Abstract
Underlying spatial and habitat attributes of a river network are crucial to comprehend the bio-spatial arrangements within it, the study of which suffers from a paucity of information. Despite several reports on various piscine assemblages, no study contributes to understanding the characteristic attributes of the freshwater habitats of the sub-Himalayan Terai–Dooars ecoregion. Therefore, this study aims to uncover such underlying features through a precise understanding of the spatial profile of freshwater habitats and additive partitions of piscine beta diversity. A significant spatial association is found in the upper stretches of most of these torrential freshwater reaches confined to the eastward of the River Teesta basin to the tributaries of River Jaldhaka. Such a pattern is aligned with a higher local contribution to beta diversity (LCBD) values. The spatial map of LCBD indicates that the mid-altitude (100 > elevation > 2000 m) region contains unique or rare species assemblages. This fact is further confirmed by the spatial aggregation of characteristically adapted hill stream fish species with higher species contribution to beta diversity (SCBD) values. The results are further explained by relevant climatic, topographic, nutrients (sediments), and habitat attributes of which climate, topographic, substrate, and land cover features are the most contributory factors. Such variables are subjected to severe modulation following increasing anthropogenic pressure and changing climatic conditions, leading to the jeopardy of these freshwater habitats. Therefore, prime importance should be accorded to the ecological restoration value of these spatially structured torrential freshwater habitats for conservation and monitoring in the coming days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial arrangements of biodiversity varying upon the different spatial scales need to be understood for efficient conservation assessment and planning (Ferrier et al. 2007; Margules and Pressey 2000). The assemblage of both native and exotic species within a given landscape is contingent on the degree of spatial heterogeneity (Davies et al. 2005). However, the spatial process directly corresponds to the species sorting following the dynamics of environmental gradients, attributed by various factors at a given spatial extent (Cottenie 2005; Heino et al. 2015a; Jackson et al. 2001). Therefore, understanding the active spatial filters for a species at varied habitats is crucial, resulting from dispersal limitation, neutral dynamics, and spatially structured environmental attributes (Leprieur et al. 2009; Sharma et al. 2011). As in-field sampling over a large space is not convenient with time and effort, it persuades the employment of choosing surrogates for the species under study (Ferrier et al. 2007). Such surrogates include habitat types, stream sections, landscapes often stemmed from remote sensing imagery, abiotic environmental classes, climate, and terrains (Ferrier et al. 2007).
Beta diversity accounts for the change in species composition between certain places representing the differentiation component of diversity and assemblage (McKnight et al. 2007). Being central to a wide array of ecological and evolutionary understanding (McKnight et al. 2007), beta diversity has long been used on many aquatic organisms to describe crucial turnover of species with the response to specific environmental characteristics resulting from temporal and spatial dissimilarities (Angeler 2013; Melchior et al. 2017; Viana et al. 2016). Legendre and De Cáceres (2013) have developed another two components to emphasize the contribution of habitat towards the existing pattern of dissimilarities in biodiversity (Vilmi et al. 2017; Yao et al. 2020). These components are described as a local contribution to beta diversity (LCBD), indicating the uniqueness of the site and its degradation as well as the species contribution to beta diversity (SCBD), indicating unique and rare assemblages of species with conservation values (Legendre and De Cáceres 2013; Sor et al. 2018; Vilmi et al. 2017).
The freshwater ecosystem depicts an epitome of researches concerning distinct species assemblage, spatial heterogeneity, and insuperable barriers (Leprieur et al. 2009). The freshwater fish communities are regulated by niche differences and dispersal limitations following ecological changes limited at a connected stretch of waters (Chase and Leibold 2003; Heino et al. 2009; Hubbell 2001). Climate, energy, and habitat diversity work in close association, resulting in a diverse piscine assemblage globally (Guégan et al. 1998; Leprieur et al. 2011). Therefore, dismantling spatial features arising at a particular site and relevant local environmental, catchment, and climatic factors is critical (Heino et al. 2007; Perez Rocha et al. 2018) in shaping freshwater fish biodiversity. The lotic habitats of freshwater fishes are under serious threat. They are subjected to climate change, channel modification, fragmentation, flow alteration, and degradation (Bhatt et al. 2016; Goswami et al. 2012a, b).
Such threats, coupled with the observed trend of anthropogenic influences, pose more considerable influence over the Himalayas (Singh 2015). The freshwater network primarily prevalent in the sub-Himalayan Terai–Dooars (TED) ecoregion has been prioritized for conservation based on the richness of fish species with higher conservation values, endemism, and vulnerabilities (Bhatt et al. 2016). The major rivers of the TED ecoregion in Northern Bengal, India, are River Teesta, Jaldhaka, Torsha, and Mahananda (Jayaram and Singh 1977). However, understanding the spatial distribution and underlining species sorting in such exclusive freshwater habitats is overlooked due to much research effort into the identification of fish species, reporting exclusive alpha diversity at various local scales (Bhowmik et al. 2016; Dey et al. 2015a ,b, c, d, 2019), eco-physiological studies in captivity (Dey et al. 2015a; b, c, d), preserving germplasm and reporting genetic variabilities (Dey et al. 2015a; b, c, d; Kundu et al. 2019). A firm understanding of spatial arrangements of fish communities would reveal the complex interaction of multiple factors, which shapes their habitats in a more varied or nested manner considering their adaptations, invasions, dispersal limitations, and mass effects (Leprieur et al. 2009; Leroy et al. 2019; Planque et al. 2011; Shurin et al. 2009; Wiersma and Urban 2005). Such a pattern gives direct insight into the response of biological communities to climate and environment, which often serves as a basis of sound management of their commercial or conservation interest (Leprieur et al. 2009; Planque et al. 2011). Therefore, the lack of such foundation stumbles conservation planning and monitoring over this freshwater habitat, becoming more vulnerable following changing geo-climatic conditions (Akhter et al. 2019; Barman and Das 2014; Goswami et al. 2019) and increasing anthropogenic pressure (Karmakar 2011; Naha et al. 2019; Singh 2015).
