Abstract
Clavulanic acid (CA) is a naturally occurring antibiotic produced by Streptomyces clavuligerus. Statistical optimization of the fermentation medium for CA production by Streptomyces clavuligerus was carried out. Multiple carbon sources, glycerol, dextrin, and triolein, were considered simultaneously. A two-level fractional factorial design experiment was conducted to identify the significant components of medium on CA production. Statistical analysis of the results showed that soybean meal, dextrin, and triolein were the most significant medium ingredients on CA production. The optimal level of these screened components was obtained by RSM based on the result of a Box-Behnken design, in which the values of dextrin, soybean meal, and triolein in CA fermentation medium were 12.37 g/L, 39.75 g/L, and 26.98 ml/L, respectively. Using the proposed optimized medium, the model predicted 938 mg/L of CA level and via experimental rechecking the model, 946 mg/L of CA level was attained in shake flask fermentation, significantly high than 630 mg/L of original medium. The optimized medium was further verified in 50-L stirred fermenter, and compared with performance of original medium in parallel, CA titer was increased from 889 to 1310 mg/L; a 47% increase was achieved through medium optimization by statistical approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clavulanic acid (CA) is a naturally occurring antibiotic produced by Streptomyces clavuligerus. CA is a potent β-lactamase inhibitor and has been widely used to co-formulate with β-lactam antibiotics to treat infections caused by b-lactamase-producing pathogens [1, 2].
With the development of genome technology and knowledge of biosynthesis pathway of CA [1, 3, 4], two main approaches have been extensively researched to improve the production of CA during past 10–20 years. One is to develop strains by targeted gene modification, e.g., amplification of gene ceaS2 for encoding the first enzyme of CA biosynthesis, overexpression of CA-specific regulator ClaR, and deletion of the lat gene encoding lysine epsilon-aminotransferase, by which 2–25 folds of productivity increase can be seen in literature [5,6,7,8]. The other approach is based on the knowledge of CA biosynthesis pathway to develop defined medium containing glycerol and several relevant amino acids [9, 10].
However, the knowledge-based approach both in genetic modification and metabolism regulation is still somewhat empirical because overproduction of metabolites involves a multitude of genes and pathways; we have not seen any gene modified strain or chemical defined medium industrialized successfully. Improvement through classical mutation start from wild strain and optimization complex medium and process condition is still the most reliable and cost-competitive approach for CA production in industry.
Complex medium containing soybean meal and glycerol is usually used for CA production [10,11,12,13,14]. Soybean protein was proved to present advantages on CA production compared with other nitrogen sources since it can provide abundant C-5 precursors through the urea cycle [15]. With regards to the energy and carbon sources, glycerol plays important role in the CA biosynthesis of Streptomyces clavuligerus [10, 11]. Other carbon resources were also investigated, Butterworth [16] suggested to use lipids instead of glycerol. In addition to, palm oils, their olein and stearin fractions [17], olive pomace oil [18], vegetable oil [19, 20], and triolein [21] were also evaluated for CA fermentation. In our own experiments, we found that starch plays an important role in regulation of cell growth and CA production of Streptomyces clavuligerus, and dextrin was more favorable carbon source than starch. Most of these published researches used one-factor-at-time experiment [17,18,19,20,21]; few researches optimized complex medium using statistical method.
In the present work, statistical methods were applied to optimize the complex medium components for the production of CA by Streptomyces clavuligerus. Soybean meal was chosen as nitrogen source, and multiple carbon sources, including glycerol, dextrin, and triolein, were considered simultaneously.
Materials and Methods
Microorganism
Streptomyces clavuligerus ATCC 27,064 was used in this study. Suspensions containing approximately 107 spores per milliliter in Cryotubes (glycerol 20%) stored at − 80℃ were used as work cell bank throughout this work.
Culture Media and Culture Conditions
Seed Medium (g/L)
glycerol, 5.0; dextrin, 10.0; soybean meal, 20.0; yeast extract, 10.0; KH2PO4, 1.0; MOPS buffer, 8.0 pH adjusted to pH7.1 with 4 M NaOH prior to sterilization at 121℃, 25 min.
