Abstract
Boron (B) toxicity often limits crop yield and the quality of production in agricultural areas. Here, we investigated the effects of calcium (Ca), silicon (Si) and salicylic acid (SA) on development of B toxicity, B allocation in canola (Brassica napus cultivar Sarw 4) and its role in non-enzymatic antioxidants in relation to yield of this cultivar under B toxicity. Canola seedlings were subjected to four B levels induced by boric acid in the absence or presence of Ca, Si and SA. The results showed that Ca, Si and SA addition ameliorated the inhibition in canola growth, water content (WC), and improved siliqua number, siliqua weight and seed index. The B content in shoots and roots and total B accumulation in the whole plant were increased in control plants under B-toxicity-stress, and these parameters were significantly decreased by addition of Ca, Si and SA. The shoot ascorbate pool (ascorbate, AsA, and dehydroascorbate, DHA), α-tocopherol and phenolics (free and bound) were increased under B toxicity, and were significantly decreased in most cases by addition of Ca, Si and SA, except α-tocopherol, which increased at low B levels (0, 25 and 50 mg kg soil−1). The glutathione content did not obviously change by B stress, while added Ca, Si and SA inhibited its accumulation under B stress. In addition, B toxicity reduced the shoot flavonoids content; however, this reduction was not alleviated by the use of Ca, Si and SA treatments. It could be concluded that growth and yield of canola plants grown under high B concentration improved after external application of Ca, Si or SA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron (B) is an essential micronutrient for growth and development of vascular plants, but excessive B in the soil or in irrigation water occurs in many dry areas of the world, including Egypt, Turkey, Iraq, Jordan, Syria, Morocco, Chile, USA, Spain and South Australia, and causes B toxicity (Ayvaz et al. 2013; Landi et al. 2012). Boron toxicity often limits crop yield and the quality of production in agricultural areas (Miwa et al. 2007). Even for boron-tolerant plants, boosting soil conditions by adding amendments is a needful step of reclamation (Kayama 2010).
Most of the ionic stresses including B trigger the formation of reactive oxygen species (ROS), which lead to the establishment of oxidative stress in plants. Plants with high levels of antioxidants have a great resistance to this oxidative damage (Foyer and Shigeoka 2011). Non-enzymatic antioxidants may act directly in the detoxification of ROS and radicals, or they can reduce substrates for antioxidant enzymes (Mittler 2002). Non-enzymatic components include the major cellular redox buffers ascorbate (AsA) and glutathione (GSH) as well as tocopherol, carotenoids, and phenolic compounds (Gratão et al. 2005; Mittler et al. 2004; Yıldırım and Uylaş 2016). AsA is a crucial component of the detoxification of ROS in the aqueous phase due to the ability to donate electrons in enzymatic and non-enzymatic reactions (Caverzan et al. 2016). Glutathione is oxidized by ROS to form oxidized glutathione (GSSG), which is present in all cellular compartments and along with its oxidized form maintains the redox balance in cellular compartments (Caverzan et al. 2016). Tocopherols is a group of lipophilic antioxidants (Sharma et al. 2012) and the α-tocopherol present in the membrane of chloroplasts protects them against photooxidative damage (Blokhina et al. 2003). Phenolic compounds are abundantly found in plant tissues and possess antioxidant properties (Grace and Logan 2000). The antioxidant activity of flavonoids is attributed to their reaction with free radicals as a hydrogen donor (Zhang et al. 2017). Few studies are available on the antioxidant response of plants submitted to toxic levels of B (Cervilla et al. 2012). This prompted us to conduct a broader study on non-enzymatic antioxidant protection of canola plants under high B stress.
Calcium (Ca) is an essential element that plays an important role in stress tolerance (Tuteja 2009). Also, Ca is an important signaling compound, regulates cellular metabolism, moreover, is important for energy production within mitochondria and chloroplasts (Batistič and Kudla 2010). Previous studies showed a major role of Ca in alleviating the negative effects of B (Kanwak et al. 2008; Turan et al. 2009). The interactions between Ca and B are still being discussed (Bolaños et al. 2004). Thus, studies of the interactive effects of Ca and B on plant growth are essential.
Silicon (Si) is the second most abundant element in the earth’s crust (Zhu and Gong 2014) and is taken up in the form of silicic acid, Si(OH)4, by plants. It is ultimately irreversibly precipitated throughout the plant as amorphous silica and is the only nutrient element that is not detrimental when accumulated excessively in plants (Ma et al. 2001). The positive roles of Si have been confirmed against biotic (Fauteux et al. 2006; Fallah 2012; Liang 1999) and abiotic stresses (Shi et al. 2016; Van Bockhaven et al. 2013; Zhu and Gong 2014). Si also protects the plant by other processes, which can boost the defense mechanisms, including the accumulation of lignin, phenolic compounds, and phytoalexins (Epstein 1999; Fawe et al. 2001; Ma and Yamaji 2006). Formation of B-Si (boron-silicate) complexes in the soil leads to lower B availability and consequently lowers tissue B accumulation (Inal et al. 2009). A limited number of investigations have been carried out with Si supplied to plants suffering from B toxicity. Therefore, the present study aimed to study the metabolic effect of Si in counteracting canola growth retardations induced under excess B toxicity.
Salicylic acid (SA) is a plant hormone that regulates many aspects of plant growth and development, as well as resistance to (a)biotic stress (Dempsey and Klessig 2017). The role of the SA in defense mechanisms under both biotic and abiotic stresses suggests that it also alleviates salt stress, B and cadmium toxicity in plants (Al-Hakimi and Hamada 2001; El-Feky et al. 2012, 2014; Eraslan et al. 2007; Metwally et al. 2003; Mimouni et al. 2016; Radi et al. 2014). Exogenous application of SA enhanced growth, physiological process and antioxidant activity and increase plant tolerance to the abiotic stress (Eraslan et al. 2007; He et al. 2002). In lower concentrations (less than 1 mM), it has been reported to be beneficial for plant growth (Rivas-San Vicente and Plasencia 2011). Recently, Yıldırım (2017) concluded that endogenous increase in SA attributed to lower B uptake, accumulation and higher B toxicity tolerance in Populus alba.
Canola quality rapeseed is one of the main oil crops world-wide but canola is a new crop in Egypt. Traditionally, Brassica napus is unsuitable as a source of food for either humans or animals due to the presence of two naturally occurring toxicants, erucic acid and glucosinolates. However, in the 1970s, very intensive breeding programs in several countries including Australia produced high quality varieties that were significantly lower in these two toxicants. Those cultivars must yield oil low in erucic acid (below 2%) and meal low in glucosinolates (total glucosinolates of 30 µmoles/g toasted oil free meal), and are often referred to as “double low” varieties. Due to the strong competition between canola and other strategic winter season crops on the limited arable land in Nile Valley and Delta the cultivated area of canola in Egypt is relatively small (Mekki 2013). Ghallab and Sharaan (2002) reported that the cultivation of canola in Egypt may provide an opportunity to overcome some of, the local deficit of edible vegetable oil production. Particularly, it could be successfully grown during winter season in newly reclaimed land outside the Nile Valley soils to get around the competition with other crops engaged the old cultivated area. Since, 1978 some spring types of Brassica napus L. have been introduced from Europe and evaluated under Egyptian conditions and the crop has great promise as an oilseed crop in the winter season in Egypt (Mekki 2007; Sharaan and Ghallab 2002). Some canola genotypes were evaluated under newly reclaimed sandy soil conditions in Egypt and were found that Serw 4 had the highest seed yield, out of six, test cultivars (Mekki 2013).