Therefore, this study reveals the pattern and reasons behind the unique fish assemblage in the sub-Himalayan TED ecoregion freshwater network. We delineated the significant spatial variables explaining the spatial structure of fish assemblages using spatial distance among the sites, identifying if the scales have a significant association. Then, we conducted a decomposition of the spatial model at relevant scales into submodels, aiming to reveal species–environment relationships. Next, using beta dissimilarities, we analyzed the individual contribution of fish species and their habitat through partitioning the total beta diversity while explaining the latter by relevant environmental variables. The results were compared to identify the freshwater habitats and their correspondence in unique piscine assemblage, subjected to ecological conservation and management considering the threats of dynamic climatic and anthropogenic events in the sub-Himalayan TED ecoregion. This study signifies the first attempt to construct such a profile from this region to explore ecological sensitivity in species conservation values and eco-restorations.
Materials and methods
Study area
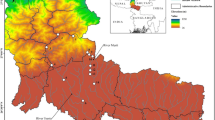
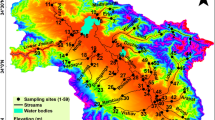
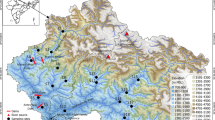
The sub-Himalayan TED ecoregion has moist and dense riverine forests along the foothills of the snow-capped Kanchenjunga range along the Eastern Himalayas (Barman and Das 2014; Kandel et al. 2016). Northern Bengal (NB) comprises the areas within West Bengal, India, confined to the north of the river Ganges (Barman and Das 2014). Innumerable streams are draining these alluvial floodplains of the TED ecoregion in NB (Barman and Das 2014; Paul et al. 2009; Rudra 2018). The freshwater reaches of this region are more dynamic due to continuous deposition in the channel, increasing the height of the riverbeds (Chakraborty and Datta 2013). The river channels are experiencing frequent shifts following anabranching and changing river courses (Akhter et al. 2019; Goswami et al. 2019) Sub-tropical monsoon climate causes excessive precipitation in the sub-Himalayan regions leading to the higher flow in these torrential courses. In the summer months, they are replenished by snow-melt waters (Akhter et al. 2019; Bhatt et al. 2012; Panja et al. 2020; Rudra 2018). This study has been conducted on a vast drainage network of Teesta–Neora–Jaldhaka rivers, including watersheds of River Teesta, River Chel, River Neora, River Dharala, River Murti, and River Jaldhaka, draining through the TED ecoregion in NB, India (Fig. 1). Along the banks of these rivers, the TED ecoregion has several reserve forests and national parks, such as Singalila National Park, Mahananda Wildlife Sanctuary, Neora Valley National Park, Gorumara National Park, and Chapramari Wildlife Sanctuary, which indicate the importance of this ecoregion (Bhattacharya 2019). A stream network of these freshwater reaches was derived using digital elevation layers (MERIT-HYDRO DEM) (Yamazaki et al. 2019) in the Arc GIS platform (V.10.1).
Fish sampling
A total of 31 sampling sites were selected based on a pilot study upon these freshwater reaches. Later, a tri-seasonal fish sampling, i.e., pre-monsoon, monsoon, and post-monsoon, was conducted during 2016–2019. A 90 m reach, at each location was sampled using the electro-fishing method by electro-fisher (300 V, 3–4A, DC) followed by gill nets, cast nets, and dragnets, respectively. The dimensions of the nets used in samplings were constant (obtained from the same source) for all the areas surveyed. Fishes were identified following existing literature (Barman and Das 2014; Jayaram and Singh 1977; Menon 1999; Shaw 1938; Talwar and Jhingran 1991). The removal method of estimation was applied in three consecutive efforts (Bohlin et al. 1989). The captured fish specimens were counted, and a single representative was preserved in a 10% formalin solution.
Environmental data
Seven bioclimatic variables, four topographic, two substrates, and two land-cover attributes had been considered under the environmental profile. The ecological success, reproductive behaviors, and physiology of freshwater fishes are significantly driven by variability in temperature and precipitation (Barbarossa et al. 2021; Ficke et al. 2007). Despite being coarse-scale modulators, previous studies (Domisch et al. 2011, 2015, 2013, 2011; Durance and Ormerod 2007; Leprieur et al. 2009; Oberdorff et al. 1999; Reyjol et al. 2007) found the significant contribution of annual mean temperature, the max temperature of the warmest month, min temperature of the coldest month, annual precipitation, precipitation of wettest month, precipitation of driest month and evatransportation in shaping the spatial assemblage of freshwater fishes. Furthermore, the topographical characteristics of streams, i.e., elevation, slope, stream order, and terrain position index, directly correspond to local ecological attributes of freshwater habitats viz. water temperature, dissolved oxygen, and flow regimes (Austin 2007; Domisch et al. 2011, 2013; Kuemmerlen et al. 2014). On the other note, stream substrate characteristics (upland & valley bottom characteristics, soil sediment) and landcover attributes (normalized difference vegetation index and land cover) control the productivity, pH, turbidity, and nutrient dynamics of water which are responsible for various species-specific adaptations (Brooks et al. 2005; Effenberger et al. 2006; Fausch et al. 2002; Kozel and Hubert 1989). Therefore, these fifteen variables (Table 1) are pertinent to understand freshwater species distribution. All these variables were obtained in 30 arc-second resolution and sampled for the selected 31 sampling sites. The respective sources of the environmental data (Haynes et al. 2018; Huntington et al. 2017; Karger and Zimmermann 2019; Pelletier et al. 2016; Trabucco and Zomer 2019; Yamazaki et al. 2019) are listed in Table 1. Slope, stream order (Strahler), and topographic position index were calculated using the DEM raster (Yamazaki et al. 2019) in the QGIS platform (QGIS version 3.10.0-A Coruà ± a) (https://www.qgis.org/en/site/). Before fitting into analytical models, these variables were resampled, standardized, and stacked for the study region and sampled against the 31 sites to conduct a Pearson’s correlation among them. Predictors with high collinearity (Pearson’s r ≥ 0.8) (Domisch et al. 2011; Thuiller et al. 2014) were discarded (See Supporting Information: Fig. S1).
