Abstract
Like other abiotic stresses, boron (B) toxicity is an important environmental constraint that limits crop productivity worldwide. B toxicity alters many physiological processes necessary for plant survival. The aim of the present study was to investigate the individual and combined effects of calcium (Ca) and B on morphological and physiological attributes of radish (Raphanus sativus L.) under normal and boron-toxicity conditions. The application of 30 mM Ca and 0.5 mM B, alone and in combination, enhanced plant growth, physiological and biochemical attributes. However, 5 mM B was detrimental to most growth and physiological parameters. The application of 30 mM Ca was most effective in alleviating the harmful effects of B toxicity by decreasing malondialdehyde and hydrogen peroxide levels and electrolyte leakage and by enhancing the activities of the antioxidant enzymes superoxide dismutase, catalase, peroxidase, glutathione reductase, and ascorbate peroxidase. Ca clearly induced plant protection mechanisms by enhancing the accumulation of proline, total soluble carbohydrates, and photosynthetic pigments in leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron (B) plays a vital role in plant tissue growth and development, including cell wall formation and function, because it forms crosslinks between dimers of the pectin rhamnogalacturonan II (a low-molecular-mass pectic polysaccharide) (Matoh 1997; O’Neill and others 2004). However, the available literature reflects that the physiological role of B in plants is not fully understood. B is a unique essential mineral element for plants with a small window of suitable concentrations between deficiency and toxicity. The requirement for B varies among plants species and among genotypes of the same species. For example, according to Keren and Bingham (1985), sensitive plants (that is, avocado, apple, and bean) can be safely grown at concentrations of 0.3 mg B L−1, semitolerant plants (that is, oat, maize, and potato) at 1–2 mg B L−1, and tolerant plants (that is, carrot, alfalfa, and sugar beet) at 2–4 mg B L−1. Torun and others (2006) also reported that large genetic variations were found in the tolerance of wheat to B toxicity. B-deficient plants showed altered growth and physiological and reproductive processes (Camacho-Gristobal and others 2008).
Worldwide, crop production is limited because B in the soil is either insufficient or at toxic levels (Rerkasem and others 2003). Like other nutrient deficiencies, B deficiency is very common and widespread around the world and causes large losses in crop production both quantitatively and qualitatively (Shorrocks 1997). Large areas of India, China, and the US are suffering from B deficiency (Liu and others 1980; Singh 2006; Yau and Ryan 2008). B toxicity is an especially serious threat to agriculture in arid and semiarid regions; B is frequently associated with saline soils and inland desert areas where native soils high in B coexist with low levels of rainfall and irrigation with high-B groundwater (Gupta 1979). The main sources of elevated B are surface mining, fly ash, and industrial chemicals (Nable and others 1997). Areas of Peru, Chile, India, Israel, South Australia, West Asia, North Africa, Egypt, Iraq, Jordon, Libya, Morocco, Syria, Turkey, and the west coast of Malaysia are all suffering from B toxicity (Yau and Ryan 2008).
High B levels in the soil inhibit seed germination and polyphenol oxidase activity (Ölçer and Kocaçaliskan 2007), cell wall expansion, and photosynthetic pigment synthesis, and reduce lignin and suberin contents (Nable and others 1997; Reid 2007). B toxicity impairs nitrogen assimilation pathways by affecting key enzymes involved in those processes (Herrera-Rodríguez and others 2010) and disrupts RNA splicing (Shomron and Ast 2003; Reid 2007). Like other abiotic stressors (that is, salinity, heavy metals, drought, cold, and heat), B also causes oxidative damage induced by the formation of reactive oxygen species (ROS) such as superoxide (O•−), hydroxyl (OH•−) radicals, which are strong oxidizers of lipids, proteins, and nucleic acids (Ardic and others 2009; Cervilla and others 2007), and destabilization of cellular homeostasis (Tombuloglu and others 2011). B maintains cell membrane integrity and also improves the cellular defense mechanism (Xuan and others 2001).
Despite the obvious importance of B toxicity, the mechanisms of B tolerance and toxicity in plants are poorly understood (Reid and others 2004; Cervilla and others 2007; Reid 2007; Fitzpatrick and Reid 2009). The interrelationships among nutrients and between nutrient and plant growth regulators are very important for plant growth and development under both normal and extreme environmental conditions (Siddiqui and others 2008b, 2009b, 2010, 2011, 2012). Calcium (Ca) plays an important role in stress tolerance and also induces antioxidant enzyme activities and reduces lipid peroxidation of cell membranes under abiotic stress (Khan and others 2010; Siddiqui and others 2010). Ca also affects B availability and its utilization by plants (Kanwak and others 2008). Balanced nutrient uptake and concentrations within plant tissues are important for proper plant growth and development (Siddiqui and others 2008b). Turan and others (2009) reported that B and Ca concentrations in the soil affected the availability of and requirement for normal plant growth. Moreover, an imbalanced supply of B and Ca is toxic to the plant and impairs the growth and translocation of Ca to the shoot and fruit (Yamauchi and others 1986). However, the interactions between Ca and B are still debated (Bolaños and others 2004). Thus, studies of the interactive effects of Ca and B on plant growth are essential; Ca could alleviate the oxidative damage induced by B toxicity. Therefore, in the present experiment, the effect of B was studied in both the presence and the absence of Ca. Our main objective was to investigate the effect of Ca and/or B on the morphological and physiological attributes of radish under normal and B-toxicity conditions.