Canola has a higher requirement for B than do cereals (Grant and Bailey 1993). B is essential to development of both male and female reproductive organs in canola (Asad et al. 2002; Dell and Huang 1997) and is an important factor in the fertilization process of flowers. B-deficient plants may grow normally but seed yield may be severely reduced (Gupta 1993). Application of B to canola has been found to significantly increase grain oil content (Pageau et al. 1999). However, if soluble B fertilizer placed close to seed and on sands that have limited B sorption capacity it may cause toxicity in crops (Moraghan and Mascagni 1991). Hughes-Games (1991) reported larger variations in tolerance of canola to B toxicity than barley and wheat. Tolerance was suggested to be related to the origin of a cultivar (Du et al. 2002).
Boron toxicity is an important nutritional disorder that can limit plant growth and productivity in arid and semi-arid environments. Elbehiry et al. (2017) found that B toxicity representing more than 50% of northern western part of Egypt Nile Delta profiles. This area is dominated by fine texture, irrigated by mixed and contaminated water, and has low content of organic matter, which contributes as a contamination source of B. Thus, in this investigation it seemed necessary to consider some physiological and biochemical responses of canola cultivar Serw 4 and how far these responses are correlated with the B-tolerance mechanisms at different B levels. Particular attention was focused to investigate the role of Ca, Si, and SA in counteracting the adverse effects of B toxicity on canola plants at vegetative growth and productivity stages, and consequently to cultivate these plants in the newly reclaimed Egyptian soil.
Materials and methods
Two experiments were carried out in a greenhouse of Faculty of Science, Assiut University (27°11′00′’N 31°10′00′’E) with day/night temperature of 25–30/12–16 °C, relative humidity of 42–48% and daily photon flux density of about 900–1100 µmol m−2 S in order to assess the possible involvement of 15 mM Ca as CaCl2 (this concentration was adjusted from preliminary experiments), 2 mM Si as Na2SiO3 (Eraslan et al. 2008), and 0.5 mM SA as sodium salicylate (Metwally et al. 2003), in adaptation of canola plant to B toxicity. The seeds of canola (Brassica napus L.) cultivar Sarw 4, commonly used in Egypt, were kindly supplied by the Agricultural Research Center, Giza, Egypt.
Experiment 1: short duration treatment (vegetative stage)
The seeds of canola were planted in plastic pots lined with polyethylene bags and filled with 2 kg of soil. Experimental soil was loam in texture (46% sand, 24% silt and clay 30%), containing 7.95% CaCO3 and had a pH (1: 1 water extract) of 7.8, 3.86 ds m−1 EC (1: 1 soil: water), 1.3 mg kg−1 B, 3412.5 mg kg−1 CO3−2 + HCO3−, 816.5 mg kg−1 Cl−, 360 mg kg−1 Ca2+, and 144 mg kg−1 Mg2+ mg kg−1. Treatments, with six replicates, consisted of four groups. The first group was B only in final concentration of: 0 (control), 25, 50 and 100 mg kg−1 soil B as boric acid. Ca group was Ca (15 mM) combined with B levels as: 0 + Ca, 25 + Ca, 50 + Ca and 100 + Ca. Si group was Si (2 mM) combined with B levels as: 0 + Si, 25 + Si, 50 + Si and 100 + Si. SA group was SA (0.5 mM) combined with B levels as: 0 + SA, 25 + SA, 50 + SA, and 100 + SA. All these treatments were applied and incorporated into the soil before seed sowing with the basal fertilization of 120 mg N kg soil−1, 100 mg P kg soil−1, and 50 mg K kg soil−1, and the pH was buffered to 5.7. Canola seeds were sown at the rate of 15 seeds to each pot. After a good stand of plants developed, they were thinned to 10 plants per pot. Soil was kept at approximately 90% field capacity by watering with tap water under the above mentioned condition.
Harvesting and plant growth yield
At the end of the experimental period (1 month), plants were fractionated into roots and shoots. Plant height and root length were measured. The roots were only briefly rinsed with deionized water, in order to avoid washing out of freshly acquired B, blotted gently with filter paper. The shoots and roots were quickly weighed separately for fresh weight (FW) determination, immediately frozen in liquid nitrogen and stored at − 80 °C for further analysis. Another fraction of freshly harvested roots and shoots were oven-dried at 60 °C for 48 h in order to determine the dry weight (DW), relative water content and to follow some analysis.
Experiment 2: long duration treatment (fruiting stage)
Canola plants were grown under the growth conditions mentioned above. Treatments, with six replicates, consisted of following combinations: six final B levels, 0, 10, 20, 30, 40 and 50 mg kg soil−1. One Ca2+ level (15 mM) combined with B levels (mg kg soil−1), 0 + Ca, 10 + Ca, 20 + Ca, 30 + Ca, 40 + Ca and 50 + Ca. One Si level (2 mM) combined with B levels, 0 + Si, 10 + Si, 20 + Si, 30 + Si, 40 + Si and 50 + Si. One SA level (0.5 mM) combined with B levels, 0 + SA, 10 + SA, 20 + SA, 30 + SA, 40 + SA and 50 + SA. At the end of the experimental period (4 months) a number of parameters such as number of siliqua, siliqua weight, seed index [100 seed weight (g)] were determined.
Physiological and biochemical analysis
Total flavonoid content
Total flavonoid content was measured as described in Moreno et al. (2000) with a slight modification, and the results were expressed as quercetin equivalents. Methanol extract was added to test tubes containing 0.1 ml of 10% aluminum nitrate, 0.1 ml of a 1 M potassium acetate solution and 3.8 ml of methanol. After 40 min at room temperature, the absorbance was measured at 415 nm. Quercetin was used as a standard. The results were expressed as mg g FW−1.
Determination of ascorbate and dehydroascorbate
Ascorbate (AsA) and dehydroascorbate (DHA) were measured according to Kampfenkel et al. (1995) with minor modifications. Briefly, total ascorbate (TA) (AsA + DHA) was determined after reduction of DHA to AsA with dithiothreitol (DTT), and the concentration of DHA was estimated from the difference between the total ascorbate pool (TA) and AsA. Shoot samples (0.3 g) were homogenized in 6% trichloroacetic acid (TCA) pre-chilled on ice. The homogenate was then centrifuged at 12,000g for 10 min and the resulting supernatant was used for the determination of TA and AsA. The reaction mixture for the TA pool contained 0.1 ml aliquot of the supernatant, 0.25 ml of 50 mM phosphate buffer (pH 7.5) containing 2.5 mM ethylenediamine tetraacetic acid (EDTA), and 0.05 ml of 10 mM DTT. After incubation for 10 min at room temperature, 0.05 ml of 0.5% N-ethylmaleimide was added to remove excess DTT. AsA was determined in a similar reaction mixture, except that 0.1 ml of H2O was added rather than DTT and N-ethylmaleimide. Color was developed in both reaction mixtures after the addition of the following reagents: 0.2 ml of 10% TCA, 0.2 ml of 44% ortho-phosphoric acid, 0.2 ml of 4% a,a′-dipyridyl in 70% ethanol, and 0.3% (w/v) FeCl3. After vortexing, the mixture was incubated at 40 °C for 40 min. Then, TA and AsA were determined when monitored at 525 nm using a spectrophotometer (Unico UV-2100 spectrophotometer). The results were expressed as mg g FW−1.