Cultivation
0.3 mL WCB was inoculated into a 500 mL Erlenmeyer flask with 50 mL seed medium, and cultivated 48 h at 28℃ in shake incubator at 250 rpm. The cultivated seed was inoculated into a fermentation medium with inoculation rate 8% (v/v). Fermentations were carried out in 500 mL Erlenmeyer flask with 30 mL medium as per experiment design and cultivated under the same conditions as seed cultivation but duration of 108 h.
Fermentation medium was composed of soybean meal, glycerol, dextrin, KH2PO4, triolein, trace element solution, and MOPS buffer 10.5 g/L. pH was pre-adjusted to 7.0 before sterilization. The nitrogen present in the medium originates from the soybean meal, which contains about 63.4% of protein.
Trace Element Solution (g/L)
MgCl2·6H2O, 25.0 g; MnCl2·4H2O, 1.25 g; FeCl3, 7.5 g; ZnSO4·4H2O, 12.5 g; CuCl2, 1.25 g; CaCl2·2H2O, 25 g; NaCl, 25 g.
Original medium was used as base of optimization and the composition was as following (g/L): glycerol, 20; dextrin, 15; soybean meal, 40; KH2PO4, 1.2; triolein, 15; trace element solution, 2.
Biomass (Packed Mycelium Volume) Measurement
Packed mycelium volume (PMV) was measured according to method of Lee [22]. Ten-milliliter samples of the culture broth were centrifuged for 20 min at 2100 g and separated into supernatant fluids and sediments. The volume of sediments cells was expressed as a percentage.
Residual Glycerol
The residual glycerol was measured with glycerol enzymatic kit.
CA Assay
CA in the fermentation broth was measured by Agilent HPLC using following procedure (Weiqida Pharma).
Sample Preparation
Accurately weigh 0.6 ± 0.02 g of fermentation broth in 100 ml volumetric flask, then add in diluent liquid(20 mM NaH2PO4, pH5.4, 5.7% acetonitrile)to 100 ml and shake to mixing. Then, filter the mixture with 0.22um filter and collect the filtrate for HPLC assay.
Pure CA (96.17%, Weiqida Pharma) is used as standard.
HPLC Measurement
Agilent 1260 with SB-C18 column (4.6 × 100 mm, 5 μm) was used. The mobile phase was 10 mM Tetrabutylammonium hydrogen sulfate (Sinopharm), 11.5% acetonitrile (Tedia), pH adjusted to pH6.0 by adding NaOH. Flowrate was maintained at 1.0 ml/min. Detection wavelength was 210 nm and column temperature was 33℃. A twenty-microliter sample was injected to HPLC for measurement.
Fractional Factorial Design
Fractional factorial design was used to evaluate the relative importance of several nutrients for the production of CA. Experimental designs were performed using the Design Expert software (Stat-Ease Inc., Minneapolis, MN, USA, Version 6.0.). As shown in Tables 1 and 26–2 fractional factorial design (FFD) was used to investigate the statistical significance of the factors of soybean meal, glycerol, dextrin, KH2PO4, triolein, trace element solution (containing per liter: MgCl2·6H2O, 25.0 g; MnCl2·4H2O, 1.25 g; FeCl3, 7.5 g; ZnSO4·4H2O, 12.5 g; CuCl2, 1.25 g; CaCl2·2H2O, 25 g; NaCl, 25 g). In FFD, six independent variables representing six nutritional components were employed, each variable was tested in two levels, low and high level was coded as − 1 and + 1, and the midpoint was coded as 0. All experiments were carried out in triplicates. For statistical calculations, the relation between the coded values and actual values is described as the following equation:
where Xi is the coded value of independent variable, Ai is the actual value of independent variable, A0 is the actual value on the center point of independent variable, and ∆A is the step change of variable.
The settings of step change of variables for factors were based on the result of our previous single factor experiment and published literature [13].
Response Surface Analysis
Once the critical variables were screened based on the results of the FFD experiment, response surface methodology (RSM) was performed to further optimize the screened medium components to improve CA production using Box-Behnken design. A set of 17 experiments was required with three variables and each variable being at three levels (− 1, 0, + 1). The design matrix was shown in Table 3. The CA titre was taken as the response.