Data analysis
Identification of the spatial structures
The spatial arrangements of habitats significantly structure the fish community composition considering space, scale, and connections within a landscape (Jackson et al. 2001; Legendre and Legendre 2012; López‐Delgado et al. 2019). This widely recognized concept would reveal the role of space in habitat heterogeneity and spatial aspects of biotic and abiotic conditions (Hanski 2001; Jackson et al. 2001). The framework of Moran’s eigenvectors map (MEM) has gained popularity as a recent family of spatial analysis techniques (Ali et al. 2010; Jackson et al. 2001; Legendre and Legendre 2012; Perez Rocha et al. 2018). This machinery aims to model the correlation structure present at each scale, linking with the spatial heterogeneity of environmental factors (Ali et al. 2010). Based on this framework, a distance-based Moran’s eigenvectors map (dbMEM) (Dray et al. 2006) would identify the characteristic spatial scales through a spatial filtering technique (Blanchet et al. 2008) to define a set of spatial proxy variables and their selection to explain the spatial structure of the response variable under study, i.e., fish species assemblage. The dbMEMs corresponding to smaller eigenvalues usually represents very fine-scale spatial patterns, where spatial autocorrelation is presumed low. On the contrary, dbMEMs corresponding to larger eigenvalues signify coarse spatial variability scales, often selected to define prominent spatial structures. Furthermore, dbMEMs corresponding with positive eigenvalues (Positive Moran’s I) depicts a positive spatial association, which is more crucial to consider than a negative spatial association (Ali et al. 2010; Griffith and Peres-Neto 2006; Legendre and Legendre 2012).
Initially, the spatial coordinates of the sites and a Hellinger transformed presence–absence data set was subjected to define dbMEMs (Blanchet et al. 2008; Dray et al. 2006; Legendre and Legendre 2012) and their spatial association with the fish species assemblage of the freshwater habitats. The data was checked for linear trends and detrended if present. The dbMEM eigenvectors were computed, and those with positive association (positive Moran’s I) were retained. Then the dbMEMs were tested in a redundancy analysis (RDA) with species data for significance and run a forward selection with double-stopping criteria (adjusted R square of the RDA and α level of rejection) (Blanchet et al. 2008) to select significant dbMEMs. Now a new RDA was run with the significant dbMEMs to tests the significance of the axes. Based on the results, maps were drawn for each significant axes identifying spatial structures among the sites. (Borcard and Legendre 2002; Borcard et al. 1992; Dray et al. 2012; Legendre and Legendre 2012). This analysis was performed in the R platform with the quickMEM function from package adespatial. The total variation in the fish species assemblage was further partitioned using the spatial (dbMEMs) and environmental variables (Perez Rocha et al. 2018; Vilmi et al. 2017) to assess their relative and cumulative roles through partial RDA testing.
The site scores of each significant RDA axes were further explained by the environmental variables (Borcard and Legendre 2002; Borcard et al. 1992; Legendre and Legendre 2012) to identify the significant association of environmental variables resulting in positive-broad scale spatial structures. This assessment was achieved by applying boosted regression trees (BRT) models (Elith et al. 2008). The boosted regression tree (BRT) is much exploited as an excellent modeling tool for the predictive purpose of ecological researches (Elith et al. 2008). However, BRT modeling with smaller data set would face a slight penalty for using larger trees which are usually overcome using low learning rates and smaller decision trees (Elith et al. 2008). BRT is advantageous in accommodating different predictors, with no dependencies on response data transformation, outlier removal, and handling complex non-linear relationships while considering interactions between the predictors to reduce predictive errors (Carslaw and Taylor 2009; Elith et al. 2008; Jafari et al. 2014; Panja et al. 2020). Considering such an advantage, previous studies have appropriately used this machine learning model using a small data set (Jafari et al. 2014; Panja et al. 2020, 2021b, a; Zhang and Ling 2018). A combination of learning rates (high to low) was tried to achieve the minimum 1000 trees initially based on the tenfold cross-validation method. Since the present study dealt with a comparatively smaller data set, tree complexity was set to 3 (Elith et al. 2008). A pseudo determinant factor, D2 was calculated for the fitted model accounting for their credibility (Nieto and Mélin 2017). The environmental variables contributing highest to the model are identified.