Glutathione
Total glutathione (GSH + GSSG) levels were determined according to the method of Griffith and Meister (1979). The results were expressed as µmol min−1 g FW−1.
Determination of α-tocopherol (vitamin E) content
Fresh shoots were harvested from each treatment (0.5 g) and stored in liquid nitrogen at − 80 °C. Plant α-tocopherol was extracted and purified by following procedure as described by Schmieden and Wild (1994).
HPLC analysis was carried out using Agilent HPLC 1200 (Santa Clara, CA) system consisting of degasser, quaternary pump, and a fluorescence detector (FLD). HPLC chemstation software (Agilent) was used for instrument control, data acquisition, and data analysis. α-Tocopherol was separated on Zorbax Eclipse -C18 column 4.6 × 150 mm, 5 µm particle size. The column temperature was maintained at 30 °C. The injection volume was 20 µl and the flow rate was 1 ml/min. Each analysis was carried out in triplicate. The elution system consisted of solvent A: methanol and B: acetonitrile with a flow rate as following: 0–4 min, 10–14 min 70% B at a flow rate 1 ml/min and 4–10 min 0% B at a flow rate 1.2 min. The eluates were monitored by FLD at 295 nm, and emission wavelength of 330 nm. α-Tocopherol was calculated from a standard curve prepared with α-tocopherol.
Determination of free and cell wall-bound phenolics
Free and cell wall-bound phenolics were determined according to Kofalvi and Nassuth (1995). Phenolic concentration in the extract was determined from standard curve prepared with gallic acid. The results were expressed as µg g FW−1.
Determination of boron
Shoot and root samples (0.01 g DW) were dry ashed in a muffle furnace at 500 °C for 6 h by a method described by Gaines and Mitchell (1979). The ash was then dissolved in 0.1 N HCl. For B concentration measurement, B was determined using flame atomic absorption spectrometry (AAS).
Statistical analysis
Each pot contained ten plants in the short duration treatments (vegetative stage), and 5 plants in the long duration treatments (fruiting stage). Each treatment contained six replicates pots. Two way ANOVA was performed on the data with six replicates of six measurements from two independent experiments. Analysis of two-way ANOVA was performed using the SPSS statistical 22.0 package.
For comparison of the means, a posthoc test (Tukey’s multiple range tests) (P < 0.05) were used for significant differences. Analysis of correlation (Pearson’s correlation) was performed to obtain the relation between mean values of different parameters of canola cultivar under boron and Ca, Si or SA treatments.
Results
Development of B toxicity in canola
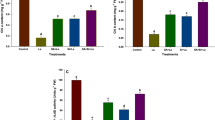
Results of the first experiment revealed that plant height and root length did not exhibit any significant reduction at low concentration of B (25 mg kg soil−1), as compared to control (experiment 1; Fig. 1, Fig. S1 (at 15-day), Tables S1, S2). On the other hand, higher concentrations (50 and 100 mg kg soil−1) caused a 47 and 66% reduction in the height of the tested plants, respectively. For the same respective B treatments, inhibition in root elongation was measured as 66 and 88%, compared to control.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on root and shoot lengths of 30-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD-post-hoc)
Treatments with Ca, Si or SA significantly increased the height and root length of canola plants more than those of stressed plants. B toxicity-dependent reductions in the height of canola at the higher B concentrations (50–100 mg kg soil−1) were measured as 8.8, 26.87 and 22.09% for Ca, Si and SA treatments, respectively. Similar improvement in Ca, Si and SA applications were also recorded for root growth at higher B toxicity treatments. The reductions in root length at 100 mg kg soil−1 B treatment for each respective application was 59.86, 74.05 and 75.02%, compared to control.
The successive increase in B concentration induced an inhibitory effect on the shoots and roots dry weight and relative water content of canola plants (experiment 1; Fig. 2, Tables S1, S2). Compared to control, the highest reduction in dry weight of the shoots (90.77%) and roots (96.87%) were recorded at 100 mg kg soil−1 B treatment. Similarly, relative water content of shoots and roots severely reduced at the same B toxicity and reached to 58.43 and 73.56% reductions, respectively.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on root and shoot dry weight (DW) and relative water content (WC) of root and shoot of 30-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD-post-hoc)
Application of Ca, Si or SA to B-stressed plants reduced the negative effect of low and moderate B levels (25 and 50 mg kg soil−1) on shoots and roots dry weight and relative water content. Although Ca, Si, and SA applications had significant improvement in shoot dry weight and relative water content, their effects on root dry weight and root relative water content were found to be insignificant, compared to 100 mg kg soil−1 B treated plants. Compared to control plants, reduction in shoot dry weight with application of Ca, Si and SA were recorded to be 66.52, 75.53 and 81.68%, while the decrease in shoot relative water content for each respective applications was 14.67, 16.09 and 15.72% at 100 mg kg soil−1 B treatment.
The results of this study indicated that shoot (–0.79) and root (–0.94) relative water content represented a strong negative correlation with increase in B concentration in the soil. As it was expected, Ca and SA applications also exhibited a similar but smaller negative correlations between shoot (–0.39) relative water content and increase in soil-B concentration. Interestingly, Si application conversely revealed a significantly positive correlation (0.24) between the same parameters.
B allocation in plants
Results indicated that the plant shoots contain a higher concentration of B than roots. These results confirmed by B accumulation in shoots, which recorded its highest value (sixfold) at 100 mg kg soil−1, compared to their B toxicity treated roots (experiment 1; Fig. 3, Tables S1, S2). Also, B in roots (3.3-fold) and total accumulation in whole plant (1.8-fold) were increased with increasing B concentration in the medium.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on root and shoot boron concentrations and % boron accumulation in whole plant of 30-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD-post-hoc)
Ca supply reduced B accumulation in both shoots (56.09%) and roots (46.03%), compared to B-treated plants at 100 mg kg soil−1 B concentration (Fig. 3). At the same B toxicity treatment, 54.68% reduction in total B accumulation were also recorded with Ca application in comparison to B-treated plants.
Similar to Ca application, Si also reduced total B uploading to the entire plant (34.13%) and to the shoots (41.48%), compared to B treated plants at 100 mg kg soil−1 B treatment (Fig. 3). Contrary to shoots, B accumulation at the same treatment increased 10.73% in the roots, compared to B-treated plants.
