Abstract
We investigated effect of silicon (Si) on the growth, uptake of sodium (Na), chloride (Cl), boron (B), stomatal resistance (SR), lipid peroxidation (MDA), membrane permeability (MP), lipoxygenase (LOX) activity, proline (PRO) accumulation, H2O2 accumulation, non-enzymatic antioxidant activity (AA) and the activities of major antioxidant enzymes (superoxide dismutase, SOD; catalase, CAT and ascorbate peroxidase, APX) of spinach and tomato grown in sodic-B toxic soil. Si applied to the sodic-B toxic soil at 2.5 and 5.0 mM concentrations significantly increased the Si concentration in the plant species and counteracted the deleterious effects of high concentrations of Na, Cl and B on root and shoot growth by lowering the accumulation of these elements in the plants. Stomatal resistance, MP, MDA and the concentrations of H2O2 and PRO were higher in the plants grown in sodic-B toxic soil without Si: LOX activity of excised leaves of both species was increased by Si. Antioxidant activities of both species were significantly affected by Si, with the activities of SOD, CAT and APX decreased and AA increased by applied Si. For most of the parameters measured, it was found that 5 mM Si was more effective than the 2.5 mM Si. Based on the present work, it can be concluded that Si alleviates sodicity and B toxicity of the plants grown in sodic-B toxic soil by preventing both oxidative membrane damage and also translocation of Na, Cl and B from root to shoots and/or soil to plant, and lowering the phytotoxic effects of Na, Cl and B within plant tissues. It was concluded that tomato was more responsive to Si than spinach since it was more salt sensitive than spinach. To our knowledge, this is the first report that Si improves the combined salt and B tolerance of spinach and tomato grown in naturally sodic-B toxic soil, and which describes membrane-related parameters and antioxidant responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saline-sodic soils are characterized by an excess of sodium (Na) to a level that can adversely affect soil structure, and this can affect the availability of nutrients to plants. Adverse effects of salinity or sodicity, or both, on crop growth arise from two major causes: (1) increasing the osmotic potential and thereby making the water in the soil less available for plants and (2) specific effects of some elements (Na, Cl, B etc.) present in excess concentrations (Yamaguchi and Blumwald 2005). Over 800 million hectares of land throughout the world are salt-affected, either by salinity or the associated condition of sodicity. Most of this salinity, and all of the sodicity, is natural. However, a significant proportion of cultivated agricultural land has become saline because irrigation continues to be practised more (Munns 2005). Boron is also often found in high concentrations in association with saline-sodic soils and is removed more slowly than salt ions during leaching. Therefore, it may still be present at excessive concentration in some reclaimed soils (Alpaslan and Gunes 2001; Ben-Gal and Shani 2002; Nable et al. 1997). Hence, increased salt and B tolerance of crops is needed to increase food production in many parts of the world. Although of considerable agronomic importance, our understanding of B toxicity mechanisms in saline-sodic soils is still incomplete, and remains an open topic.
According to Mittler (2002), stress conditions favour the accumulation of reactive oxygen species (ROS), such as superoxide radicals (O ·−2 ), hydroxyl radicals (OH·−) and hydrogen peroxide (H2O2). Accumulation of ROS was reported in apple rootstock (Molassiotis et al. 2006), citrus leaves (Keles et al. 2004), and barley plants (Karabal et al. 2003) under B-toxic conditions, and in potato (Rahnama and Ebrahimzadeh 2005), wheat genotypes (Sairam et al. 2005) tomato (Al-Aghabary et al. 2004) and rice seedlings (Tsai et al. 2004) under saline conditions. ROS are strong oxidizing species that cause oxidative damage to biomolecules such as lipids and proteins, and eventually lead to cell death (Molassiotis et al. 2006). Malondialdehyde (MDA), a decomposition product of polyunsaturated fatty acids, has been utilized as a biomarker for lipid peroxidation (Mittler 2002). Lipid peroxidation can also be initiated enzymatically through a sequential action of lipoxygenase (LOX; EC 1.13.11.12), a ubiquitous plant enzyme that incorporates molecular oxygen into polyunsaturated fatty acids to form lipid hydroperoxides (Axelrod et al. 1981). The antioxidant defence system in the plant cell includes both enzymatic antioxidants such as superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6) and ascorbate peroxidase (APX, EC 1.11.1.11), and non-enzymatic antioxidants such as ascorbate, glutathione and α-tocopherol. As a major scavenger, SOD catalyses the dismutation of superoxide to hydrogen peroxide and oxygen. However, H2O2 is also toxic to the cells and has to be further detoxified by CAT or peroxidase, or both, to water and oxygen (Halliwell and Gutteridge 1999; Zhu et al. 2004). In the ascorbate–glutathione cycle, APX reduces H2O2 using ascorbate as an electron donor. Altered activities of these antioxidant enzymes and antioxidants have been commonly reported, and are used frequently as indicators of oxidative stress in plants (Mittler 2002). Under stress conditions plants, in addition to producing antioxidants, also accumulate in the cytosol-compatible solutes such as proline that originally were thought to function as osmotic buffers. However, apart from osmotic adjustment they seem to play a role in maintaining the functional state of macromolecules, probably by scavenging ROS (Xiong and Zhu 2002). There is good evidence that the alleviation of oxidative damage and increased resistance to environmental stresses is often correlated with an efficient antioxidative system (Cakmak et al. 1993).