The Design Expert software was applied to analyze the obtained results. The quadratic model for the variables was as follows:
where Y is the predicted response, β0 is an intercept term, βi is the linear coefficient, βii is the squared coefficient, and βij is the interactive coefficient.
The above quadratic equation was used to build surfaces for variables. The optimal values of these factors were determined by response surface and point prediction with the Design Expert software.
Verification of the Optimized Model in Erlenmeyer Flask and 50-L Stirred Fermenter
After statistical optimization, the final optimized medium was verified in both scales of Erlenmeyer flask and 50-L stirred fermenter, respectively, and compared with performance of original basal medium.
The fermentation in Erlenmeyer flask was performed according to aforementioned condition in cultivation.
The 50-L stirred fermenter was equipped with two 6-bladed impellers. 1.4 L seed was transferred to the fermenter resulting in 35 L fermentation volume. Temperature was kept at 28 ℃ constantly during the whole fermentation. Aeration and agitation were started with 10 L/min and 300 RPM firstly, then automatically adjusted by controlling dissolved oxygen (DO) above 20%. pH was adjusted to 7.0 before inoculation and controlled at pH7 ± 0.1 with 20% ammonia. The fermentation was ended when pH rose up to 7.4.
Results and Discussion
Fractional Factorial Design
The significance of glycerol (X1), dextrin (X2), soybean meal (X3), KH2PO4 (X4), triolein (X5), and trace element (X6) was investigated using a 26–2 fractional factorial design. The experimental designs with 16 runs and six factors and their corresponding yield of CA were indicated in Table 1. On the basis of the response values, analysis of variance (ANOVA) was employed for the analysis of significant variables using Fisher’s statistical test. Final equation in terms of coded factors:
where Yca is the predicted CA yield; X1, X2, X3, X4, X5, and X6 are the factors of glycerol, dextrin, soybean meal, KH2PO4, triolein, and trace element, respectively.
The regression equation Eq. (3) was obtained from analysis of variance, if linear coefficient is positive, the impact of the variable on CA yield is greater at a high level; if it is negative, the impact of the variable on CA yield is greater at a low concentration. It was found that high concentrations of X1, X2, X4, X5, and X6 enhanced CA production, respectively, and that low concentration of soybean meal resulted in high CA production.
As seen from Table 2, the P values of X2 (dextrin), X3 (soybean meal), and X5 (triolein) are smaller than 0.001, which indicates they are the most significant variables to influence CA production. It is common knowledge that glycerol is critical for CA fermentation as it can provide C3 precursor for bio-synthesis of CA [10, 15, 22]. The non-significance of glycerol of this study is not conflict with literature and mature industrial process, since the concentration of glycerol in the FFD experiment is 15 and 25 g/L respectively, higher than 10–15 g/l of normal dosage of literature [15, 16, 23] and our own full-scale process. 0.004 of P value actually proves that glycerol is critical for CA fermentation, but 10–15 g/L in batch medium of our full-scale fermentation is a reasonable dosage. Dextrin is widely used [24] whereas few literatures investigated the impact of glycerol combining with dextrin on CA fermentation. Bellão et al. [25] found that glycerol promotes CA titers up to fivefold the observed in cultivations with starch as carbon source, but another study by Thakur et al. [26] demonstrated that the addition of dextrin or glycerol as a sole carbon source neither improved nor decreased the production of CA. The present study is the first time to find that dextrin can significantly increase titer of CA fermentation when combine with glycerol. Soy protein including soybean meal, isolated soya protein has been fully recognized as an ideal nitrogen source for CA fermentation [24] as soy protein can provide sufficient C5 precursor for CA biosynthesis [15]; this is agreed very well with the present study.
The predicted model fits the experimental data well as F value and P value of the model are 64.78 and 0.0001 respectively; the coefficient of determination R2 of the model is calculated to be 0.9878 indicates that the reliability of the variability in the data is 98.78%. In addition, the “curvature F value” of 45.96 implies that there is significant curvature (as measured by difference between the average of the center points and the average of the factorial points) in the design space. Thus, it was no need to perform the steepest ascent experiment.