Application of SA also caused a reduction in B accumulation in shoots (67.85%) and roots (23.35%) as well as total accumulation in whole plant (61.59%) of the 100 mg kg soil−1 B-stressed plants, compared to their B-toxicity-treated controls (Fig. 3).
Non-enzymatic antioxidants
As B was transported and accumulated in shoot system, all the studied parameters were done on shoots of canola plants.
Ascorbate pool (vit. C)
The data presented in Fig. 4 and Table S1 and S2 (experiment 1) showed that B with or without Ca, Si and SA did not enhance AsA content (reduced form) over their corresponding absolute control of canola shoots; only the highest level (100 mg kg soil−1) without Ca, Si or SA enhanced it.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on shoot ascorbate and dehydro-ascorbate of 30-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD-post-hoc)
On the other side, DHA (oxidized form) of canola plant increased gradually with the rise of B level (Fig. 4). The highest DHA content in shoots was consistently found in plants grown at the highest B level. However, application of Ca, Si and SA caused an inhibition in DHA content in B-stressed plants as evidenced by 61.95, 71.52 and 62.29%, respectively, compared to stressed controls.
Tocopherol (Vit. E)
B toxicity had stimulatory effects on shoot α-tocopherol content at the highest B level (100 mg kg soil−1), while 25 and 50 mg kg soil−1 B failed to induce the same response, when compared with their absolute control (experiment 1; Fig. 5, Tables S1, S2). Overall, Ca, Si and SA in this investigation had significant stimulatory effects on α-tocopherol content in canola shoots under both B toxicity and non-B toxicity conditions, compared to absolute controls. The most effective role was played by SA under both B toxicity (in most cases) and non-B toxicity conditions. In the presence of SA, the highest stimulation in α-tocopherol content was found to be 2494.59% over the control without B toxicity and 2422.96% at 25 mg kg soil−1 B treatment, as compared to B-treated plants.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on shoot alpha-tocopherol, flavonoids and glutathione (GSH) of 30-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD- post-hoc)
Flavonoids
Increasing B level from 25 to 100 mg kg soil−1 reduced the biosynthesis of flavonoids in shoots of the tested plant (experiment 1; Fig. 5, Tables S1, S2). However, this reduction was not alleviated by the use of Ca, Si and SA treatments. In this respect, under non-B toxicity conditions, Ca and Si decreased flavonoids content in shoots of canola plants, compared to absolute control.
Glutathione
The results of the current study (experiment 1; Fig. 5, Tables S1, S2) revealed that increase in soil B (25–100 mg kg soil−1) concentrations did not induce any significant alteration in glutathione accumulation in shoots of canola plants (P ≤ 0.1686). On the other hand, application of Ca, Si and SA caused an inhibition (3.30, 14.45 and 11.62%, respectively) in glutathione content of the 100 mg kg soil−1 B-stressed plants, compared to their B toxicity treated controls.
Free phenolics
Boron toxicity has a major effect on free phenolic compounds content. The data in Fig. 6 and supplementary Tables 1 and 2 (experiment 1) clearly demonstrated that free phenolics compounds accumulation in shoots of canola plants was stimulated by all the B levels. In addition, free phenolics compounds accumulation increased gradually with the rise of B level. The highest free phenolics compounds content in shoots was consistently found in plants grown at the highest B level (100 mg kg soil−1). Application of Ca, Si and SA significantly controlled the rise of free phenolics accumulation in B toxicity applied plants. As can be seen Fig. 6, especially Ca and SA had the most significant effect on the reduction of free phenolics accumulation in B toxicity treated canola shoot.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on shoot free and bound phenolics of 30-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD-post-hoc)
Bound phenolics
Based on the data presented in Fig. 6 and Table S1 and S2 (experiment 1), it can be observed that the increase in B supply, in the range studied, had a considerable stimulatory effect on bound phenolics compounds accumulation in shoots of canola plants. The highest bound phenolics compounds accumulation was consistently found in plants grown in the highest soil B concentration.
Ca, Si and SA in this investigation had a noticeable effect on bound phenolics content. At 50 and 100 mg kg soil−1 B, Ca, Si and SA treatments showed a uniform pattern in their response through reducing the bound phenolics content under B toxicity stresses to a significant level, compared to their corresponding controls. The minimum values were recorded in canola for plants treated with Ca and SA and grown under B toxicity conditions, respectively.
Crop yield
Siliqua number
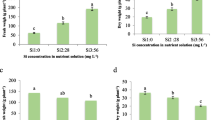
Under B toxic conditions, external application of Ca, Si and SA significantly alleviated the inhibitory effect of B toxicity on siliqua number up to 40 mg kg soil−1 B concentration (experiment 2; Fig. 7, Tables S1, S2). At the highest B level (50 mg kg soil−1), the most effective alleviation treatments were Ca, and SA, respectively. For instance, at 50 mg kg soil−1 B with Ca and SA treatments the siliqua number increased to 134.61 and 63.46%, respectively, than that of respective B-stressed control, which decreased 56.70% than control.
Effect of calcium (Ca), silicon (Si) or salicylic acid (SA) on siliqua number, siliqua weight, seed index and siliqua size of 120-day-old canola plants under different boron concentration. The data are means ± SD (n = 6). Different letters, capital for B treatments and small for Ca, Si and SA, indicate significant (two way ANOVA; Tukey HSD-post-hoc)
Siliqua weight
From the data in Fig. 7 and Tables S1 and S2 (experiment 2), it can be observed that B toxicity stress caused a significant reduction in siliqua weight of canola plants, reaching to 91.28%, as compared to control. Application of Ca, Si and SA alleviated the inhibitory effect of B toxicity up to 40 mg kg soil−1 concentration. Only Ca and SA were able to alleviate the negative effect of B toxicity on siliqua weight at 50 mg kg soil−1 concentration (29.51 and 64.98%, respectively, less than control).
Seed index
The results depicted in Fig. 7 and Table S1 and S2 (experiment 2) showed that application of Ca, Si and SA succeeded in alleviating the inhibitory effect of B toxicity on crop yield (seed weight) of canola plants. Only, Ca and SA were found to be effective in enhancing crop yield under B toxicity conditions, where they increased the seed weight. Specifically, the seed index was decreased to 56.34 and 61.47% at 50 mg kg soil−1 B with Ca and SA treatments, respectively, of control. In addition to inhibition of crop production under B toxicity stress, the highest B concentration (50 mg kg soil−1) with or without Si treatment led to collapse in plant yield.
Discussion
In this study, the impacts of Ca, Si and SA as an agriculturally effective fertilizer element on mitigation of B toxicity were studied in canola.
Development of B toxicity in canola
In the current study, canola did not show great visible shoot injury symptoms (Fig. S 1). The symptoms restricted as an injured zone all around the margin accompanied by leaf curling, while the adverse effects of B excess were alleviated by Ca, Si, and SA treatments. In certain species, however, yield reductions may occur without visible shoot injury symptoms (Francois 1986).