Silicon is the second most prevalent element within the soil. Although abundant, silicon is never found in a free form and is always combined with other elements, usually forming oxides. The importance of silicon (Si) has been recognized (Epstein 1999) and the beneficial effects of Si in enhancing the tolerance of plants of biotic and abiotic stresses in several crops, and its relevance to agriculture, have been widely described (Epstein 1999; Ma 2004).
To our knowledge, there is currently no information available about the possible beneficial effects of Si on the antioxidative system and stress markers in the performance of spinach and tomato plants grown in sodic-B toxic soils. The effect of Si on the salt tolerance of crops has been studied in hydroponics in many experiments (Zhu et al. 2004; Al-Aghabary et al. 2004; Romero-Aranda et al. 2006). However, we used original sodic soil containing toxic levels of B in this study in order to find out the effects of Si under real growing conditions. The aim of the present work was to investigate the impact of Si on the growth, Na, Cl and B uptake, MP, lipid peroxidation, activity of LOX, PRO and H2O2 concentration, SR, non-enzymatic and antioxidative enzyme (SOD, CAT and APX) activity of spinach and tomato plants grown under sodic-B toxic conditions. It was hoped that this study would provide a basis for developing strategies for reducing the risks associated with sodicity and B toxicity and maintaining sustainable plant production.
Materials and method
Growth conditions and treatments
Spinach (Spinacia oleracea L. cv. Matador) and tomato (Lycopersicon esculentum Mill. cv. H2274) plants were grown from 12th October 2005 to 1st January 2006 in a naturally lighted glasshouse at the Faculty of Agriculture, Ankara University (39o57′44.51′′ N; 32o51′46.95′′ E). Experimental soil, typic Natrargids, was collected from the plough layer (0–30 cm) of the Akgol depression (560.110 E, 4.156.151 N, UTM 36. Zone) of the Great Konya Basin (Central Anatolia). Some characteristics of the soil were as follows: water retention capacity at 1/3 and 15 atm 31.7% and 22.1%, respectively, texture clay, CaCO3 460.8 g kg−1, pH (1:2.5 water) 8.48, EC 7.76 dS m−1, CEC 54.4 cmol kg−1, SAR 37 (mmolc l−1)1/2, organic matter 9.0 g kg−1, total N 0.5 g kg−1. The concentrations of NH4OAc-extractable K, Ca, Mg and Na were as follows (cmol kg−1): 1.70, 10.4, 3.60 and 14.3, respectively. The concentrations of Cl, SO4, HCO3, Ca, Mg, Na and K in saturation extracts were as follows (cmol L−1): 9.50, 4.04, 0.79, 0.42, 2.58, 14.32 and 0.21, respectively. The NaHCO3-available P was 7.75 mg kg−1, and DTPA-extractable Zn, Fe and Mn were as follows (mg kg−1); 0.95, 4.19 and 6.51 respectively and the concentration of citric acid-extractable Si was 6.4 mg kg−1. The NaOAc-extractable B concentration was 18.28 mg kg−1. All soil analyses were carried out according to Page et al. (1982). pH, EC, SAR and B concentration had values that make this soil marginal for plant growth. PVC pots (16 cm in length, 17.5 cm in top and 12.0 cm in bottom diameter) lined with polyethylene were filled with 2 kg of air-dried soil. The roots were exposed to (1) control, (2) 2.5 mM Si and (3) 5.0 mM Si. Si was applied to the soil as Na2Si3O7 and was incorporated into the soil before seed sowing. For the basal fertilization, 200 mg N kg−1 soil as NH4NO3 and 50 and 62.5 mg P and K kg−1 soil as KH2PO4 were applied before sowing. Spinach seeds were sown at the rate of 25 seeds to each pot. After a good stand of plants had emerged they were thinned to 18 plants per pot. Two uniformly grown four-week-old tomato seedlings were transplanted to individual pots. During the experiment, soil was kept at approximately 60% of field capacity by watering with tap water.