Response Surface Analysis
X2 (dextrin), X3 (soybean meal), and X5 (triolein) were the most effective variables for enhancing the production of CA and further optimized using a Box-Behnken design. Experimental design and results were shown in Table 3. By analysis on the experimental data using the Design Expert software, the quadratic equation model Eq. (4) is given as follows:
where Yca is the predicted CA yield; X1, X2, and X3 are the screened factors of dextrin, soybean meal, and triolein, respectively. As shown in Table 4, it is clear that the line terms of dextrin, the square terms of dextrin, the interactive terms of dextrin and triolein, dextrin, and triolein are not significant (P > 0.1). The liner terms of soybean meal and triolein, the square terms of soybean meal and triolein, the interactive terms of dextrin and soybean meal had high significance on correlation of coefficients (P < 0.05), and the R2 (0.9342), F value (11.04), and P value (< 0.05) for the model and lack of fit F value (8.03) indicate that the model is a good representation of the data. The low coefficient of variation (CV) value of 7.03% indicates great degree of precision with which the treatments are compared. The model can be used to navigate the design space with the value of adequate precision (10.79) greater than 4.0.
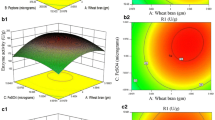
The effect of these three important medium components on CA production by Streptomyces clavuligerus was further analyzed using response surface plots, which were the graphical representations of the regression model. The response surface curves and its contour curves for the three variables are shown in Fig. 1. The response surface representing CA yield was a function of concentrations of the two medium components with another nutrient being at an optimal level. With these response surfaces, it was convenient to understand the interactions between any two medium components and to locate their optimum levels. The response surface plots showed that the highest CA production was obtained when the concentration of soybean meal was at the central level (Fig. 1a), and higher concentration of triolein and lower dextrin can enhance CA yield (Fig. 1a–c). By the Design Expert software, the maximum CA production of 938 mg/L was obtained when the optimum values of dextrin, soybean meal, and triolein were employed at 12.37 g/L, 39.75 g/L, and 26.98 ml/L, respectively. It was clear that the optimal values obtained from response surface plots were almost consistent with those obtained from the quadratic equation model. Validate experiments were performed in triplicates in shake flask under the optimized medium, and an average yield of CA went up to 946 mg/L, suggesting that the model was adequate. The final composition of medium optimized was (/L): glycerol, 20 g; dextrin, 12.37 g; soybean meal, 39.75 g; KH2PO4, 1.2 g; triolein, 26.98 ml; trace element solution 2 ml.
Verification of Optimized Medium in 50-L Stirred Fermenter
In general, a 47% increase was achieved through medium optimization by statistical approaches (Fig. 1). Comparing with performance of fermentation with original medium, CA titer in 50-L stirred fermenter was increased from 889 to 1310 mg/L (Fig. 1C) by fermentation with optimized medium. Although the duration of fermentation with optimized medium was 8 h longer than that of original medium, 30% increase of general productivity was obtained by fermentation with optimized medium. Specific productivity was calculated based on different phases of fermentation and it was found that specific productivity of both fermentations reached peak at 46 h, but it was 1.43 mg/kg/h for fermentation with optimized medium which was significantly higher than 0.91 mg/kg/h of fermentation with original medium (Fig. 1D). The main differences between original medium and optimized medium were dextrin and triolein. Dextrin was decreased from original 15 to 12.37 g/L while triolein was increased from original 15 g/L to 26.98. The result of verification fermentation with optimized medium agreed to some extent with Kim’s research [21] in which triolein was found the most suitable fatty acid for CA production comparing with tripalmitin, trilinolein, corn oil, olive oil, and palm oil.
Conclusion
The optimization of medium components is a critical process to improve the productivity of microbial fermentation. The one-factor approach usually does not depict the combined effect of all the factors involved; it is also a time-consuming process and requires a lot of experiments and fails to identify the optimal conditions for the bioprocess. Compared with the one-factor approach, the fractional factorial design and response surface methodology enable us to screen the significant factors from a large number of experimental factors and find out the best nutrients for maximizing the CA production. In the present work, three factors, soybean meal, dextrin, and triolein, were proved to be the most significant components for CA production by Streptomyces clavuligerus. The optimal medium concentrations were obtained by using response surface methodology and the CA production was increased from 630 mg/L (Table 1) to 946 mg/L, with 50.2% increase compared to that before optimization. The performance of fermentation with optimized medium in 50-L stirred fermenter under strictly control conditions shows that CA titer was increased to 1310 mg/L, 47% higher than that of parallel fermentation with original medium (Fig. 2c).