Our results showed that B treatments significantly decreased the growth as well as shoot and root relative water content. A similar adverse effect of B toxicity on shoot growth was previously reported in canola (Koohkan and Maftoun 2016). The strong negative correlation between water content and B concentration in shoots and roots confirms earlier reports of Pandey (2013) who concluded that excess B resulted in poor water uptake leading to water stress in brassica.
Ca application alleviated B toxicity, as manifested by increases in shoot and root lengths, shoot and root dry weight and water content of shoot and root, compared to B-stressed plants. These results suggest that Ca plays an important role in alleviating the damage to canola incurred under B toxicity conditions. Similar alleviation activity by applied CaCl2 has been reported for different plant species under B toxicity stress conditions (El-Feky et al. 2012; Siddiqui et al. 2013; Turan et al. 2009). The negative weak and strong correlations between water content and B concentration in shoots and roots, respectively, confirmed that maintaining critical Ca levels may be required to keep the water channels open through gating processes such as phosphorylation (Azad et al. 2004; Martínez-Ballesta et al. 2008). As most plant Ca is stored in leaves and root to shoot transfer of Ca2+ has been elaborated at length (Gilliham et al. 2011). The results of this study suggest that Ca alleviates B toxicity by improving plant-water status.
Data reported here showed beneficial effects of Si on canola plant growth under B toxicity conditions, and corroborate the beneficial effects of Si observed on barley plant under 20 mg B kg soil−1 stress (Inal et al. 2009). Furthermore, Hossain et al. (2002) and Hattori et al. (2003) suggested that Si increased plant growth by increasing the cell wall extensibility in the plant roots and shoots. According to the weak and strong correlations between water content and B concentration in shoots and roots, respectively, we speculate the improved water status in shoot was due to silicate crystals deposited in the epidermal cells form a barrier that reduces water loss through the cuticle and improve water relations in plant tissues (Romero-Ananda et al. 2006). Under salt stress, Si can improve plant tolerance through enhancing root water uptake, which contributes to the regulation of aquaporin activity and gene expression (Liu et al. 2015; Zhu et al. 2015). These results suggest that the Si-improved plant-water status could be the major reason for the increased growth parameters under the B- toxicity condition. Accordingly, Akcay and Erkan (2016) found that PIP1 type aquaporin upregulation in B, Si, and B + Si treated barley shoots at day 1 might have provided the tissues with water and CO2 to cope with stress and helped their adjustments to changing environmental conditions.
In the present study, SA application significantly inhibited the decrease in growth under B-toxicity condition, and water content was also alleviated in SA-treated plants compared with SA-untreated plants, indicating that SA application alleviated the B- toxicity. The present result is consistent with the previous findings of El-Feky et al. (2012). The improvement of water content of stressed plants by SA could be another explanation for growth enhancement in B-stressed plants. In this respect, Boursiac et al. (2008) suggested that SA can regulate root water transport as a regulator of aquaporin activity.
B allocation in plants
In our study, an increase of B supply concentration resulted in a correspondent increase in the B concentration of all plant organs. B accumulated principally in the shoots (six-fold) than roots, which reveal that the distribution of B significantly correlated with the increase of B levels. Moreover, total B accumulation in whole plant was increased by increasing B supply, these results have also been observed in previous studies (Koohkan and Maftoun 2016).
The application of Ca reduced accumulation of B in both root and shoot, distribution and total B accumulation in whole plant in the present study at high B concentration (50 and 100 mg kg soil−1). This might be explained as a protective effect resulting from the essential role of Ca in the physiological process i.e. Ca plays an important role in the cell wall and controls the translocation of root-B to leaves. Whereas under B toxicity, the aquaporins nodulin 26-like intrinsic proteins (NIPs), the tonoplast intrinsic proteins (TIPs), and the plasma membrane intrinsic proteins (PIPs) are involved in reducing the accumulation of toxic boric acid levels in plant tissues (Wang et al. 2016). In the response of plants to environmental stimuli, the functions of aquaporin could be regulated by Ca2+ via reversible phosphorylation (Wang et al. 2016). Similar alleviation by applied Ca has been reported for wheat, barley and radish species under B toxicity (El-Feky et al. 2012; Siddiqui et al. 2013; Turan et al. 2009). Gupta and Macleod (1981) and Taban et al. (1995) also concluded that the application of Ca could reduce the availability of B, resulting in decreased uptake of B. Moreover, Turan et al. (2009) concluded that localization of Ca in cell wall leads to decreased cell wall B permeability.
In the present study, the B content in root and shoot, B distribution and total B accumulation in whole plant were lower in plants treated with Si than in untreated ones, except at 100 mg kg soil−1 the B content in root was unaffected by Si treatment. The application of Si could reduce the availability of B, resulting in decreased uptake of B. Supplementation of Si could lead to thick lignified/suberized exodermis, which could directly delay the entry of B into the roots. This suggestion was confirmed by the result of Fleck et al. (2011) who concluded that Si nutrition of rice plants reduced the oxidation power of roots and enhanced the development of casparian bands in the exodermis and endodermis, as well as lignin depositions in the sclerenchyma. Also, the result of Cheng et al. (2014) who concluded that the relatively higher metal (Pb, Zn and Cu) tolerances in rhizophoraceous mangrove species are ascribed to their thick lignified/suberized exodermis, which could directly delay the entry of metals into the roots. Both Si and B are classified as metalloids. They both form uncharged acidic compounds at neutral pH and were absorbed into plant tissues in nonionic form, which is uncommon with other nutrients (Reid 2010). Akcay and Erkan (2016) showed that the presence of Si under B stress reduces shoot and root B levels, when compared to B accumulation under B stress alone. They also suggested that some form of competition through a channel protein and preference of transporter protein in favor of Si intake might be another likely explanation. In accordance with these results Farooq et al. (2015) suggested that the positive effect of Si application in decreasing B uptake might be attributed to the formation of B-silicate complexes in the soil. Gunes et al. (2007) also found that supplemental Si reduced B translocation from the roots to shoots of spinach plants and this effect could be related to Si being irreversibly precipitated as amorphous silica (SiO2∙nH2O) in the cell walls and lumens (Epstein 1999).
From our results, it was clear that SA reduced the B-toxicity stress condition by reducing the accumulation of B inside the plant organs. Also, SA mediated the reduction in the root-to-shoot B distribution. These results are in agreement with the findings of El-Feky et al. (2012, 2014) who found that SA decreased B content of barley tissues under B toxicity. Recently, Yıldırım (2017) reported that lower B accumulation, better growth performance and higher chlorophyll content of Populus alba was attributed to endogenous increase in SA production and its contribution on gene regulation under B toxicity. We speculated that SA may work through some special mechanism, such as diminishing the uptake, or activating the efflux from the roots, i.e. by mechanisms leading to lower cytoplasmic B content. However, the role of exogenously applied SA under B stress in plants is not yet clear and needs further investigations.