Materials and Methods
Plant Cultures and Ca and B Treatments
Experiment was performed under glasshouse conditions using radish (Raphanus sativus L. cv. Prolsy Kafrelsheikh) obtained from a local market in Riyadh, Saudi Arabia. Healthy seeds were surface sterilized with 1 % sodium hypochlorite for 10 min then vigorously rinsed with sterilized double-distilled water (DDW) before sowing. The seeds were sown in plastic pots (25 cm diameter, 25 cm height) filled with perlite and supplied with Raukura’s nutrient solution (Smith and others 1983). The pots were arranged in a simple randomized design in the greenhouse with a single factor and four replicates. The pots were covered with black plastic to reduce evaporation. One week after sowing, seedlings were thinned so that each pot contained healthy plants of uniform size. Pots were irrigated every 2 days with DDW (100 mL) to keep the perlite moist. Ca and B treatments were initiated 15 days after germination as follows [the concentration (in mM) of each element is indicated as a subscript]: (1) Ca0 + B0 (control), (2) Ca20 + B0, (3) Ca30 + B0, (4) Ca40 + B0, (5) Ca0 + B0.5, (6) Ca0 + B5, (7) Ca20 + B0.5, (8) Ca20 + B5, (9) Ca30 + B0.5, (10) Ca30 + B5, (11) Ca40 + B0.5, and (12) Ca40 + B5. The range of concentrations of B (that is, 0.05–5 mM) selected for this study was based on earlier experiments (Ardic and others 2009). The sources of Ca and B were calcium chloride and boric acid, respectively. Plants were sampled on the seventh day after treatment to assess their growth characteristics [plant height (PH), fresh weight (FW), and dry weight (DW); leaf area (LA); and leaf area index (LAI)] and physiological attributes [concentrations of B, Ca, photosynthetic pigments [chlorophyll (Chl) a and Chl b], total soluble carbohydrates (TSC), proline (Pro), hydrogen peroxide (H2O2), and malondialdehyde (MDA); electrolyte leakage; and activity levels of carbonic anhydrase (CA) and the antioxidants catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione reductase (GR), and ascorbate peroxidase (APX)].
Plant Growth Characteristics
Plant height was measured using a meter scale after removal of plants from the pots. After recording the FW with a balance, plants were placed in a 60 °C oven for 48 h and then were weighed for DW. LA was measured using a LI-3000 Portable Leaf Area Meter (LI-COR, Lincoln, NE, USA). LAI was calculated according to the method of Watson (1958).
Physiological and Biochemical Parameters
Chemical Content of Leaves
All the chemical reagents used in this procedure were of analytical grade. Absorbances were determined using a UV–Vis spectrophotometer, unless otherwise specified.
Chlorophyll (Chl) was extracted from fresh leaves using the DMSO method of Barnes and others (1992). Chl a and Chl b concentrations were calculated based on the absorbance of the extract at 663.8 and 646.8 nm.
To determine B and Ca concentrations, we followed the digestion approach of Hseu (2004). A leaf sample (0.5 g) was placed in a 250 mL digestion tube, and 10 mL of 2:1 concentrated nitric acid:perchloric acid was added. Samples were heated for 45 min at 90 °C, then the temperature was increased to 150 °C and boiled for 2–3 h until a clear solution was obtained. At intervals, 5 mL of concentrated nitric acid:perchloric acid with hydrogen peroxide was added to the sample (at least three times), and the digestion continued until the volume was reduced to about 1 mL. The interior walls of the tube were washed down with a little double distilled water (DDW) and the tubes were swirled throughout the digestion to keep the walls clean and prevent loss of the samples. After cooling, 5 mL of 1 % HNO3 was added to each sample. Thereafter, the solution was filtered through Whatman No. 42 filter paper and smaller than 0.45 μm Millipore filter paper. The filtrate was diluted to a total of 25 mL with distilled water. After dilution, the concentrations of Ca and B were determined with an atomic absorption spectrometer (Model 300, Perkin-Elmer, Waltham, MA, USA) and an inductively coupled plasma optical emission spectroscope (Model iCAP6000, Thermo-Scientific, Thermo-Fisher Scientific, Waltham, MA, USA).