Beta diversity measures
Legendre and De Cáceres (2013) emphasized the advantages of estimating beta diversity (BDTotal) as the total variation of the community matrix (Z). They showed that Z could be linked with the beta diversity assessments computed from the dissimilarity matrix of community composition while establishing the closer relevance of other beta diversity measures (Anderson et al. 2006; Ricotta and Marignani 2007; Whittaker 1972). Based on such principle, BDTotal could be disintegrated into two attributes accounting for contributions of single sites to overall beta diversity and individual species to overall beta diversity (Heino and Grönroos 2017; Legendre and De Cáceres 2013). The former is referred to as local contributions to beta diversity (LCBD) which comparatively indicates the ecological uniqueness of the study sites. The latter attribute is defined as species contributions to beta diversity (SCBD) which denotes the degree of variation of individual species across the study region (Legendre and De Cáceres 2013). The LCBD values identify sites with more (or less) contribution than the mean to beta diversity, which comparatively indicates the uniqueness of species composition at a particular site. LCBD directly corresponds to the number of rare species across geographical space, while were negatively related to the occurrences of common species, therefore, accounting for dispersal limitation as well as the local environment and community characteristics (Legendre and De Cáceres 2013; Vilmi et al. 2017; Yao et al. 2020). On the other note, SCBD indices are not the same as indicator species for a given group of sites. Instead, it directly corresponds to significant variations imposed by each species across the study area (Cáceres and Legendre 2009; Dufrêne and Legendre 1997; Legendre and De Cáceres 2013).
Z could be obtained by calculating a matrix of squared deviations along the column means based on computing community dissimilarity matrices from the transformed species presence–absence data. Then the total sum of squares (SSTotal) is obtained when summing all the squared values, which forms the initial basis of BDTotal.
\({\text{BD}}_{{{\text{Total}}}} = {\raise0.7ex\hbox{${{\text{SS}}_{{{\text{Total}}}} }$} \!\mathord{\left/ {\vphantom {{{\text{SS}}_{{{\text{Total}}}} } {\left( {n - 1} \right)}}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${\left( {n - 1} \right)}$}},\)n represent the number of sites (Legendre and De Cáceres 2013).
Computation of SSTotal is advantageous to directly assess the contributions of individual species and individual sampling units to the overall beta diversity (Heino and Grönroos 2017; Legendre and De Cáceres 2013). Therefore, SCBD and LCBD indices were developed on total sum squares of species composition, thereby calculating the proportion of jth species and the ith sampling unit for SCBD and LCBD viz. SCBDj = SSj/SSTotal and LCBDi = SSi/SSTotal (Legendre and De Cáceres 2013). Sites with higher LCBD values are identified. The fish species with higher SCBD values than the average value of all are identified.
BRT was fitted similarly with the LCBD values against the predictor environmental data set. The significant variables were identified, contributing highest to explain the LCBD attributes of each site and compared with the spatial model. LCBD site scores were further assessed through a correlation approach with the significant RDA axes about surrogacy in conserving and monitoring these freshwater ecosystems. Based on the best trees of the suited model, the LCBD was predicted over the restacked spatial map of selected environmental variables to construct a spatial LCBD profile for this study area.
All the statistical analyses have been performed in the R platform (Team R 2015; Team RC 2013) using packages, namely, raster, maptools, rgeos, dismo, and gbm. The spatial maps are created using the Arc GIS platform (version 10.1).
Results
Initially, a total of 175 fish species were recorded from the sampling. However, discarding exotic species (See Supporting Information: Table S1) from the study (Bhowmik et al. 2016; Sarkar and Pal 2018), a total of 170 indigenous fish species with 11,560 individuals has been recorded from the sampling along 31 sites (See Supporting Information: Table S2 and Fig. S2). River Jaldhaka has the highest species richness with 158 fish species. In comparison, the lowest has been observed in River Neora with 22 fish species.
Partitioning spatial component of ecological variation
A total of nine spatial variables, i.e., dbMEM eigenvectors (Table 2), have been produced with R2 of the global model = 0.484. Among them, four dbMEM eigenvectors have been forward selected (Table 2) at p < 0.001. In the final RDA model, two canonical axes are significant, i.e., RDA 1 (Axes1) at p < 0.001 and RDA 2 (Axes 2) at p < 0.05 (Table 2; Fig. 2), explaining the underlying spatial association of sites resulting into species sorting in these selected freshwater habitats. It appears that Axes 1 (Fig. 2) indicates substantial positive spatial influence on the species assemblages around the upper stretches of most of these freshwater reaches confined to the TED ecoregion. However, Axes 2 (Fig. 2) indicates a secondary spatial pattern occurring specifically in River Teesta and its tributaries around the mid altitudinal zone.
Significant canonical axis of redundancy analysis: Axes 1 and 2 of four forward selected dbMEM eigenvectors in Distance-based Moran’s Eigenvector Maps (dbMEM) analysis explaining the spatial association of freshwater habitats regarding differential fish assemblages in the freshwater network of sub-Himalayan Terai–Dooars ecoregion. (Darker and larger the circle indicates the larger and positive eigenvalues depicting stronger positive spatial association)
In variation partitioning, the selected spatial variables significantly influence the fish species variation in the partial RDA model at p < 0.001 (See Supporting Information: Table S3). The purely spatial components have explained 22%, while purely environmental factors have explained the variation of 11%. However, they both cumulatively address 2% of the total constrained variation (Fig. 3).