Non-enzymatic antioxidants
In this respect, Mittler (2002) concluded that non-enzymatic antioxidant activity is represented by a series of antioxidant molecules that the plant uses against ROS formation. In the current study, AsA and α-tocopherol significantly increased in shoots under the highest B level, while, DHA and phenolics (free and bound) of canola shoot increased with the rise of B level. Our results, further revealed that B levels used failed to change the glutathione content in shoots (P ≤ 0.1686) and the correlation between B in shoot was weak (0.233), however increasing B level reduced flavonoids content in canola shoots. These antioxidants could be important in protecting against oxidative stress triggered by high B concentrations. Molassiotis et al. (2006) concluded that excess B stimulates the non-enzymatic antioxidant mechanism in the apple rootstock EM 9 (Malus domestica Borkh) explants. In tomato, Cervilla et al. (2007) suggested that oxidative stress caused by B toxicity both increases the AsA pool and increases its oxidation. According to De Gara et al. (2000), DHA accumulation is generally considered a negative event for the cell metabolism. In contrast, Keles et al. (2004) found that the excessive B concentration inhibits the synthesis of α-tocopherol in the leaves of the orange plant. Munné-Bosch et al. (1999) also recorded that the increases in α-tocopherol observed in water-stressed plants might prevent chlorophyll photooxidation. Regarding glutathione, Yadav (2010) suggested that the availability of GSH is linked to the production of phytochelatins, which are used to alleviate the toxicity by nutrients and heavy metals.
In this context, Yıldırım (2017) suggested that higher up regulation of glutathione S-transferase, heavy metal-associated isoprenylated plant protein and ABC transporters in Populus nigra associated to internal detoxification of excess B under toxic condition. Cervilla et al. (2007) also showed that GSH amount increased in tomato specie (Kosaco), while no significant change was observed in the other (Josefina). Our result reveals that there might be no relationship between B content of canola and GSH accumulation. In dissimilarity with our result, Cervilla et al. (2012) recorded an increase in flavonoids content in tomato plants under the treatment with 2 mM of B. Our result suggested that the reduction of flavonoids resulted in impairment of B tolerance. Similar to our result, Cervilla et al. (2012) found that total concentration of phenols significantly increased under B treatment in tomato plants, this result suggesting a role of these secondary metabolites in the defence mechanisms against B stress.
Ca, Si and SA caused an inhibition in accumulation of AsA, DHA, tocopherol, phenolics and glutathione however did not alleviate the reduction of flavinoids in 100 mg kg soil−1 B-stressed plants, compared to corresponding stressed controls. Decreases in AsA, an antioxidant molecule capable of directly quenching ROS (Salin 1988) and H2O2 (Noctor and Foyer 1998), might be attributed to the reduction in B uptake and accumulation.
Ca considerably protected B-induced oxidative injury in canola shoots, and this protection might be related to the avoidance of ROS generation and the reduction of B uptake. These results ask for further investigation.
We speculated that Si-mediated alleviation of oxidative damage by strengthening the structural integrity of cell membranes, particularly during B stress that lead to hampered B uptake. In this respect, Liang et al. (2015) suggested that Si might help to maintain membrane integrity and decrease permeability. Ma et al. (2016) also suggested that Si supplement wheat plant showed lower lipid peroxidation. Further study is needed to elucidate how Si initiates these responses.
Similarly, the effect of SA on non-enzymatic antioxidant in SA-treated plants could be responsible for the lower accumulation of H2O2 and MDA at SA application, which suggests that oxidative damage induced by B stress is alleviated by the addition of SA.
Crop yield
Our results showed that excess B induced a significant reduction in yield components of canola plants, however external application of Ca, Si and SA alleviated these inhibitory effects to varying degrees. In this respect, the most effective alleviation treatment was Ca followed by SA and Si. The results of the current study suggested that the inhibition of canola plant yield by B toxicity stress was associated with reduced seedling growth, water content and increased B ions. Boron treatments in our earlier studies were found to cause a reduction in tiller number of two wheat cultivars (Metwally et al. 2017). Kaya et al. (2009) observed that B toxicity caused a remarkable reduction in fruit yield and growth in tomato plants. Canola seed yield varied significantly among the cultivars and B application decreased the seed yield (Öztürk et al. 2010).
The pronounced effects of Ca, Si and SA on the improvement of yield under B stress may also be due to its role in enhancement of the water uptake, which was impaired by B stress. Results of the present study indicated a significant decrease in the uptake of the water by B stress in both shoots and roots of canola plants. However, application of Ca, Si and SA remarkably alleviated the negative effect of B on the water uptake. Also, when Ca, Si and SA were co-applied with B, a significant decrease in B accumulation was observed in B-stressed canola plants, resulting in a significant increase of the growth parameters of the stressed plants as well as yield.
Our results are in agreement with the results reported in other plant species Sotiropoulos et al. (2002) reported that Ca partially protected kiwifruit plants against the harmful effects of B excess. Moreover, El-Feky et al. (2014) recorded an increase in weight of straw, grains and 100-grains of B-stressed (3.0 mg l−1 B) barley treated with 5 mM CaCl2.
Si enhances growth, yield and crop quality in response to abiotic and biotic stresses (for review, see Coskun et al. 2016). Little is known about the mechanism underlying Si-increased crop yield under B toxicity in plants. The role played by Si in counteracting the damage on yield induced under B stress was evident in our results. Similarly, Ahmed et al. (2016) reported that yield parameters such as plant height, spike length, a hundred grain weight, biological and grain yield were significantly improved by adding Si through seed priming under water deficit conditions.
Enhancement in yield productivity by application of SA was reported in other plants. El-Feky et al. (2014) found an increase in weight of straw, grains and 100-grains of B-stressed barley treated with 1 mM SA.
Conclusion
From the previous discussions, it could be concluded that growth and yield of canola plants improved after external application of Ca, Si or SA. The reversal of inhibitory effect of excess B by Ca, Si and SA treatments was conferred by preventing growth inhibition or various forms of reducing B uptake and translocation to shoot system. In addition, adding treatments might enhance the non-enzymatic antioxidant and protect plants. Finally, the increase in grains number by treatment with Ca, Si and SA could be attributed to enhancing water content and other physiological activities.