Proline concentration was determined spectrophotometrically by adopting the ninhydrin method of Bates and others (1973). We first homogenized 300 mg of fresh leaf samples in sulphosalicylic acid, then added 2 mL each of acid ninhydrin and glacial acetic acid. The samples were heated at 100 °C. The mixture was extracted with toluene and the free toluene was quantified at 528 nm using L-proline as a standard.
Total soluble carbohydrate concentration (TSC) was estimated by the absorbance at 490 nm as described by Dubois and others (1956), using glucose as a standard. TSC was expressed as mg g−1 DW.
Malondialdehyde (MDA) content was determined according to the method of Heath and Packer (1968). Leaves were weighed and homogenates containing 10 % trichloroacetic acid (TCA) and 0.65 % 2-thiobarbituric acid were heated at 95 °C for 60 min then cooled to room temperature and centrifuged at 10,000×g for 10 min. The absorbance of the supernatant was read at 532 and 600 nm against a reagent blank.
Hydrogen peroxide (H2O2) content was measured as described by Velikova and others (2000). Fresh leaf samples (0.5 g) were homogenized in 5 mL of 0.1 % (w/v) TCA. The homogenate was centrifuged at 12,000 rpm for 15 min and the supernatant was added to 10 mM potassium phosphate buffer (pH 7.0) and 1 M potassium iodide. The absorbance of the supernatant was recorded at 390 nm. The content of H2O2 was calculated by comparison with a standard calibration curve plotted using known concentrations of H2O2.
Electrolyte Leakage
Electrolyte leakage was used to assess membrane permeability, as described by Lutts and others (1995). Samples were washed three times with DDW to remove surface contamination. Leaf discs were cut from young leaves and placed in sealed vials containing 10 mL of DDW and incubated on a rotatory shaker for 24 h; subsequently, the electrical conductivity of the solution (EC1) was determined. Samples were then autoclaved at 120 °C for 20 min and the electrical conductivity was measured again (EC2) after cooling the solution to room temperature. Electrolyte leakage (%) was calculated as (EC1/EC2) × 100 %.
Enzyme Activity
The activity of CA (EC 4.2.1.1) was determined by the method of Dwivedi and Randhawa (1974). Leaf samples were cut into small pieces and suspended in cysteine hydrochloride solution. The samples were incubated at 40 °C for 20 min. The pieces were blotted and transferred to test tubes containing phosphate buffer (pH 6.8), then alkaline bicarbonate solution and bromothymol blue indicator were added. The test tubes were incubated at 50 °C for 20 min. After the addition of 0.2 mL of methyl red indicator, the reaction mixture was titrated against 0.05 N HCl. The results were expressed as μmol CO2 kg−1 FW s−1.
To determine the enzymatic activities of the antioxidant proteins, a crude enzyme extract was prepared by homogenizing 500 mg of leaf tissue in extraction buffer containing 0.5 % Triton X-100 and 1 % polyvinylpyrrolidone in 100 mM potassium phosphate buffer (pH 7.0) using a chilled mortar and pestle. The homogenate was centrifuged at 15,000 g for 20 min at 4 °C. The supernatant was used for the enzymatic assays described below. For APX, the extraction buffer was supplemented with 2 mM ascorbate. All enzyme activities were expressed as mg−1 protein min−1.
We used the method of Chance and Maehly (1955) to determine POD (EC 1.11.1.7) activity by using 5 mL of an assay mixture containing phosphate buffer (pH 6.8), 50 M of pyrogallol, 50 mM of H2O2, and 1 mL of the enzyme extract diluted 20 times. This was incubated for 5 min at 25 °C, after which the reaction was stopped by adding 0.5 mL of 5 % (v/v) H2SO4. The amount of purpurogallin formed was determined by measuring absorbance at 420 nm. A unit of peroxidase activity was the amount of purpurogallin formed mg−1 protein min−1.
Aebi’s (1984) method was used to measure CAT (EC 1.11.1.6) activity. The decomposition of H2O2 was monitored by the decrease in absorbance at 240 nm. For the assay, a 50 mM phosphate buffer (pH 7.8) and 10 mM H2O2 were used.
The activity of SOD (EC 1.15.1.1) was determined by measuring its ability to inhibit the photoreduction of nitro blue tetrazolium (NBT) according to the methods of Giannopolitis and Ries (1977). The reaction solution (3 mL) contained 50 μmol NBT, 1.3 μmol riboflavin, 13 mmol methionine, 75 nmol EDTA, 50 mmol phosphate buffer (pH 7.8), and 20–50 μL enzyme extract. The reaction solution was irradiated under a bank of fluorescent lights at 75 μmol m−2 s−1 for 15 min. The absorbance at 560 nm was read against a blank (nonirradiated reaction solution). One unit of SOD activity was defined as the amount of enzyme that inhibited 50 % of NBT photoreduction.