Additive components of beta dissimilarity
The higher LCBD values are projected in the upper stretches of River Chel, Neora, Murti, Jaldhaka, and mid altitudinal reaches of River Teesta (Fig. 4a). RDA Axes 1 depicting substantial spatial patterns in the sites are highly correlated (Pearson’s r > 0.8) with the respective LCBD values (Fig. 4b). The higher SCBD values are observed for 54 fish species. The top ten fish species with significant contribution are Neolissochilus hexastichus, Neolissochilus hexagonolepis, Garra lamta, Tor tor, Badis badis, Devario aequipinnatus, Garra annandalei, Crossocheilus latius, Amblyceps mangois, and Barilius vagra (Fig. 5).
a Local contribution to beta diversity (LCBD) values of freshwater habitats in the freshwater network of sub-Himalayan Terai–Dooars ecoregion (Larger the circle indicates the higher values). b Correlation between the LCBD values and significant RDA axes (A1: Axes1 & A2: Axes 2) of the spatial model depicting the spatial association of freshwater habitats in the sub-Himalayan Terai–Dooars ecoregion
Boosted regression models
A Pearson’s correlation test has identified the highly correlated predictors among fifteen predictor environmental variables. As the highly correlated variables would increase the uncertainty in the model and decrease robustness, they were removed from the predictor set (See Supporting Information: Fig. S1). Therefore, eight variables have been retained, which are annual mean temperature (B1), annual precipitation (B12), precipitation of driest month (B14), slope (SL), stream order (SO), land cover (LC), upland & valley bottom characteristics (UV) and soil sediment (SS).
B14, SO, UV, LC, B1, B12, and SL have a more substantial influence in the spatial model (Table 3), explaining Axes 1 with BRT (See Supporting Information: Fig. S3). However, B12, UV, B14, SO, LC & B1 are relevant (Table 3) to explain Axes 2 in the BRT model (See Supporting Information: Fig. S3). Therefore, B14, B12, B1, SO, UV, and LC are cumulatively responsible for the underlying spatial profile in the freshwater habitats of differential fish species assemblage. On the other note, LCBD values of these freshwater habitats are best explained by B14, SO & B1 (Table 3) in the BRT model (See Supporting Information: Fig. S3).
The projected spatial map of LCBD (Fig. 6) following the best trees projects higher values in the upstream reaches of River Jaldhaka, Murti, Chel, Neora. Comparatively, lower LCBD values are dispersed around the plains of Terai and Dooars, confined into the district of Jalpaiguri and Cooch Bihar. Moderate LCBD values are predicted within mid altitudinal stretches of River Teesta.
Discussion
In this study, a prominent spatial association determining the fish assemblages among the water reaches is observed. Such spatial features contribute at large to the differential fish species assemblage in these freshwater habitats. At the same time, they are substantially associated with climatic (precipitation of driest month, annual precipitation, annual mean temperature), topographical (stream order), substrate (upland & valley bottom characteristics), and land cover (landcover) characteristics of these freshwater reaches of TED ecoregion of NB. On the other note, the LCBD profile is aligned with axes 1 of significant spatial association of these freshwater habitats. However, such profile is best explained by climatic (precipitation of driest month, annual mean temperature) and topographical (stream order) attributes. Furthermore, higher SCBD values emphasized 54 fish species which are differentially distributed among these freshwater reaches of the TED ecoregion of NB.
The freshwater habitats of this study belong to different freshwater river reaches that are not necessarily connected (Fig. 1). Our result suggests a spatial metacommunity structure among the freshwater habitats presumed to result in these broad-scale spatial associations (Grönroos et al. 2013; Heino et al. 2015a, b; Thompson and Townsend 2006). However, the more delicate spatial association is not achieved as they are hard to discern (Ali et al. 2010; Legendre and Legendre 2012) and might be associated with local mass effect dynamics (Borcard and Legendre 2002; Heino et al. 2015a, b). Such an approach is apposite considering the identification of broad-scale spatial structures and coarse-scale resolution of relevant environmental variables explaining them. Broad-scale spatial variables tend to be associated with dispersal limitation (Heino et al. 2015a, b; Heino et al. 2015a, b; López‐Delgado et al. 2019) as the watercourse distances are more stringent for stream organisms (Altermatt 2013). Therefore, the community dynamics of each habitat rely significantly upon spatial autocorrelation (SA) (Shurin et al. 2009) than changes in rates of movements.
The results indicate a substantial role of climate, topography, landscape, and substrate features behind the prominent positive spatial association of the habitats. Such findings are relevant as a significant geomorphological recess has been observed in the east of River Teesta to River Jaldhaka–Diana channel, further segmented by Chel–Mal, Mal–Murti, Jaldhaka–Gathia interfluves (Goswami et al. 2019). This region is predominantly manifested by a transitional zone between the Eastern Himalayan mountains and the upper Gangetic plains (Chakraborty and Datta 2013), where a marked difference exists in annual temperature, precipitation, and climate extremities (Das 2020; Panja et al. 2021b, a; Rudra 2018; Sam and Chakma 2019). The substrate composition of freshwater channels is characterized by the piedmont fans, channel deposition, frequent shifting of courses (Chakraborty and Datta 2013; Goswami et al. 2019) which cumulatively modulated by topography. Such characteristics might have resulted in the recurrence of high and low flow regimes and a pronounced temperature gradient in these freshwater habitats (Bandyopadhyay et al. 2014; Chakraborty and Datta 2013; Goswami et al. 2012a, b; Guha et al. 2007; Panja et al. 2020). The spatial structure in the mid altitudinal freshwater habitats of River Teesta and Chel might be associated with the stream frequency, braiding, and drainage density (Akhter et al. 2019; Dhali et al. 2020) which has previously exhibited a positive spatial pattern autocorrelation (Akhter et al. 2019). Goswami et al. (2019) explained that two extrinsic factors, i.e., tectonism and climate coupled with extreme anthropogenic events (Dhali et al. 2020), have been causing land cover changes and interfluve characteristics of these rivers, a significant modulator of the observed spatial structures of our results. Such variables are presumed to create the spatial association and segregation among these freshwater reaches by modulating flow regimes, pH, dissolved oxygen, turbidity, and the ecological integrity of the river channel and aquatic habitats (Akhter et al. 2019; Comiti et al. 2011; Dhali et al. 2020), leading to more spatially structured piscine assemblages. Such finding is further strengthened by the higher influence of spatial variables than environmental factors in variation partitioning (Fig. 3) (de Campos et al. 2019; Erős et al. 2012; Leonidas et al. 2020). However, the unexplained remnant variation is comparatively large and could be often described for several local factors, biotic interactions, and their lack of fidelity to include in the study (López‐Delgado et al. 2019; Perez Rocha et al. 2018).
