 14:334–342
Wang R, Peng B, Huang K (2015) The research progress of CO2 sequestration by algal bio-fertilizer in China. J CO2 Utilization 11:67–70
Waterbury JB (2006) The cyanobacteria-isolation, purification and identification. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The Prokaryotes. Springer, New York, pp 1053–1073
Welker M, Dӧhren H (2006) Cyanobacterial peptides–nature’s own combinatorial biosynthesis. FEMS Microbiol Rev 30:530–563
Whitton BA (2000) Soils and rice-fields. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria. Kluwer, Dordrecht, pp 233–255
Willis BF, Rodrigues BF, Harris PJC (2013) The Ecology of arbuscular mycorrhizal fungi. Crit Rev Plant Sci 32:1–20
Wutipraditkul N, Waditee R, Incharoensakdi A, Hibino T, Tanaka Y, Nakamura T, Shikata M, Takabe T (2005) Halotolerant cyanobacterium Aphanothece halophytica contains Nap A-type Na+/H+ antiporters with novel ion specificity that are involved in salt tolerance at alkaline pH. Appl Environ Microbiol 71:4176–4184
Yadav RK, Thagela P, Tripathi KN, Abraham G (2016) Physiological and proteomic analysis of salinity tolerance of the halotolerant cyanobacterium Anabaena sp. World J Microbiol Biotechnol 32:147
Yang J, Kloepper JW, Ryu CM (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Yang J, Ma LA, Jiang H, Wu G, Dong H (2016) Salinity shapes microbial diversity and community structure in surface sediments of the Qinghai-Tibetan lakes. Sci Rep 6:25078
Yang J, Wang F, Fang L, Tan T (2007) Synthesis, characterization and application of a novel chemicals and fixing agent-poly (aspartic acid) and its composites. Environ Pollut 149:125–130
Yoshimura H, Kotake T, Aohara T, Tsumuraya Y, Ikeuchi M, Ohmori M (2012) The role of extracellular polysaccharides produced by the terrestrial cyanobacterium Nostoc sp. strain HK-01 in NaCl tolerance. J Appl Phycol 24:237–243
Zarezadeh S, Riahi H, Shariatmadari Z, Sonboli A (2020) Effects of cyanobacterial suspensions as bio-fertilizers on growth factors and the essential oil composition of chamomile, Matricaria chamomilla L. J Appl Phycol 32:1231–1241
Zerrouk IZ, Benchabane M, Khelifi L, Yokawa K, Ludwig-Muller J, Baluska F (2016) A Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J Plant Physiol 191:111–119
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528:51–59
Zhang X, Zhang R, Gao J, Wang X, Fan F, Ma X, Yin H, Zhang C, Feng K, Deng Y (2017) Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol Biochem 104:208–217
Zhang Y (2005) The microstructure and formation of biological soil crusts in their early developmental stage. Chinese Sci Bull 50:117–121
Zhao J, Davis LC, Verporte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Zhao J, Guo Y, Fujita K, Sakai K (2004) Involvement of cAMPsignaling pathway in elicitor-induced phytoalecin accumulation in Cupressus lusitanica cell cultures. New Phytol 161:723–733
Zhao J, Sakai K (2003a) Multiple signaling pathways mediate fungal elicitor induced β-thujaplicin accumulation in Cupressus lusitanica cell cultures. J Exp Bot 54:647–656
Zhao J, Sakai K (2003b) Peroxidases are involved in the biosynthesis and biodegradation of β-thujaplicin in fungal elicitor-treated Cupressus lusitanica suspension cultures. New Phytol 159:719–731
Zhao Y, Wang S, Li Y, Liu J, Zhuo Y, Chen H, Wang J, Xu L, Sun Z (2018) Extensive reclamation of saline-sodic soils with flue gas desulfurization gypsum on the Songnen Plain, Northeast China. Geoderma 321:52–60
Zheng J, Fu J, Gou M, Huai J, Liu Y, Jian M, Huang Q, Guo X, Dong Z, Wang H, Wang G (2010) Genome-wide transcriptome analysis of two maize inbred lines under drought stress. Plant Mol Biol 72:407–421
Acknowledgements
We gratefully acknowledge the authorities of ICAR-Indian Agricultural Research Institute, New Delhi, India, for facilities and encouragement. Financial assistance from CSIR (HRDG) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Both authors Sneha GR and Ravindra Kumar Yadav contributed equally.
Rights and permissions
About this article
Cite this article
GR, S., Yadav, R.K., Chatrath, A. et al. Perspectives on the potential application of cyanobacteria in the alleviation of drought and salinity stress in crop plants. J Appl Phycol 33, 3761–3778 (2021). https://doi.org/10.1007/s10811-021-02570-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02570-5