Glutathione reductase (EC 1.6.4.2) activity was assayed as described by Foyer and Halliwell (1976), with minor modifications. The assay mixture consisted of 50 μL of the enzyme extract, 100 mM phosphate buffer (pH 7.8), 0.1 μM EDTA, 0.05 mM nicotinamide adenine dinucleotide phosphate-oxidase (NADPH), and 3.0 mM oxidized glutathione in a total volume of 1.0 mL. The NADPH oxidation rate was monitored by reading the absorbance at 340 nm at the moment of H2O2 addition and 1 min later. The difference in absorbance (A340) was divided by the NADPH molar extinction coefficient (6.22 mM cm−1) and the enzyme activity expressed as 1 μmol of NADPH mg−1 protein min−1.
We measured APX (EC 1.11.1.11) activity following the method of Nakano and Asada (1981). The reaction buffer solution contained 50 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, 0.1 mM H2O2, and 0.5 mM ascorbate. The reaction was started by adding the sample solution to the reaction buffer solution. The H2O2-dependent oxidation of ascorbate was tracked by the decrease in absorbance at 290 nm (absorbance coefficient at 2.8 mM−1 cm−1).
Statistical Analysis
Each pot was treated as one replicate and all the treatments were repeated four times. The data were analyzed statistically with SPSS v17 statistical software (SPSS Inc., Chicago, IL, USA). Means were statistically compared by Duncan’s multiple-range test (DMRT) at the p < 0.05 % level.
Results
Boron toxicity was assessed on the basis of plant growth and physiological and biochemical characteristics of R. sativus L.
Plant Growth Characteristics
We measured five plant growth characteristics (PH, FW, DW, LA, and LAI; Table 1). The application of B0.5 significantly enhanced all five traits relative to the control (Ca0 + B0). However, at B5, FW was significantly reduced; PH, LA, and LAI were lower but not significantly so; and DW was unchanged. Calcium at C30 enhanced all five growth attributes relative to the control, and other concentrations generally improved growth traits, but not always to a significant level.
The combined treatments of Ca20 + B0.5 and Ca30 + B0.5 significantly improved all growth traits relative to the control, although Ca30 + B0.5 yielded larger plants by all measured parameters. Ca40 + B0.5 significantly enhanced all traits except DW relative to the control. The application of Ca20 + B5 and Ca30 + B5 significantly ameliorated PH and FW compared to the application of B5 alone, whereas the other plant traits were generally improved, although not always statistically so. Ca40 + B5 was harmful to plants according to all of our measured traits (although the decrease in FW was not statistically significant).
Physiological and Biochemical Parameters
All concentrations of Ca alone (that is, Ca20, Ca30, and Ca40) significantly increased leaf concentrations of Chl a and Chl b, as did B0.5 (Fig. 1a). Ca30 induced the synthesis of significantly more Chl a than any other Ca treatment, although its effects on Chl b were statistically indistinguishable from those of Ca20. Plants treated with B5 had significantly lower concentrations of both chlorophylls than the control. The addition of Ca20 and Ca30 to the B0.5 and B5 treatments significantly improved the concentrations of both chlorophylls relative to boron alone, and plants exposed to Ca30 + B0.5 had significantly higher concentrations of both Chl a and Chl b than any other treatment.
Effect of Ca and/or B on the concentration of Chl a and Chl b (a), Ca and B (b), and activity of CA (c) in leaves of Raphanus sativus L. (mean of four replicates). Bars followed by the same letters show no statistical difference at p < 0.05 (Duncan’s multiple-range test). Average of four determinations are presented with bars indicating SE. Ca1 = 20 mM, Ca2 = 30 mM, Ca3 = 40 mM, B1 = 0.5 mM, B2 = 5 mM
All Ca and B treatments significantly increased the levels of B in leaves relative to the control (Fig. 1b). The highest concentration of B was recorded at B5, whereas the addition of Ca to B5 significantly lowered the levels of B in leaves, in the order Ca40 + B5, Ca20 + B5, then Ca30 + B5. Both B0.5 and B5 significantly lowered the leaf Ca concentration relative to the control, with B5 having the larger effect (Fig. 1b). All other treatments significantly enhanced Ca levels in leaves relative to the control and to either the B0.5 or the B5 treatment. The maximum value of Ca in leaves was found with the Ca30 + B0.5 treatment.
The activity of CA relative to the control was significantly inhibited by the addition of boron alone, especially at B5 (Fig. 1c). The application of Ca alone significantly increased the activity of CA relative to the control at Ca20 and Ca30, and all concentrations of Ca in combination with B0.5 or B5 significantly improved CA activity compared to boron alone. Ca30 + B0.5 enhanced CA activity significantly more than any other treatment.