Stomatal resistance measurements
Stomatal resistance (SR) of the experimental plants was measured by a ΔT AP4 Porometer (DELTA-T DEVICES, UK). The youngest fully developed intact leaflets were used in the SR measurements. Measurements were made on three and two plants in each pot for spinach and tomato, respectively, and were performed in the morning (10.30–11.30 a.m.) at a steady photon flux density (>150 μmol m−2 s−1), while leaf temperature varied between 18°C and 20oC.
Sampling and harvest procedure
For fresh matter used for assays, samples were taken from whole shoots of one spinach plant and fully matured leaves from tomato chosen at random. All the measurements with fresh matter were carried out during the last week of November 2005. At the end of the experiment, plants were harvested and separated into shoot and root. After weighing of fresh mass, the shoots and roots were washed once with tap water and twice in deionized water. They were then dried in an air-forced oven at 60oC until constant mass was reached. They were then weighed for dry weight determination, and subsequently ground (40 mesh sieve) for non-enzymatic antioxidant (AA), and Na, Cl, B and Si analysis.
Enzyme extraction and assay
All the enzymatic measurements were carried out at 0–4°C. Fresh samples (0.5 g) were homogenized in a Heidolph, Diax 900 homogenizer in 5 ml 100 mM potassium phosphate buffer (pH 7.6) containing 1 mM EDTA-Na2 and 0.5 mM ascorbate. The homogenized samples were centrifuged at 10,000g for 5 min. The supernatant was used as a crude enzyme extract in SOD, CAT, APX and LOX enzyme analyses. All colorimetric measurements (including enzyme activities) were made at 20oC in a Shimadzu UV/VIS 1201 spectrophotometer. Enzyme activities were expressed as units per gram dry weight of tissue.
Superoxide dismutase (SOD) activity was assayed by the nitroblue tetrazolium (NBT) method (Giannopolitis and Reis 1977). The reaction mixture (3 ml) contained 50 mM Na-phosphate buffer, pH 7.3, 13 mM methionine, 75 μM NBT, 0.1 mM EDTA, 4 μM riboflavin and enzyme extract (0.2 ml). The reaction was started by the addition of riboflavin, and the glass test tubes were shaken and placed under fluorescent lamps (60 μmol m−2 s−1). The reaction was allowed to proceed for 5 min and was then stopped by switching off the light. The absorbance was measured at 560 nm. Blanks and controls were run in the same manner but without illumination and enzyme, respectively. One unit of SOD was defined as the amount of enzyme that produced 50% inhibition of NBT reduction under assay conditions.
Ascorbate peroxidase (APX) activity (EC 1.11.1.11) was determined by following the decrease of ascorbate and measuring the change in absorbance at 290 nm for 1 min in 2 ml of a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 1 mM EDTA-Na2, 0.5 mM ascorbic acid, 0.1 mM H2O2 and 50 μl of crude enzyme extract at 25°C (Nakano and Asada 1981). The activity was calculated from the extinction coefficient (2.8 mM−1 cm−1) for the ascorbate.
Catalase (CAT) activity (EC 1.11.1.6) was determined as a decrease in absorbance at 240 nm for 1 min following the decomposition of H2O2 (Cakmak et al. 1993). The reaction mixture (3 ml) contained 50 mM phosphate buffer (pH 7.0), 15 mM H2O2 and 50 μl of crude enzyme extract at 25°C. The activity was calculated from the extinction coefficient (40 mM−1 cm−1) for H2O2.
Lipoxygenase (LOX) activity (EC 1.13.11.12) was measured according to Axelrod et al. (1981). The reaction was initiated by the addition of 0.2 ml enzyme extract in 4 ml of reaction mixture containing 50 mM sodium phosphate buffer (pH 6.5) and 0.4 mM linoleic acid. The absorbance was recorded at 234 nm (coefficient of extinction, 25 mM−1 cm−1).