The degree of dispersal limitation (Cottenie 2005; Villéger et al. 2013) is arduous to fathom for each fish species; instead, the focus has been given to its additive partitions of beta dissimilarities, i.e., LCBD and SCBD (Heino 2011; Leibold et al. 2004). Previously LCBD indices were accounted to assess the uniqueness of the aquatic habitats of a fish community (Legendre and De Cáceres 2013). The higher LCBD values indicate unusual species composition and species-poor sites requiring ecological restoration (Legendre and De Cáceres 2013; Panja et al. 2021a; Vilmi et al. 2017), which is reflected for the freshwater habitats of the transitional zone between the Eastern Himalayan mountains and the upper Gangetic plains. Such a pattern is aligned with the spatial association of the sites. This alignment is also supported by the positive correlation between significant spatial Axes 1 and LCBD values. Therefore, the LCBD analysis captures the broad-scale spatial association of these freshwater habitats and infers factual information about these freshwater habitats. The LCBD attribute is explained by precipitation of driest month, annual mean temperature, and stream order. The facts support these findings that most Eastern Himalayan foothill rivers become feeble during the post-monsoon season due to low discharge (Ayaz et al. 2018; Dhali et al. 2020; Rudra 2018). These torrential freshwater reaches harbor strong temperature gradients as they are replenished by snow-melt waters and originated under dense canopies at higher elevations (Akhter et al. 2019; Barman and Das 2014; Bhatt et al. 2016, 2012; Das 2020; Panja et al. 2020; Rudra 2018). Following the requirements colder, higher flow modulated oxygen-rich habitats of some characteristically adapted fish species; the assemblages might become spatially segregated (Jackson et al. 2001), leading to unique assemblage structures. On the other note, higher order streams are prevalent in the torrential upland rivers, viz. Chel, Neora, Mal, and Murti, while lower order streams are predominant in large-scale rivers, such as River Jaldhaka and Teesta (Akhter et al. 2019; Dhali et al. 2020; Goswami et al. 2019). The lower stream orders are usually negatively associated with species richness (Beecher et al. 1988; Platts 1979). Therefore, stream order has a significant relationship with LCBD, negatively correlated species richness (Legendre and De Cáceres 2013). However, these variables are similar to the variables explaining spatial association except for substrate and land cover attributes. Such difference is presumed to be raised due to the more direct role of substrate and land cover in shaping these freshwater habitats (Akhter et al. 2019; Ali et al. 2010; Biswas and Paul 2020; Dhali et al. 2020; Goswami et al. 2019) in comparison to the differential distribution of fish species (Chakrabarty and Homechaudhuri 2015; Panja et al.2021b, 2020).
It is apparent that these habitat attributes have facilitated the enriched assemblage of rare and characteristically adapted fish species from genus Neolissocheilus, Garra, Tor, Devario, Barilius, Psilorhynchus, Crossochelius, and Glyptothorax of the EH (Barman and Das 2014; Chakrabarty and Homechaudhuri 2015; Goswami et al. 2012a; Panja et al. 2021a, b). The SCBD analysis emphasized these genera, which have driven the higher contribution towards the beta dissimilarity (Fig. 5), resulting in higher LCBD of their habitats (Heino and Grönroos 2017; Legendre and De Cáceres 2013). SCBD values also associate with general species characteristics (for example, species niche & degree of occupancy) and adaptive traits of a species (Heino and Grönroos 2017; Legendre and De Cáceres 2013). Considering the top contributors in SCBD analysis, Neolissocheilus hexastichus and N. hexagonolepis are migratory Mahseer fish species that prefer riffles and pools, characterized by the higher water current and substrate coarseness (Arunachalam 2010; Froese and Pauly 2011; IUCN 2020). They are also experiencing threats of extinction, being categorized as near threatened in IUCN red list (IUCN 2020). Although least concerned (IUCN 2020), Garra lamta and G. annandalei dwell in swift and clear torrential hill streams and exhibit a high degree of adaptation against rocky substratum (Froese and Pauly 2011; Nagar et al. 2012). Unlike the other Mahseer species, Tor tor inhabits rapid streams with rocky substrate exhibiting upstream spawning migration into more oxygen-rich cascades, riffles, deep pools, and reservoirs (Froese and Pauly 2011; Menon 1999). Amblyceps mangois, Devario aequipinnatus, Barilius vagra, and Crossocheilus latius also inhabit hill streams and prefer mid-hill clear waters with coarser bedrock pebbles, gravel, and stones (Froese and Pauly 2011; Menon 1999; Talwar and Jhingran 1991). In contrast, Badis badis dwells in tropical freshwaters with a moderate temperature and lower pH. Therefore, the choice of freshwater habitats characterized by a similar range of variables in these fish species might have led to spatial aggregation and unique or rare species composition in the upper reaches of River Chel, Neora, Murti, Jaldhaka, and upper-west stretches of River Teesta. Such inference is reflected in both the spatial and LCBD models, which are fairly explained by characteristic climate, topography, substrate, and land cover attributes. However, the predictive model with LCBD is overfitted, indicating reduced fidelity and the need for higher resolution studies in his field (Elith et al. 2008; Nieto and Mélin 2017). Previous studies (Milardi et al. 2018, 2019) accorded a higher native species richness in upland sites with significant SCBD than exotic fish species. Besides, exotic fishes usually override the critical environmental drivers (relevant to native) as they uniquely rely upon geography and human-mediated dispersal limitations (Gavioli et al. 2019; Leprieur et al. 2009). However, evidence regarding the impact of exotic fishes on functional diversity, predation, and trophic overlap with the native fish lack from this region; therefore, the present inferences are solely based on native fish species and presumed to be less moderated by exotics considering the spatial scale of the study (Davies et al. 2005; Gavioli et al. 2019; Milardi et al. 2019).