The level of lipid peroxidation was indicated by the MDA concentration in leaves, and membrane damage and alteration was assessed by H2O2 content and electrolyte leakage (Fig. 2a–c). In all cases, B5 was significantly more damaging to plants than any other treatment. However, the addition of Ca20 and Ca30 to B0.5 significantly improved all parameters relative to B0.5 alone, and all three concentrations of Ca combined with B5 significantly ameliorated damage to plants. The combined application of Ca30 + B0.5 yielded the lowest values of MDA, H2O2, and electrolyte leakage.
Effect of Ca and/or B on content of MDA (a), electrolyte leakage (b), and concentration of H2O2 (c) of Raphanus sativus L. Bars followed by the same letters show no statistical difference at p < 0.05 (Duncan’s multiple-range test). Average of four determinations are presented with bars indicating SE. Ca1 = 20 mM, Ca2 = 30 mM, Ca3 = 40 mM, B1 = 0.5 mM, B2 = 5 mM
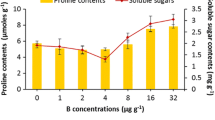
All treatments significantly increased the concentration of Pro in leaves relative to the control, although the effects were most notable with B5 alone and when Ca and B were combined (Fig. 3a). The highest value occurred with the Ca30 + B0.5 treatment. Levels of TSC were higher than the control in all treatments, although the differences were not significant for Ca40 or B0.5 (Fig. 3b). Values significantly higher than all others were seen with the Ca30 + B0.5 and Ca30 + B5 treatments.
Effect of Ca and/or B on the concentration of Pro (a) and TSC (b) in leaves of Raphanus sativus L. Bars followed by the same letters show no statistical difference at p < 0.05 (Duncan’s multiple-range test). Average of four determinations are presented with bars indicating SE. Ca1 = 20 mM, Ca2 = 30 mM, Ca3 = 40 mM, B1 = 0.5 mM, B2 = 5 mM
The application of Ca and B significantly influenced the activities of antioxidant enzymes (Fig. 4a–c). The application of all concentrations of Ca and B, alone and in combination, significantly increased relative to the control the activities of SOD, CAT, POD, GR, and APX (Fig. 4a–c). The activities of CAT and POD were significantly higher than any other treatment at Ca20 + B0.5, whereas the activities of SOD, GR, and APX were significantly maximized at Ca30 + B5.
Effect of Ca and/or B on the activity of SOD (a), CAT and POD (b), and GR and APX (c) of Raphanus sativus L. Bars followed by the same letters show no statistical difference at p < 0.05 (Duncan’s multiple-range test). Average of four determinations are presented with bars indicating SE. Ca1 = 20 mM, Ca2 = 30 mM, Ca3 = 40 mM, B1 = 0.5 mM, B2 = 5 mM
Discussion
The application of various concentrations of Ca and a low concentration of boron (B0.5), individually as well as in combination, to radish seedling substrate generally improved growth (Table 1), physiological characteristics, and biochemical processes (Figs. 1, 2, 3, 4); however, high levels of boron alone (B5) were generally harmful.
We found that supplying Ca and low levels of B to radish plants improved plant growth. This effect may be explained by their physiological roles; both are required for the formation of cell walls and membranes (Cakmak and Römheld 1997). The role of Ca is very similar to that of a hormone; it regulates the protein pumps that take up nutrients into roots and move those nutrients among cells within the plant (White and Broadley 2003). Boron forms an important structural component of the peptic rhamnogalacturon II complex in cell walls (Matoh 1997). High levels of B (B5) suppressed plant growth, which might be due to its toxic effects on root cell division, cell wall expansion, chlorophyll content, and photosynthetic rate (Liu and Yang 2000; Herrera-Rodriguez and others 2010). Combining Ca and B was most effective at improving PH, FW, DW, LA, and LAI, especially at C30 + B0.5 concentrations.
In the present study, the synthesis of both chlorophyll a and b was severely affected by the higher dose of B5 (Fig. 1a). This inhibition of photosynthetic pigments might be due to lipid peroxidation and hydrogen peroxide accumulation, which were both significantly affected by B5 (Fig. 2a–c) and could damage reaction centers (Kyle 1987). In other studies, oxidative damage in apple and grape (Gunes and others 2006) and photooxidation damage to organic molecules in orange plants were induced by B toxicity (Cervilla and others 2007). Boron toxicity caused the inhibition of protein synthesis through the formation of borate esters with ribose (Reid 2007) and also altered the activities of several enzymes, and consequently the plant metabolism (Herrera-Rodrigues and others 2010). Interestingly, we found that the application of Ca in combination with B notably improved the levels of photosynthetic pigments as well as CA enzyme activity, and Ca application even helped to alleviate the toxic effects of B on these traits (Fig. 1a, c). Supplying Ca to the plants may have reduced the loss of photosynthetic pigments by reducing the photooxidation of organic molecules (Wise and Naylor 1987) or by maintaining membrane integrity (Hirschi 2004). Improved CA activity might have improved various physiological processes such as ion exchange, acid-base balance, inorganic carbon diffusion within cells and between them and their environments, and reversible hydrogenation of CO2, thus maintaining its constant supply to RuBisCO. All these scenarios may explain the improved tolerance of radish to B toxicity.