Determination of membrane damage and non-enzymatic antioxidants
Lipid peroxidation (MDA) and membrane permeability (EC%) in shoot samples were measured to assess the membrane damage. For the measurement of lipid peroxidation, the thiobarbituric acid (TBA) test, which determines MDA as an end product of lipid peroxidation was used (Hodges et al. 1999). For this, sub-samples (500 mg) were homogenized in 4.0 ml of 1% TCA (trichloroacetic acid) solution and centrifuged at 10,000g for 10 min. The supernatant was added to 1 ml 0.5% (w:v) TBA in 20% TCA. The mixture was incubated in boiling water for 30 min and the reaction was stopped by placing the tubes in an ice bath. The samples were centrifuged at 10,000g for 5 min, and the absorbance of the supernatant was read at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The amount of MDA–TBA complex (red pigment) was calculated from the extinction coefficient of 155 mM−1 cm−1.
Membrane permeability (EC%) was measured by using an electrical conductivity method for the shoot disc samples as described by Yan et al. (1996).
The H2O2 content of shoots was colorimetrically measured as described by Mukherjee and Choudhuri (1983). To determine H2O2 levels, shoot samples were extracted with cold acetone. An aliquot (3 ml) of the extracted solution was mixed with 1 ml of 0.1% titanium dioxide in 20% (v:v) H2SO4 and the mixture was then centrifuged at 6,000g for 15 min. The intensity of yellow colour of the supernatant was measured at 415 nm. The concentration of H2O2 was calculated from a standard curve plotted within the range of 100–1,000 nmol H2O2.
Free proline was extracted from 0.5 g of fresh shoot samples in 3% (w:v) aqueous sulphosalicylic acid and estimated by ninhydrin reagent (Bates et al. 1973). The absorbance of the fraction with toluene aspired from the liquid phase was read at 520 nm. Proline concentration was determined from a calibration curve and was expressed as μmol proline g−1 fw.
The non-enzymatic total antioxidant activity was estimated by the method of Prieto et al. (1999). The assay is based on the reduction of Mo(VI) to Mo(V) and subsequent formation of a green phosphate/Mo(V) complex at acidic pH. 0.5 g of dry shoot samples was homogenized in 10 ml ethanol and centrifuged at 10,000g for 5 min. 0.1 ml ethanolic extract was combined with 3 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were incubated at 95oC for 90 min. After the mixture had cooled to room temperature, the absorbance was measured at 695 nm. The antioxidant activity was expressed as the number of equivalents of ascorbic acid on a dry weight basis.
Sodium, chloride, boron and silicon determination
Dried shoot and root samples (500 mg) were dry-ashed in a muffle furnace at 500oC for 6 h. The ash was then dissolved in 0.1 M HCl, and B was determined colorimetrically at 420 nm by the azomethine-H method of Wolf (1971). Sodium was determined by flame photometry (Jenway PFP7, ELE Instrument Co. Ltd) and Cl concentration in ground samples was determined by a titrimetric procedure.
Silicon in shoot and root tissues was determined by the blue silico-molybdate procedure as described by van der Vorm (1987). Dried plant samples (300 mg) were placed in porcelain crucibles and incinerated for 3 h at 550oC. The ash was washed into 100 ml polycarbonate test tubes, then 50 ml of 0.08 M H2SO4 and 2 ml of 40% HF was added. Colour development was accomplished by adding 1.5 ml of this solution to 1.5 ml of reagent mixture of the 0.08 M H2SO4 and ammonium molybdate (20 g l−1), then 1.5 ml of 0.25 M tartaric acid (C4H6O6) and finally 1.5 ml of 0.2 M ascorbic acid was added. After mixing the tubes, absorbance at 811 nm was measured and Si concentration was expressed as g kg−1 (of dry weights).
Statistical analysis
The experiments were set up in a completely randomized design. Each pot contained 18 plants in the spinach experiment and 2 plants in the tomato experiment, and each treatment contained three replicate pots. Analysis of variance was performed on the data, and significant differences among treatment means were calculated by Duncan’s multiple range test (P < 0.05).
Results
Shoot and root dry weights
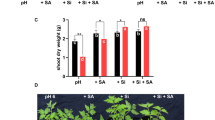
Shoot and root dry weights of spinach and tomato plants grown in sodic-B toxic soil in the presence of 0, 2.5 and 5.0 mM Si are shown in Fig. 1. The shoot and root dry weights of both spinach and tomato plants were significantly increased by the Si treatments.