References
Ahmed M, Qadeer U, Ahmed ZI, Hassan F (2016) Improvement of wheat (Triticum aestivum) drought tolerance by seed priming with silicon. Arch Agron Soil Sci 62:299–315
Akcay UC, Erkan IE (2016) Silicon induced antioxidative responses and expression ofBOR2 and two PIP family aquaporin genes in barley grown under boron toxicity. Front Plant Mol Biol Rep 34:318–326
Al-Hakimi AM, Hamada AM (2001) Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate. Biol Plantarum 44:253–261
Asad A, Blamey FPC, Edwards DG (2002) Dry matter production and boron concentrations of vegetative and reproductive tissues of canola and sunflower plants grown in nutrient solution. Plant Soil 243:243–252
Ayvaz M, Avci M, Yamaner C, Koyuncu M, Guven A, Fagerstedt K (2013) Does excess boron affect the malondialdehyde levels of potato cultivars? Eur Asian J Biosci 7:47–53
Azad AK, Sawa Y, Ishikawa T, Shibata H (2004) Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol 45:608–617
Batistič O, Kudla J (2010) Calcium: not just another ion. In: Hell R, Mendel RR (eds) Cell biology of metals and nutrients. Plant Cell Monographs 17. Springer, Berlin, pp 17–54
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bolaños L, Lukaszewski K, Bonilla I, Blevins D (2004) Why boron? Plant Physiol Biochem 42:907–912
Boursiac Y, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Maurel C (2008) Stimulus-induced down regulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J 56:207–218
Caverzan A, Casassola A, Brammer SP (2016) Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In Shanker AK, Shanker C (eds) Agricultural and biological sciences “abiotic and biotic stress in plants—recent advances and future perspectives”. Intech Open Agricultural and Biological Sciences
Cervilla LM, Blasco B, Rios JJ, Romero L, Ruiz JM (2007) Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Ann Bot 100:747–756
Cervilla LM, Blasco B, Rios JJ, Rosales MA, Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Romero L, Ruiz JM (2012) Parameters symptomatic for boron toxicity in leaves of tomato plants. J Bot 2012:1–17
Cheng H, Jiang ZY, Liu Y, Ye ZH, Wu ML, Sun CC, Sun FL, Fei J, Wang YS (2014) Metal (Pb, Zn and Cu) uptake and tolerance by mangroves in relation to root anatomy and lignification/suberization. Tree Physiol 34:646–656
Coskun D, Britto DT, Huynh WQ, Kronzucker HJ (2016) The role of silicon in higher plants under salinity and drought stress. Front Plant Sci 7:1072
De Gara L, Paciolla C, De Tullio MC, Motto M, Arrigioni O (2000) Ascorbate-dependent hydrogen peroxide detoxification and ascorbate regeneration during germination of a highly productive maize hybrid: Evidence of an improved detoxification mechanism against reactive oxygen species. Physiol Plant 109:7–13
Dell B, Huang L (1997) Physiological response of plants to low boron. Plant Soil 193:103–120
Dempsey DA, Klessig DF (2017) How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol 15:23
Du CW, Wang YH, Xu FS, Yang YH, Wang HY (2002) Study on the physiological mechanism of boron utilization efficiency in rape cultivars. J Plant Nutr 25:231–244
Elbehiry F, Elbasiouny H, El-Henawy A (2017) Boron: Spatial distribution in an area of north Nile Delta, Egypt. Commun Soil Sci Plant Anal 48:294–306
El-Feky SS, El-Shintinawy FA, Shaker EM, Shams El-Din HA (2012) Effect of elevated boron concentrations on the growth and yield of barley (Hordeum vulgare L.) and alleviation of its toxicity using different plant growth modulators. Aust J Crop Sci 6:1687–1695
El-Feky SS, El-Shintinawy FA, Shaker EM (2014) Role of CaCl2 and salicylic acid on metabolic activities and productivity of boron stressed barley (Hordeum vulgare L.). Int J Curr Microbiol Appl Sci 3:368–380
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Eraslan F, Inal A, Gunes A, Alpaslan M (2007) Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci Hortic 113:120–128
Eraslan F, Inal A, Pilbeam DJ, Gunes A (2008) Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. cv. Matador) grown under boron toxicity and salinity. Plant Growth Regul 55:207–219
Fallah A (2012) Silicon effect on lodging parameters of rice plants under hydroponic culture. Int J Agric Sci 2:630–634
Farooq MA, Saqib ZA, Akhtar J (2015) Silicon-mediated oxidative stress tolerance and genetic variability in rice (Oryza sativa L.) grown under combined stress of salinity and boron toxicity. Turk J Agric For 39:718–729
Fauteux F, Chain F, Belzile F, Menzies JG, Bélanger RR (2006) The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. PNAS 103:17554–17559
Fawe A, Menzies JG, Chérif M, Bélanger RR (2001) Silicon and disease resistance in dicotyledons. In: Datnoff LE, Snyder GH, Korndöfer GH (eds) Silicon in agriculture. Elsevier, Amsterdam, pp 159–170
Fleck AT, Nye T, Repenning C, Stahl F, Zahn M, Schenk MK (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J Exp Bot 62:2001–2011
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Francois LE (1986) Effect of excess boron on broccoli, cauliflower, and radish. J Am Soc Hortic Sci 111:494–498
Gaines TP, Mitchell GA (1979) Boron determination in plant tissue by the azomethine-H method. Comm Soil Sci Plant Anal 10:1099–1108
Ghallab KH, Sharaan AN (2002) Selection in canola (Brassica napus L.) germplasm under conditions of newly reclaimed land. II. Salt tolerant selections. Egypt J Plant Breed 6:15–30
Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, Leigh RA, Tyerman SD (2011) Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exp Bot 62:2233–2250
Grace SC, Logan BA (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos Trans R Soc Lond B Biol Sci 355:1499–1510
Grant CA, Bailey LD (1993) Fertility management in canola production. Can J Plant Sci 73:651–670
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Function Plant Biol 32:481–494
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (s-n-butylhomocysteine sulfoximine). J Biol Chem 254:7558–7560
Gunes A, Inal A, Bagci EG, Pilbeam JD (2007) Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 290:103–114
Gupta UC (1993) Deficiency, sufficiency, and toxicity levels of boron in crops. In: Gupta UC (ed) Boron and its role in crop production. CRC Press, Boca Raton, pp 137–145
Gupta UC, Macleod JA (1981) Plant and soil boron as influenced by soil pH and calcium sources on podzol soils. Soil Sci 131:20–25
Hattori T, Inanaga S, Tanimoto E, Lux A, Luxová M, Sugimoto Y (2003) Silicon-induced changes in viscoelastic properties of sorghum root cell walls. Plant Cell Physiol 44:743–749
He YL, Liu YL, Chen Q, Bian AH (2002) Thermo-tolerance related to antioxidation induced by salicylic acid and heat hardening in tall fescue seedlings. J Plant Physiol Mol Biol 28:89–95
Hossain MT, Mori R, Soga K, Wakabayashi K, Kamisaka S, Fujii S, Yamamoto R, Hoson T (2002) Growth promotion and an increase in cell wall extensibility by silicon in rice and some other Poaceae seedlings. J Plant Res 115:23–27
Hughes-Games G (1991) Boron for field crops. Soil Factsheet. Order No. 631.012–1, Agdex 540
Inal A, Pilbeam DJ, Gunes A (2009) Silicon increases tolerance to boron toxicity and reduces oxidative damage in barley. J Plant Nutr 32:112–128
Kampfenkel K, Van Montagu M, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Kanwak S, Rahmatullah Aziz T, Maqsood MA, Abbas N (2008) Critical ratio of calcium and boron in maize shoot for optimum growth. J Plant Nutr 31:1535–1542