The results revealed that the content of B in leaves of plants was significantly and negatively correlated with the tolerance of plants to B toxicity. We found the highest accumulation of B in leaves at the highest applied dose of B (B5) (Fig. 1b). However, applying Ca with B significantly decreased the B content of leaves. Paull (1990) reported that B tolerance was correlated with a reduced accumulation of B in some wheat and barley genotypes. In our study, the growth medium containing B0.5 increased Ca uptake, but the growth medium containing B5 decreased Ca content in leaves (Fig. 1b). These results suggested that Ca and B have interrelated metabolic roles. This interrelationship could be responsible for the improvements in PH seen, giving plants better leaf positioning for harvesting solar energy as well as facilitating leaf expansion, leading to larger LA. Thus, the addition of both Ca and B to the growth medium was more effective at improving plant growth than B alone (Table 1). An imbalanced supply of B and Ca is toxic to plants and impairs the growth and translocation of Ca and B within plants (Yamauchi and others 1986).
Lipid peroxidation, electrolyte leakage, and H2O2 content have been used as indicators of oxidative damage induced by different environmental stresses. The increases in oxidative damage as measured by MDA, H2O2, and electrolyte leakage in plants at B5 (Fig. 2a–c) indicate that B toxicity may have caused cellular dysfunction by increasing lipid peroxidation. These results corroborate previous findings of increases in MDA and H2O2 content and electrolyte leakage in response to B toxicity (Ardic and others 2009; Cervilla and others 2007; Guimarães and others 2011). It is well established that Ca, an important nutrient, helps plants to protect against lipid peroxidation of the membrane by stabilizing the plasmalemma against lipolytic enzymes (Hirschi 2004; Siddiqui and others 2011). A high concentration of Ca in the cytosol of plants enhances physiological processes by triggering several enzymes, by affecting oxidative signal transduction, and by regulating antioxidant enzymes under different environmental conditions (Khan and others 2010; Siddiqui and others 2011). Thus, we postulate that the application of Ca protected the plants from B toxicity.
The functions of osmolytes in osmotic adjustment vary from species to species and even within plants species (Siddiqui and others 2008a, 2009a). Compatible solutes can lower or balance the osmotic potential within cells. In our study, both Pro and TSC levels increased relative to the control when plants received a high concentration of B (Fig. 3a, b). Both Pro and TSC were maximized with a combined application of C30 + B0.5. Khan and others (2010) reported similar results. Pro, a universal osmoprotectant, acts as an antioxidant and a source of energy (Matysik and others 2002) and regulates gene expression for osmotic adjustment (Iyer and Caplan 1998). TSCs are the major soluble constituents that help plant cells in maintaining osmotic balance. This work indicates that alleviation of B toxicity is associated with increases in the concentrations of Pro and TSC caused by the addition of Ca to the growth medium.
The generation of ROS is metabolically induced by abiotic and biotic stresses in plant cells (Foyer and Noctor 2000). ROS are harmful to plants because they react with a large variety of biomolecules, including DNA, proteins, and carbohydrates. Plants must limit the production of ROS, detoxify them once formed, and repair damage caused by ROS. The levels of MDA, H2O2, and electrolyte leakage were substantially higher in plants subjected to B5 (Fig. 2a–c). MDA and H2O2 are considered indicators of oxidative damage. Mittler (2002) suggested that membrane damage might be caused by high levels of H2O2, which could trigger the Haber–Weiss reaction, resulting in hydroxyl radical formation and thus lipid peroxidation. However, application of Ca may have lowered these factors by enhancing antioxidant systems (Fig. 4a–c). ROS scavenging is associated with antioxidant enzymes such as POD, SOD, CAT, APX, and GR (Meloni and others 2004; Siddiqui and others 2009a). In the present study, the activities of SOD, GR, and APX were highest in plants receiving Ca30 + B5. These results substantiate the findings of Khan and others (2010) and Siddiqui and others (2011). The balance among SOD, GR, and APX activities in the cell could be crucial for determining the steady-state levels of O2 − and H2O2. The accumulated Ca may have been responsible for the decreased content of MDA and H2O2; the unique importance of Ca for stabilizing membranes is well known (Marschner 1995; Hirschi 2004). The application of Ca30 + B0.5 enhanced POD and CAT activities, suggesting that Ca alone did not affect these two enzymes. SOD constitutes the first line of defense against superoxide and ROS, and GR also plays an important role in maintaining the ratio of cellular antioxidants and pro-oxidants. Thus, enhancing the antioxidant systems of plants by applying Ca could induce plant defenses against oxidative damage by H2O2 under B toxicity.