Shoot and root dry weights of spinach and tomato plants grown in sodic boron toxic soil in the presence or absence of 2.5 and 5.0 mM Si. The values are means of three replicates ± standard error (SE). Different letter above each bar (for each treatment) represent significant differences at P = 0.05, based on Duncan’s multiple range test
Silicon, boron, sodium and chloride concentrations
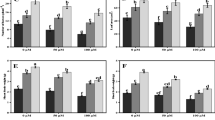
Silicon concentrations in both shoot and root tissues of spinach and tomato were significantly higher with supplemental Si (Fig. 2 a). In the control treatment, shoot Si concentrations of spinach and tomato plants were 0.74 and 2.11 g kg−1, respectively. However, shoot Si concentrations of spinach and tomato increased to 1.04 and 2.59 g kg−1 with 2.5 mM Si, and 2.09 and 2.86 g kg−1 with 5.0 mM Si supply, respectively. Silicon concentrations in the roots of spinach plants were noticeably higher than the spinach shoot Si concentrations while the root concentrations were slightly lower than the shoot concentrations in tomato.
Silicon (Si), boron (B), sodium (Na) and chloride (Cl) concentrations of the shoot and roots of spinach and tomato plants grown in sodic boron toxic soil in the presence or absence of 2.5 and 5.0 mM Si. The values are means of three replicates ± standard error (SE). Different letter above each bar (for each treatment) represent significant differences at P = 0.05, based on Duncan’s multiple range test, *non-significant
The shoot and root B concentrations of spinach and tomato were significantly reduced by Si treatments regardless of the concentrations of Si applied. Compared with spinach, the shoot B concentration of tomato was found to be higher, but was still reduced by silicon (Fig. 2b).
Sodium concentrations in the spinach and tomato shoots were lowered by applied Si. However, the decrease in concentration of Na by Si in the tomato shoots was more dramatic than the decrease in Na concentration brought about by Si in spinach shoots. Root Na concentration in the spinach was also reduced by applied Si while it was unchanged in the tomato plants (Fig. 2c).
Si treatments also significantly decreased the Cl concentrations in the shoots of both plant species. The chloride concentration of tomato was found to be higher than that of spinach. Root Cl concentrations of both species were unaffected by Si treatments (Fig. 2d).
Stomatal resistance
Stomatal resistance (SR) of spinach plants was significantly decreased from 9.03 in control plants to 7.01 s cm−1 by 5.0 mM Si treatments (Table 1). Application of Si at concentrations of 2.5 and 5.0 mM resulted in significant decreases in the SR of tomato plants from 23.21 (control) to 19.89 and 13.27 s cm−1, respectively (Table 2). Where both spinach and tomato plants were grown under the same conditions, the measured SR value of tomato leaves was higher than that of spinach (Tables 1 and 2).
H2O2 concentrations and membrane permeability (MP), lipid peroxidation (MDA), and LOX activity
In order to test the hypothesis that combined sodicity and B toxicity can induce oxidative stress, measurements were made on H2O2 concentrations, MP, lipid peroxidation (MDA), and LOX activity and the results are shown for spinach in Table 1, and for tomato in Table 2. The concentration of H2O2 was decreased from 39.76 to 34.68 μmol g−1 by 2.5 mM Si, and to 30.05 μmol g−1 by 5.0 mM Si treatment in spinach (Table 1). The corresponding values for tomato were 11.69, 10.09 and 7.76 μmol g−1, showing significant decreases in the 5.0 mM Si treatment in both species (Tables 1 and 2).
Lipid peroxidation (MDA) and the MP measured in the excised leaves of spinach plants were decreased by the Si treatments. The same parameters for the tomato did not show significant changes (Tables 1 and 2). LOX activities of spinach and tomato were 0.84 and 0.78 mmol g−1, respectively, in sodic-B toxic conditions. The activities of LOX were significantly increased to 1.13 mmol g−1 by 2.5 mM Si for tomato and 1.75 mmol g−1 by 5.0 mM Si for spinach plants (Tables 1 and 2).
Proline accumulation
The concentrations of PRO in spinach and tomato plants are presented in Tables 1 and 2. Compared with spinach, tomato accumulated higher amounts of proline. However, in both species PRO concentrations were significantly decreased by the Si treatments (Tables 1 and 2).