The torrential freshwater reaches of these vast TED ecoregions are experiencing a frequent change in river courses with increasing habitation and altered land use pattern (Chakraborty and Datta 2013; Dhali et al. 2020; Naha et al. 2019). A severe trend of deforestation due to natural and anthropogenic hazards regarding replacement, settlements, mining, pebble displacements, and cultivation is leading to increased siltation and vulnerability of these river beds (Akhter et al. 2019; Chakraborty and Datta 2013; Dhali et al. 2020; Goswami et al. 2012a, b; Naha et al. 2019; Panja et al. 2020; Rudra 2018). Climate change would aggravate such perils more disastrously, leading to severe degradation of this spatially structured freshwater habitat with unique or rare piscine assemblage (Barman and Das 2014; Goswami et al. 2012a, b; Panja et al. 2021b). Due to their spatial association and dispersal limitation, a wide range of fish species will be experiencing immense threats, while their freshwater habitat will be in jeopardy in the future (Barman and Das 2014; Bhatt et al. 2016, 2012; Bhattacharya 2019; Chakraborty and Datta 2013; Goswami et al. 2012a; Naha et al. 2019; Panja et al. 2020; Rudra 2018).
Conclusion
This study reveals the underlying control of freshwater habitats resulting in freshwater fish species sorting as a first of its kind information in the TED ecoregion of the EH. The decomposition of spatial models and local contribution has led to identifying the spatial range of freshwater habitats with unique assemblage and higher eco-restoration values. Such habitats must be prioritized for monitoring and conservation assessments following constant alteration in tectonic, climate, and anthropogenic events. The inferences also raise concern for managing and restoring these characteristic freshwater habitats that share significant association and more nested assemblage of unique and rare fish species. Therefore, using spatial decomposition and additive beta partitions would be beneficial to demarcate the habitats and identify the characteristically adapted species for conservation and prioritization. A future application of such analytics through a multi-taxa approach across the expansive landscape would reveal important information on the freshwater habitats of ecologically sensitive ecoregion of EH.
Data availability
The raw data is not being submitted presently at this moment. It cannot be reproduced before the publication of the manuscript. However, it may be shared in the review/revision stage for better analytical clarity during review/ revision. All the necessary data for peer review has been submitted with the manuscript and supporting information.
References
Akhter S, Eibek KU, Islam S, Islam ARMT, Chu R, Shuanghe S (2019) Predicting spatiotemporal changes of channel morphology in the reach of Teesta River, Bangladesh using GIS and ARIMA modeling. Quatern Int 513:80–94
Ali GA, Roy AG, Legendre P (2010) Spatial relationships between soil moisture patterns and topographic variables at multiple scales in a humid temperate forested catchment. Water Resour Res. https://doi.org/10.1029/2009WR008804
Altermatt F (2013) Diversity in riverine metacommunities: a network perspective. Aquat Ecol 47:365–377
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of ββ diversity. Ecol Lett 9:683–693
Angeler DG (2013) Revealing a conservation challenge through partitioned long-term beta diversity: increasing turnover and decreasing nestedness of boreal lake metacommunities. Divers Distrib 19:772–781
Arunachalam M (2010) Neolissochilus hexagonolepis (errata version published in 2020). The IUCN Red List of Threatened Species 2010: e.T166479A174785418. https://doi.org/10.2305/IUCN.UK.2010-4.RLTS.T166479A174785418.en
Austin M (2007) Species distribution models and ecological theory: a critical assessment and some possible new approaches. Ecol Model 200:1–19
Ayaz S, Biswas M, Dhali MK (2018) Morphotectonic analysis of alluvial fan dynamics: comparative study in spatio-temporal scale of Himalayan foothill, India. Arab J Geosci 11:41
Bandyopadhyay S, Kar NS, Das S, Sen J (2014) River systems and water resources of West Bengal: a review. Spec Publ Geol Soc India 3:63–84
Barbarossa V, Bosmans J, Wanders N, King H, Bierkens MF, Huijbregts MA, Schipper AM (2021) Threats of global warming to the world’s freshwater fishes. Nat Commun 12:1–10
Barman RP, Das A (2014) A study on the threatened and endemic fishes of North Bengal, India with a discussion on the potential impact of climate change on them. Rec zool Surv India Occasional Paper 354:1–56
Beecher HA, Dott ER, Fernau RF (1988) Fish species richness and stream order in Washington State streams. Environ Biol Fishes 22:193–209
Bhatt JP, Manish K, Pandit MK (2012) Elevational gradients in fish diversity in the Himalaya: water discharge is the key driver of distribution patterns. PLoS ONE 7:e46237
Bhatt JP, Manish K, Mehta R, Pandit MK (2016) Assessing potential conservation and restoration areas of freshwater fish fauna in the Indian river basins. Environ Manage 57:1098–1111
Bhattacharya S (2019) Environmental Crisis in the Eastern Himalayan Landscapes in India. Consilience 21:66–85