Conclusions
In conclusion, application of Ca had a significant synergistic effect on plant growth when applied in conjunction with a lower level of B. A decrease in accumulation of B was recorded in plants supplied with Ca. In the present study, out data revealed that MDA and H2O2 contents and electrolyte leakage increased on treatment with a high dose of B. However, Ca significantly reduced the adverse effects of B toxicity by mitigating cellular oxidative damage through improved free radical scavenging by antioxidant enzymes. Calcium clearly induced protective mechanisms by enhancing the accumulation of Pro, TSC, and photosynthetic pigments in leaves. This experiment therefore provides information that supplemental irrigation with Ca is an effective crop management practice for reducing B toxicity.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ardıc M, Sekmen AH, Tokur S, Ozdemir F, Turkan I (2009) Antioxidant responses of chickpea plants subjected to boron toxicity. Plant Biol 11:328–338
Barnes JD, Balaguer L, Manrique E, Elvira S, Davison AW (1992) A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ Exp Bot 32:85–100
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bolaños L, Lukaszewski K, Bonilla I, Blevins D (2004) Why boron? Plant Physiol Biochem 42:907–912
Cakmak I, Römheld V (1997) Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 193:71–83
Camacho-Cristobal JJ, Rexach J, Gonzalez-Fontes A (2008) Boron in plants: deficiency and toxicity. J Integr Plant Biol 50:1247–1255
Cervilla LM, Blasco B, Rıos J, Romero L, Ruiz J (2007) Oxidative stress and antioxidants in tomato Solanum lycopersicum plants subjected to boron toxicity. Ann Bot 100:747–756
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Dubois N, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dwivedi RS, Randhawa NS (1974) Evolution of a rapid test for the hidden hunger of zinc in plants. Plant Soil 40:445–451
Fitzpatrick KL, Reid RJ (2009) The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ 32:1357–1365
Foyer C, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis: regulation and signaling. New Phytol 146:359–388
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Guimarães FVA, Lacerda CFD, Marques EC, Miranda MRAD, Abreu CEBD, Prisco JT, Gomes-Flho E (2011) Calcium can moderate changes on membrane structure and lipid composition in cowpea plants under salt stress. Plant Growth Regul 65:55–63
Gunes A, Soylemezoglu G, Inal A, Bagci EG, Coban S, Sahin O (2006) Antioxidant and stomatal responses of grapevine (Vitis vinifera L.) to boron toxicity. Sci Hortic 110:279–284
Gupta UC (1979) Boron nutrition of crops. Adv Agron 31:273–307
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Herrera-Rodríguez MB, González-Fontes A, Rexach J, Camacho-Cristóbal JJ, Maldonado JM, Navarro-Gochicoa MT (2010) Role of boron in vascular plants and response mechanisms to boron stresses. Plant Stress 4:115–122
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Hseu ZY (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour Technol 95:53–59
Iyer S, Caplan A (1998) Products of proline catabolism can induce osmotically regulated genes in rice. Plant Physiol 116:203–211
Kanwak S, Rahmatullah AT, Maqsood MA, Abbas N (2008) Critical ratio of calcium and boron in maize shoot for optimum growth. J Plant Nutr 31:1535–1542
Keren R, Bingham FT (1985) Boron in water, soils, and plants. Adv Soil Sci 1:230–276
Khan MN, Siddiqui MH, Mohammad F, Naeem M, Khan MMA (2010) Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant 32:121–132
Kyle DJ (1987) The biochemical basis for photoinhibition of photosystem II. In: Kyle DJ, Osmond CB, Artzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 197–226
Liu P, Yang PA (2000) Effects of molybdenum and boron on membrane lipid peroxidation and endogenous protective systems of soybean leaves. Acta Bot Sin 42:461–466
Liu Z, Zhu Q, Tong L (1980) Boron-deficient soils and their distribution in China. Tu Jang Hsuch Pao 17:228–239
Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J Exp Bot 46:1843–1852
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Matoh T (1997) Boron in plant cell walls. Plant Soil 193:59–70
Matysik J, Alia, Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Meloni DA, Gulotta MR, Martinez CA, Oliva MA (2004) The effect of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz J Plant Physiol 16:39–46
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193:181–198
Nakano G, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