Antioxidative responses
The activities of SOD, CAT and APX enzymes of spinach and tomato plants grown in sodic-B toxic soils with 0, 2.5 and 5.0 mM Si are presented in Tables 1 and 2. The spinach and tomato plants grown in sodic-B toxic soil without Si supplement exhibited higher SOD activities (0.551 and 0.256 Unit mg−1, respectively). However, with the Si treatments the activity of SOD was significantly reduced. CAT activity of spinach was higher in 2.5 mM Si and lower in 5.0 mM Si treatments compared with control plants. However, the activity of CAT was decreased in tomato by Si treatments (especially in 5.0 mM Si) compared with control plants. APX activity of tomato was 8.71 in control plants and that was decreased to 8.41 and 6.40 mmol g−1 by 2.5 and 5.0 mM Si treatments, respectively. APX activity in spinach plants was also lowered significantly by Si treatments and the lowest APX activity was found in the 2.5 mM Si treatment. In both plant species, non-enzymatic antioxidant activities (AA) were increased by the Si treatments. Tomato plants exhibited higher AA than spinach in all cases (Tables 1 and 2).
Discussion
Crop productivity in many arid and semiarid regions of the world is threatened by the occurrence of salt-affected soils, and improved management practices are needed to maintain or increase the productivity of sodic-B toxic soils. In this study we investigated the effects of Si on shoot and root growth, some physiological and enzymatic parameters symptomatic of oxidative stress and the alleviation of sodicity and B toxicity stress in tomato and spinach plants.
Shoot and root growth of spinach and tomato was significantly lower when the plants were grown without supplemental Si. Growth reduction under combined saline and B toxic conditions is well documented in tomato and cucumber (Alpaslan and Gunes 2001), maize and sorghum (Ismail 2003) and B toxic conditions in barley (Karabal et al. 2003). Si applied at 2.5 and 5.0 mM significantly improved the growth of both spinach and tomato grown in sodic-B toxic soil.
There are no reports currently dealing with the effect of Si on sodicity and B toxicity. However, Si has been shown to give yield increases under salt stress conditions in tomato (Al-Aghabary et al. 2004; Romero-Aranda et al. 2006), cucumber (Zhu et al. 2004), Prosopis juliflora (Bradbury and Ahmad 1990) and wheat (Ahmad et al. 1992), under different oxidative stress conditions such as Al toxicity in barley (Morikowa and Saigusa 2002), Mn toxicity in cucumber and cowpea (Rogalla and Römheld 2002; Iwasaki et al. 2002), As toxicity in rice (Guo et al. 2005), Cd toxicity in strawberry (Treder and Cieslinski 2005) and maize (Liang et al. 2005), and under drought stress in wheat (Gong et al. 2005) and sorghum (Hattori et al. 2005).
Application of Si to the soil at rates of 2.5 and 5.0 mM increased the shoot and root Si concentration of both plant species. Romero-Aranda et al. (2006) also reported increases in Si concentration in the shoots and roots of tomato in the presence of 2.5 mM Si. Applied Si resulted in significantly decreased concentrations of B, Na and Cl in shoot tissues of both plant species. Root B concentrations of both plants and root Na concentrations of spinach plants were also decreased by the Si treatments. The concentrations of B and Cl were found to be higher in tomato than in spinach. These decreases in B concentrations might be due to the formation of B–Si (boron-silicate) complexes in the soil, leading to lower B availability. The action of silicon in reducing Na uptake in wheat (Ahmad et al. 1992) and rice genotypes (Yeo et al. 1999) has been previously reported, and the latter authors also reported that salt-tolerant rice genotypes were least responsive to Si application. Si has previously been shown to depress B uptake in oilseed rape (Liang and Shen 1994).
Supplemental Si reduced B, and also Na and Cl, translocation from the roots to shoots of spinach and tomato plants. This ameliorative effect of Si in decreasing the transport of B, Cl and Na could be related to Si being irreversibly precipitated as amorphous silica (SiO2·nH2O) in the cell walls and lumens. This has been suggested to reduce the translocation of salts to shoots (Epstein 1999). Silicon reinforcement of cell walls also protects plants from abiotic stresses (Epstein 1999). In this study, the enhanced tolerance of sodicity and B toxicity brought about by supplemental Si can be seen to be associated with decreased Na, Cl and B concentrations, and this could be one reason for the decreased membrane damage seen in the spinach plants.
It is well known that free-radical-induced peroxidation of lipids of membranes is a reflection of stress-induced damage at the cellular level (Jain et al. 2001). Therefore, the level of MDA, produced during peroxidation of membrane lipids, is often used as an indicator of oxidative damage. In the present study, MP and lipid peroxidation (MDA concentration) was significantly decreased in spinach, although LOX activity was increased, by Si treatment. Therefore, better protection of membranes by antioxidative systems may be another reason why Si protects against sodicity and B toxicity, although the increase in LOX activity is surprising. It could be that rather than causing breakdown of membranes LOX breaks down polyunsaturated fatty acids released by membrane damage, and so could be useful in minimizing the effects of this damage. It has certainly been shown to have a role in prevention of fungal infection by plants (Rance et al. 1998), and its stimulation by Si may be related to the well-documented effect of silicon in protecting plants from biotic stress (Epstein 1999; Ma 2004). Decreased MDA content in barley by Si was reported by Liang (1999). Excessive B- and salinity-mediated membrane damage was also previously reported in onion (Inal and Tarakcioglu 2001), tomato and cucumber (Alpaslan and Gunes 2001), sorghum and maize (Ismail 2003) and barley (Karabal et al. 2003).