Data availability
Not applicable.
References
Paradkar, A. (2013). Clavulanic acid production by Streptomyces clavuligerus: biogenesis, regulation and strain improvement. The Journal of Antibiotics, 66, 411–420.
Huttner, A., Bielicki, J., Clements, M. N., Frimodt-Møller, N., Muller, A. E., Paccaud, J.-P., & Mouton, J. W. (2020). Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clinical Microbiology & Infection, 26(7), 871–879.
Valentine, B. P., Bailey, C. R., Doherty, A., Morris, J., Elson, S. W., Bagley, K. H., & Nicholson, N. H. (1993). Evidence that arginine is later metabolic intermediate than ornithine in the biosynthesis of clavulanic acid by Streptomyces clavuligerus. Journal of the Chemical Society, Chemical Communications, 15, 1210–1211.
Peng, Q., Gao, G., Lü, J., Long, Q., Chen, X., Zhang, F., Xu, M., Liu, K., Wang, Y., & Deng, Z. (2018). Engineered streptomyces lividans strains for optimal identification and expression of cryptic biosynthetic gene clusters. Frontiers in Microbiology, 9, 1–15.
Song, J. Y., Jensen, S. E., & Lee, K. J. (2010). Clavulanic acid biosynthesis and genetic manipulation for its overproduction. Applied Microbiology and Biotechnology, 88, 659–669.
Jnawali, H. N., Yoo, J. C., & Sohng, J. K. (2011). Improvement of clavulanic acid production in Streptomyces clavuligerus by genetic manipulation of structural biosynthesis genes. Biotechnology Letters, 33, 1221–1226.
Kurt Kizildogan, A., VanliJaccard, G., Mutlu, A., Sertdemir, I., & Özcengiz, G. (2017). Genetic engineering of an industrial strain of Streptomyces clavuligerus for further enhancement of clavulanic acid production. Turkish Journal of Biology, 41, 342–353.
Song, J. Y., Kim, E. S., Kim, D. W., Jensen, S. E., & Lee, K. J. (2008). Functional effects of increased copy number of the gene encoding proclavaminate amidino hydrolase on clavulanic acid production in Streptomyces clavuligerus ATCC 27064. Journal of Microbiology and Biotechnology, 18, 417–426.
Saudagar, P. S., & Singhal, R. S. (2007). Optimization of nutritional requirements and feeding strategies for clavulanic acid production by Streptomyces clavuligerus. Bioresource Technology, 98, 2010–2017.
Domingues, L. C. G., Teodoro, J. C., Hokka, C. O., Badino, A. C., & Araujo, M. L. G. C. (2010). Optimization of the glycerol-to-ornithine molar ratio in the feed medium for the continuous production of clavulanic acid by Streptomyces clavuligerus. Biochemical Engineering Journal, 53, 7–11.
Chen, K. C., Lin, Y. H., Tsai, C. M., Hsieh, C. H., & Houng, J. Y. (2002). Optimization of glycerol feeding for clavulanic acid production by Streptomyces clavuligerus with glycerol feeding. Biotechnology Letters, 24, 455–458.
Chen, K. C., Lin, Y. H., Wu, J. Y., & Hwang, S. C. (2003). Enhancement of clavulanic acid production in Streptomyces clavuligerus with ornithine feeding. Enzyme and Microbial Technology, 32, 152–156.
Wang, Y. H., Yang, B., Ren, J., Dong, M. L., Liang, D., & Xu, A. L. (2005). Optimization of medium composition for the production of clavulanic acid by Streptomyces clavuligerus. Process Biochemistry, 40, 1161–1166.