Kaya C, Levent Tuna AL, Dikilitas M, Ashraf M, Koskeroglu S, Guneri M (2009) Supplementary phosphorus can alleviate boron toxicity in tomato. Sci Hortic 121:284–288
Kayama Y (2010) Treatments of severely boron-contaminated soils for phytorestoration. Phytorestoration, Spring, pp 1–17
Keles Y, Öncel I, Yenice N (2004) Relationship between boron content and antioxidant compounds in citrus leaves taken from field with different water source. Plant Soil 265:345–353
Kofalvi SA, Nassuth A (1995) Influence of wheat streak mosaic virus infection on phenyl propanoid metabolism and the accumulation of phenolics and lignin in wheat. Physiol Mol Plant Pathol 47:365–377
Koohkan H, Maftoun M (2016) Effect of nitrogen–boron interaction on plant growth and tissue nutrient concentration of canola (Brassica napus L.). J Plant Nutr 39:922–931
Landi M, Degl’Innocenti E, Pardossi A, Guidi L (2012) Antioxidant and photosynthetic responses in plants under boron toxicity: a review. Am J Agric Biol Sci 7:255–270
Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209:217–224
Liang Y, Nikolic M, Bélanger R, Gong H, Song A (2015) Silicon in agriculture. Springer, Dordrecht
Liu P, Yin L, Wang S, Zhang M, Deng X, Zhang S, Tanaka K (2015) Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ Exp Bot 111:42–51
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma JF, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. Stud Plant Sci 8:17–39
Ma D, Sun D, Wang C, Qin H, Ding H, Li Y, Guo T (2016) Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J Plant Growth Regul 35:1–10
Martínez-Ballesta MC, Cabañero F, Olmos E, Periago PM, Maurel C, Carvajal M (2008) Two different effects of calcium on aquaporins in salinity-stressed pepper plants. Planta 228:15–25
Mekki BB (2007) The potential of yield and quality of canola (Brassica napus L.) as a new winter oil crop in Egypt. In: Proceeding of 12th International Conference; Rapeseed Congress Wuhan; China, pp 26–30
Mekki BB (2013) Yield and quality traits of some canola varieties grown in newly reclaimed sandy soils in Egypt. World Appl Sci J 25:258–263
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Metwally AM, El-Shazoly RM, Hamada AM (2017) Physiological responses to excess boron in wheat cultivars. Eur J Biol Res 7:1–8
Mimouni H, Wasti S, Manaa A, Gharbi E, Chalh A, Vandoorne B, Lutts S, Ben Ahmed H (2016) Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. OMICS 20:180–190
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Miwa K, Takano K, Omori H, Seki M, Shinozaki K, Fujiwara T (2007) Plants tolerant of high boron levels. Science 318:1417
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Moraghan JT, Mascagni HJ (1991) Environmental and soil factors affecting micronutrient deficiencies and toxicities. In: Luxmoore RJ (ed) Micronutrients in agriculture. Soil Science Society of America, Madison, pp 371–425
Moreno MI, Isla MI, Sampietro AR, Vattuone MA (2000) Comparison of the free radical scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol 71:109–114
Munné-Bosch S, Schwarz K, Alegre L (1999) Enhanced formation of α-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary Plants. Plant Physiol 121:1047–1052
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Öztürk Ö, Soylu S, Ada R, Gezgin S, Babaoglu M (2010) Studies on differential response of spring canola cultivars to boron toxicity. J Plant Nutr 33:1141–1154
Pageau D, Lafond J, Tremblay GF (1999) The effects of boron on the productivity of canola. http://www.regional.org.au/au/gcirc/2/22.htm
Pandey NA (2013) Antioxidant responses and water status in brassica seedlings subjected to boron stress. Acta Physiol Plant 35:697–706
Radi AA, Metwally AM, El-Shazoly RM, Hamada AM (2014) Some metabolic responses of boron-stressed canola plants to external application of calcium, silicon and salicylic acid at vegetative growth stage. Egypt J Exp Biol (Bot) 10:143–154
Reid R (2010) Can we really increase yields by making crop plants tolerant to boron toxicity? Plant Sci 178:9–11
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Salin ML (1988) Toxic oxygen species and protective systems of the chloroplasts. Physiol Plant 72:681–689
Schmieden U, Wild A (1994) Changes in level of α-tocopherol and ascorbate in spruce needles at three low mountain sites exposed to Mg2+ deficiency and ozone. Z Naturforsch C 49:171–180
Sharaan AN, Ghallab KH (2002) Selection in canola (Brassica napus L.) germplasm under conditions of newly reclaimed land. I. Variability and genetic parameters in the base lines. Egypt J Plant Breed 6:1–13
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H (2016) Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front Plant Sci 7:196
Siddiqui MH, Al-Whaibi MH, Sakran AM, Ali HM, Basalah MO, Faisal M, Alatar A, Al-Amri AA (2013) Calcium-induced amelioration of boron toxicity in radish. J Plant Growth Regul 32:61–71
Sotiropoulos TE, Therios IN, Daimassi KN, Bosabalidis AM, Kofidis G (2002) Nutritional status, growth, CO2 assimilation and leaf anatomical responses in two kiwi fruit species under boron toxicity. J Plant Nutr 25:1249–1261
Taban S, Alpaslan M, Kutuk C, Inal A, Erdal I (1995) Relationship between boron and calcium on wheat (Triticum aestivum L.). 9th International Symposium of CIEC, “Soil Fertility and Fertilization Management-bridge between Science, Industry and Practice”, September 25–30, Kuşadası-Turkey. pp 85–90
Turan MA, Taban N, Taban S (2009) Effect of calcium on the alleviation of boron toxicity and localization of boron and calcium in cell wall of wheat. Not Bot Hort Agrobot Cluj 37:99–103
Tuteja N (2009) Integrated calcium signaling in plants. In: Baluška F, Mancuso S (eds) Signaling in plants. Springer, Berlin Heidelberg, pp 29–49
Van Bockhaven J, De Vleesschauwer D, Höfte M (2013) Towards establishing broad-spectrum disease resistance in plants: silicon leads the way. J Exp Bot 64:1281–1293
Wang M, Ding L, Gao L, Li Y, Shen Q, Guo S (2016) The interactions of aquaporins and mineral nutrients in higher plants. Int J Mol Sci 17:122
Yadav SK (2010) Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yıldırım K (2017) Transcriptomic and hormonal control of boron uptake, accumulation and toxicity tolerance in poplar. Environ Exp Bot 141:60–73
Yıldırım K, Uylas S (2016) Genome-wide transcriptome profiling of black poplar (Populus nigra L.) under boron toxicity revealed candidate genes responsible in boron uptake, transport and detoxification. Plant Physiol Biochem 109:146–155
Zhang Q, Liu M, Ruan J (2017) Metabolomics analysis reveals the metabolic and functional roles of flavonoids in light-sensitive tea leaves. BMC Plant Biol 17:64
Zhu Y, Gong H (2014) Beneficial effects of silicon on salt and drought tolerance in plants. Agron Sustain Dev 34:455–472
Zhu YX, Xu XB, Hu YH, Han WH, Yin JL, Li HL, Gong HJ (2015) Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep 34:1629–1646
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Metwally, A.M., Radi, A.A., El-Shazoly, R.M. et al. The role of calcium, silicon and salicylic acid treatment in protection of canola plants against boron toxicity stress. J Plant Res 131, 1015–1028 (2018). https://doi.org/10.1007/s10265-018-1008-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-018-1008-y