An increased proline level, together with enhanced H2O2 concentration, is a common response of plants to B stress treatments (Karabal et al. 2003), and combined salinity and B toxicity (Alpaslan and Gunes 2001). In our experiment, under sodicity and B toxicity both proline and H2O2 concentrations were significantly lower with supplemental Si than in Si-untreated plants, at least with Si supplied at 5.0 mM. These results are consistent with the findings of Al-Aghabary et al. (2004), who showed that Si decreased salt-induced production of H2O2 and improved rates of photosynthesis. Our data do not provide evidence about whether or not Si increases net photosynthesis, but decreased stomatal resistance by Si could increase the photosynthetic rate. Leaf SR provides sensitive comparisons and indicates the degree of stress in plants under adverse conditions (Gunes et al. 1996). In our experiment, the highest SR was detected under sodicity and B toxicity with no supplemental Si in both plant species. However, SR was decreased by the supply of Si. These results are in good agreement with those of Yeo et al. (1999) and Romero-Aranda et al. (2006), who found that SR in tomato and rice was decreased by Si under salinity, and of Papadakis et al. (2004), who reported that SR was increased by excessive B in mandarin leaves.
The dismutation of O ·−2 into H2O2 and oxygen is an important step in protecting the cell, and is catalysed by SOD (Halliwell and Gutteridge 1999). In the present study, the activities of SOD, CAT and APX in plants grown without Si were obviously higher. Application of Si, at the highest rate especially, decreased their activities. The activity of non-enzymatic antioxidants was increased in the Si-supplemented spinach and tomato plants, further confirmation that oxidative damage induced by sodicity and B toxicity was alleviated by enhanced activity of antioxidative systems. The results related to antioxidant responses are in agreement with the findings of Molassiotis et al. (2006), who reported increases in SOD and CAT activity in apple rootstocks under B toxicity. In addition to this, Garcia et al. (2001) and Karabal et al. (2003) have also shown increased SOD activity under B toxicity in tobacco and barley respectively. Rahnama and Ebrahimzadeh (2005) reported that the SOD, CAT and APX activities of potato seedlings were increased under saline conditions.
In conclusion, the results of this study highlight the role of Si in regulating the sodicity and B-toxicity stress responses of spinach and tomato, and indicate that Si could be used as a potential growth modifier to improve plant growth under sodic-B toxic conditions. The results indicate that Si is involved in metabolic or physiological activities in spinach and tomato under sodic-B toxic conditions, and also that both species accumulate lower amounts of potentially toxic Na, Cl and B with supplemental Si. Because the extent of increase in dry weight in response to applied Si was lower in spinach than in tomato we suggest that spinach is more tolerant of combined sodicity and B toxicity than tomato. Furthermore, mechanisms of exclusion of B, Na and Cl in spinach are more efficient than such mechanisms of tomato. Additionally, the physiological stress response parameters of spinach were also shown to be less extreme than in tomato. We found lower SR, MDA, PRO accumulation, CAT activity and AA and higher values of H2O2, SOD activity and APX activity in spinach than in the tomato plants.