O’Neill MA, Ishii T, Albersheim P, Darvill AG (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Ann Rev Plant Biol 55:109–139
Ölçer H, Kocaçaliskan I (2007) Excess boron reduces polyphenol oxidase activities in embryo and endosperm of maize seed during germination. J Biosci 62:111–115
Paull JG (1990) Genetic studies on the tolerance of wheat to high concentrations of boron. Ph.D. Thesis, The University of Adelaide, Adelaide
Reid R (2007) Update on boron toxicity and tolerance in plants. In: Xu F, Goldbach HE, Brown PH, Bell RW, Fujiwara T, Hunt CD, Goldberg S, Shi L (eds) Advances in plant and animal nutrition. Springer, Dordrecht, pp 83–90
Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD (2004) A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ 25:1405–1414
Rerkasem B, Nirantrayagul S, Jamjod S (2003) Increasing boron efficiency in international bread wheat, durum wheat, triticale and barley germplasm will boost production on soils low in boron. Field Crops Res 86:175–184
Shomron N, Ast G (2003) Boric acid reversibly inhibits the second step of pre-mRNA splicing. FEBS 552:219–224
Shorrocks VM (1997) The occurrence and correction of boron deficiency. Plant Soil 193:121–148
Siddiqui MH, Khan MN, Mohammad F, Khan MMA (2008a) Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J Agron Crop Sci 194:214–224
Siddiqui MH, Mohammad F, Khan MN, Khan MMA (2008b) Cumulative effect of soil and foliar application of nitrogen, phosphorus, and sulfur on growth, physico-biochemical parameters, yield attributes, and fatty acid composition in oil of erucic acid-free rapeseed-mustard genotypes. J Plant Nutr 31:1284–1298
Siddiqui MH, Mohammad F, Khan MN (2009a) Morphological and physiobiochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J Plant Interact 4:67–80
Siddiqui MH, Mohammad F, Khan MN, Naeem M, Khan MMA (2009b) Differential response of salt-sensitive and salt-tolerant Brassica juncea genotypes to N application: enhancement of N-metabolism and anti-oxidative properties in the salt-tolerant type. Plant Stress 3:55–63
Siddiqui MH, Mohammad F, Khan MN, Al-Whaibi MH, Bahkali AHA (2010) Nitrogen in relation to photosynthetic capacity and accumulation of osmoprotectant and nutrients in Brassica genotypes grown under salt stress. Agr Sci China 9:671–680
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 248:503–511
Siddiqui MH, Mohammad F, Khan MMA, Al-Whaibi MH (2012) Cumulative effect of nitrogen and sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma 249:139–153
Singh MV (2006) Emerging boron deficiency in soils and crops in India and its management. 18th World Congress of Soil Science, Philadelphia
Smith GS, Johnston CM, Cornforth IS (1983) Comparison of nutrient solutions for growth of plants in sand culture. New Phytol 94:537–548
Tombuloglu H, Semizoglu N, Sakcali S, Kekec G (2012) Boron induced expression of some stress-related genes in tomato. Chemosphere 85:433–438
Torun AA, Yazici A, Erdem H, Çakmak I (2006) Genotypic variation in tolerance to boron toxicity in 70 durum wheat genotypes. Tur J Agric For 30:49–58
Turan MA, Taban N, Taban S (2009) Effect of calcium on the alleviation of boron toxicity and localization of boron and calcium in cell wall of wheat. Not Bot Hort Agrobot Cluj 37:99–103
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Watson DJ (1958) The dependence of net assimilation rate on leaf area index. Ann Bot 22:37–54
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Wise RR, Naylor AW (1987) Chilling-enhanced photo-oxidation. The peroxidation destruction of lipids during chilling injury to photosynthesis and ultrastructure. Plant Physiol 83:278–282
Xuan H, Streif J, Pfeffer H, Dannel F, Romheld V, Bangerth F (2001) Effect of pre-harvest boron application on the incidence of CA-storage related disorders in ‘Conference’ pears. J Hort Sci Biotechnol 76:133–137
Yamauchi T, Hara T, Sonoda Y (1986) Distribution of Ca and B in the pectin fraction of tomato leaf cell wall. Plant Cell Physiol 27:727–732
Yau SK, Ryan J (2008) Boron toxicity tolerance in crops: a viable alternative to soil amelioration. Crop Sci 48:854–865
Acknowledgments
The financial support by the Deanship of Scientific Research of King Saud University, Riyadh, KSA, to the Research Group No. RGPVPP-153 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddiqui, M.H., Al-Whaibi, M.H., Sakran, A.M. et al. Calcium-Induced Amelioration of Boron Toxicity in Radish. J Plant Growth Regul 32, 61–71 (2013). https://doi.org/10.1007/s00344-012-9276-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9276-6