Ortiz, S. C., Hokka, C. O., & Badino, A. C. (2007). Utilization of soybean derivatives on clavulanic acid production by Streptomyces clavuligerus. Enzyme and Microbial Technology, 40, 1071–1077.
Silva Rodrigues, K. C., de Souza, A. T., Badino, A. C., Pedrolli, D. B., & Cerri, M. O. (2018). Microbiology Screening of medium constituents for clavulanic acid production by Streptomyces clavuligerus. Brazilian Journal of Microbiology, 49, 832–839.
Teodoro, J. C., Baptista-Neto, A., Cruz-Hernandez, I. L., Hokka, C. O., & Badino, A. C. (2006). Influence of feeding conditions on clavulanic acid production in fed-batch cultivation with medium containing glycerol. Applied Microbiology and Biotechnology, 72, 450–455.
Large, K. P., Mirjalili, N., Osborne, M., Peacock, L. M., Zormpaidis, V., Walsh, M., Cavanagh, M. E., Leadlay, P. F., & Ison, A. P. (1999). Lipase activity in Streptomycetes. Enzyme and Microbial Technology, 25, 569–575.
Young, T., Li, Y., & Efthimiou, G. (2019). Olive pomace oil can be used as an alternative carbon source for clavulanic acid production by Streptomyces clavuligerus. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-019-00719-5.
Maranesi, G. L., Baptista-Neto, A., Hokka, C. O., & Badino, A. C. (2005). Utilization of vegetable oil in the production of clavulanic acid by Streptomyces clavuligerus ATCC 27064. World Journal of Microbiology and Biotechnology, 21, 509–514.
Salem-Bekhit, M. M., Alanazi, F. K., & Alsarra, I. A. (2010). Improvement and enhancement of clavulanic acid production in Streptomyces clavuligerus using vegetable oils. African Journal of Biotechnology, 9, 6806–6812.
Kim, S.-J., Kim, J.-O., Shin, C.-H., Park, H. W., & Kim, C. W. (2009). An approach to strain improvement and enhance production of clavulanic acid in Streptomyces clavuligerus. Bioscience, Biotechnology, and Biochemistry, 73(1), 160–164.
Lee, S. D., Park, S. W., Oh, K. K., Hong, S. I., & Kim, S. W. (2002). Improvement for the production of clavulanic acid by mutant Streptomyces clavuligerus. Letters in Applied Microbiology, 34, 370–375.
Teodoro, J. C., Araujo, M. L. G. C., Hokka, C. O., & Badino, A. C. (2010). Influence of glycerol and ornithine feeding on clavulanic acid production by Streptomyces clavuligerus. Brazilian Journal of Chemical Engineering, 27, 499–506.
Ser, H.-L., Law, J.W.-F., Chaiyakunapruk, N., Jacob, S. A., Palanisamy, U. D., Chan, K.-G., Goh, B.-H., & Lee, L.-H. (2016). Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: A systematic review. Frontiers in Microbiology, 7, 522.
Bellão, C., Antonio, T., Araujo, M. L. G. C., & Badino, A. C. (2013). Production of clavulanic acid and cephamycin c by streptomyces clavuligerus under different fed-batch conditions. Brazilian Journal of Chemical Engineering, 30, 257–266.
Thakur, R., Roy, M. K., Dutta, N. N., & Bezbaruah, R. L. (1999). Coordinate production of cephamycin c and clavulanic acid by Streptomyces clavuligerus. Indian Journal of Experimental Biology, 37, 1031–1033.
Funding
This study was supported by the National Hi-Tech Program (863 Program 2006AA020302).
Author information
Authors and Affiliations
Contributions
Tao Feng: experiment design, execution, and paper writing; Jie Zhao: experiment execution for fermentation by 50 L fermentor. Ju Chu is the corresponding author and guides the research direction of the project. Yong-hong Wang supports the data statistical analysis. Ying-Ping Zhuang supports experiment design and data analysis.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, T., Zhao, J., Chu, J. et al. Statistical Optimizing of Medium for Clavulanic Acid Production by Streptomyces clavuligerus Using Response Surface Methodology. Appl Biochem Biotechnol 193, 3936–3948 (2021). https://doi.org/10.1007/s12010-021-03627-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03627-4