Abbreviations
- AA:
-
Non-enzymatic antioxidant activity
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- LOX:
-
Lipoxygenase
- MP:
-
Membrane permeability
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- PRO:
-
Proline
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SR:
-
Stomatal resistance
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Ahmad R, Zaheer S, Ismail S (1992) Role of silicon in salt tolerance of wheat (Triticum aestivum L.). Plant Sci 85:43–50
Alpaslan M, Gunes A (2001) Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 236:123–128
Al-Aghabary K, Zhu Z, Shi Q (2004) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 12:2101–2115
Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenases from soybeans. In: Lowenstein JM (ed) Methods in enzymology. Academic Press, New York, pp 441–451
Bates LS, Waldren RP, Teare JD (1973) Rapid determination of proline for water stress studies. Plant Soil 39:205–207
Ben-Gal A, Shani U (2002) Yield, transpiration and growth of tomatoes under combined excess boron and salinity stress. Plant Soil 247:211–221
Bradbury M, Ahmad R (1990) The effect of silicon on the growth of Prosopis juliflora growing in saline soil. Plant Soil 125:71–74
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinated wheat seeds. J Exp Bot 44:127–132
Epstein E (1999) Silicon. Annu Rev Plant Physiol Plant Mol Biol 50:641–664
Garcia POC, Rivero RM, Lopez-Lefebre LR, Sanchez E, Ruiz JM, Romero L (2001) Response of oxidative metabolism to the application of carbendazim plus boron in tobacco. Aust J Plant Physiol 28:801–806
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gunes A, Inal A, Alpaslan M (1996) Effect of salinity on stomatal resistance, proline and mineral composition of pepper. J Plant Nutr 19:389–396
Guo W, Huo YL, Wang SG, Zhu YG (2005) Effect of silicate on the growth and arsenate uptake by rice (Oryza sativa L.) seedlings in solution culture. Plant Soil 272:173–181
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, UK
Hattori T, Inanaha S, Araki H, An P, Morita S, Luxova M, Lux A (2005) Application of silicon enhanced tolerance in Sorghum bicolor. Physiol Plant 123:459–466
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Inal A, Tarakcioglu C (2001) Effects of nitrogen forms on growth, nitrate accumulation, membrane permeability and nitrogen use efficiency of hydroponically grown bunch onion under boron deficiency and toxicity. J Plant Nutr 24:1521–1534
Ismail AM (2003) Response of maize and sorghum to excess boron and salinity. Biol Plant 42:313–316
Iwasaki K, Maier P, Fecth M, Horst WJ (2002) Leaf apoplastic silicon enhances manganese tolerance of cowpea (Vigna unguiculata). J Plant Physiol 159:167–173
Jain M, Mathur G, Koul S, Sarin NB (2001) Ameliorative effects of proline on salt stressed lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep 20:463–468
Karabal E, Yucel M, Oktem HA (2003) Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci 164:925–933
Keles Y, Oncel I, Yenice N (2004) Relationship between boron content and antioxidant compounds in Citrus leaves taken from field with different water source. Plant Soil 265:345–353
Liang Y (1999) Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209:217–224
Liang Y, Shen Z (1994) Interaction of silicon and boron in oilseed rape plants. J Plant Nutr 17:415–425
Liang Y, Wong JWC, Wei L (2005) Silicon mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molassiotis A, Sotiropoulos T, Tanou G, Diamantidis G, Therios I (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Environ Exp Bot 56:54–62
Morikowa CK, Saigusa M (2002) Si amelioration of Al toxicity in barley (Hordeum vulgare L.) growing in two Andosol. Plant Soil 240:161–168
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193:181–198
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis, part 2; chemical and microbiological properties, 2nd edn. SSSA, Madison, WI, USA
Papadakis IE, Dimassi KN, Bosabadilis AM, Therios IN, Pataks A, Giannakoula A (2004) Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks. Plant Sci 166:539–547
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol Plant 49:93–97
Rance I, Fournier J, Esquerre-Tugaye MT (1998) The incompatible interaction between Phytophthora parasitica var. nicotianae race and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proc Natl Acad Sci USA 95:6554–6559
Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91
Treder W, Cieslinski G (2005) Effect of silicon application on cadmium uptake and distribution in strawberry plants grown on contaminated soils. J Plant Nutr 28:917–929
Tsai YC, Hong CY, Liu LF, Kao CH (2004) Relative importance of Na and Cl in NaCl-induced antioxidative systems in roots of rice seedlings. Physiol Plant 122:86–94
Van der Vorm PDJ (1987) Dry ashing of plant material and dissolution of the ash in HF for the colorimetric determination of silicon. Commun Soil Sci Plant Anal 18:1181–1189
Wolf B (1971) The determination of boron in soil extracts, plant materials, composts, manures, water and nutrient solutions. Commun Soil Sci Plant Anal 2:363–374
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant response to osmotic stress. Plant Cell Environ 25:131–139
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10:615–620
Yan B, Dai Q, Liu X, Huang S, Wang Z (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268
Yeo AR, Flowers SA, Rao G, Welfare K, Senanayake N, Flowers TJ (1999) Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for a reduction in the transpirational bypass flow. Plant Cell Environ 22:559–565
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunes, A., Inal, A., Bagci, E.G. et al. Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 290, 103–114 (2007). https://doi.org/10.1007/s11104-006-9137-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9137-9