Bhowmik S, Pal S, Das D, Chakraborty K (2016) Ichthyofaunal diversity at lower part of sub-Himalayan Terai region of West Bengal. In: Proceedings-“Biodiversity”-prospect and threats: present scenario, pp 26–40
Biswas M, Paul A (2020) Application of geomorphic indices to Address the foreland Himalayan tectonics and landform deformation-Matiali-Chalsa-Baradighi recess, West Bengal, India. Quatern Int 585:3–14
Blanchet FG, Legendre P, Borcard D (2008) Modelling directional spatial processes in ecological data. Ecol Model 215:325–336
Bohlin T, Hamrin S, Heggberget TG, Rasmussen G, Saltveit SJ (1989) Electrofishing—theory and practice with special emphasis on salmonids. Hydrobiologia 173:9–43
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153:51–68
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Brooks AJ, Haeusler T, Reinfelds I, Williams S (2005) Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles. Freshwat Biol 50:331–344
Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
Carslaw DC, Taylor PJ (2009) Analysis of air pollution data at a mixed source location using boosted regression trees. Atmos Environ 43:3563–3570
Chakrabarty M, Homechaudhuri S (2015) Resource partitioning as determining factor in structuring fish diversity pattern along ecological gradient of River Teesta in Eastern Himalaya. Int J Fish Aquat Stud 2:74–80
Chakraborty S, Datta K (2013) Causes and consequences of channel changes–a spatio-temporal analysis using remote sensing and GIS—Jaldhaka-Diana River System (Lower Course), Jalpaiguri (Duars), West Bengal, India. J Geogr Nat Disasters 3:1–13
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, USA
Comiti F, Da Canal M, Surian N, Mao L, Picco L, Lenzi M (2011) Channel adjustments and vegetation cover dynamics in a large gravel bed river over the last 200 years. Geomorphology 125:147–159
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182
Das A (2020) Water quality assessment of River Murti for the safety of wildlife, Jalpaiguri, West Bengal, India. Sustain Water Resour Manag 6:20
Davies KF, Chesson P, Harrison S, Inouye BD, Melbourne BA, Rice KJ (2005) Spatial heterogeneity explains the scale dependence of the native–exotic diversity relationship. Ecology 86:1602–1610
de Campos R, da Conceição EdO, Martens K, Higuti J (2019) Extreme drought periods can change spatial effects on periphytic ostracod metacommunities in river-floodplain ecosystems. Hydrobiologia 828:369–381
Dey A, Nur R, Sarkar D, Barat S (2015a) Ichthyofauna diversity of river Kaljani in Cooch Behar District of West Bengal, India. Int J Pure App Biosci 3:247–256
Dey A, Sarkar D, Barat S (2015b) Spawning biology and proper dose of hormone for captive breeding of vulnerable fish, Botia rostrata (Gunther), in Cooch Behar, West Bengal, India. IJAR 1:767–768
Dey A, Sarkar D, Barat S (2015c) Spawning biology, embryonic development and rearing of endangered loach, Botia lohachata (Chaudhuri) in captivity. Int J Curr Res 7:22208–22215
Dey A, Sarkar D, Singh M, Barat S (2015d) DNA Barcoding of four ornamental fishes of genus Botia from Eastern Himalaya. Int J Sci Res 6:608–611
Dey A, Roy Chowdhury B, Nur R, Sarkar D, Kosygin L, Barat S (2019) Channa amari, a new species of snakehead (Teleostei: Channidae) from North Bengal, India. Int J Pharm Biol Sci 9:299–304
Dhali MK, Ayaz S, Sahana M, Guha S (2020) Response of sediment flux, bridge scouring on river bed morphology and geomorphic resilience in middle-lower part of river Chel, Eastern Himalayan foothills zone, India. Ecol Eng 142:105632
Domisch S, Jaehnig SC, Haase P (2011) Climate-change winners and losers: stream macroinvertebrates of a submontane region in Central Europe. Freshwat Biol 56:2009–2020
Domisch S, Kuemmerlen M, Jähnig SC, Haase P (2013) Choice of study area and predictors affect habitat suitability projections, but not the performance of species distribution models of stream biota. Ecol Model 257:1–10
Domisch S, Jähnig SC, Simaika JP, Kuemmerlen M, Stoll S (2015) Application of species distribution models in stream ecosystems: the challenges of spatial and temporal scale, environmental predictors and species occurrence data. Fundam Appl Limnol 186:45–61
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Model 196:483–493
Dray S, Pélissier R, Couteron P, Fortin M-J, Legendre P, Peres-Neto PR, Bellier E, Bivand R, Blanchet FG, De Cáceres M (2012) Community ecology in the age of multivariate multiscale spatial analysis. Ecol Monogr 82:257–275
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Durance I, Ormerod SJ (2007) Climate change effects on upland stream macroinvertebrates over a 25-year period. Global Change Biol 13:942–957
Effenberger M, Sailer G, Townsend CR, Matthaei CD (2006) Local disturbance history and habitat parameters influence the microdistribution of stream invertebrates. Freshwat Biol 51:312–332
Elith J, Leathwick JR, Hastie T (2008) A working guide to boosted regression trees. J Anim Ecol 77:802–813
Erős T, Sály P, Takács P, Specziár A, Bíró P (2012) Temporal variability in the spatial and environmental determinants of functional metacommunity organization–stream fish in a human-modified landscape. Freshwat Biol 57:1914–1928
Fausch KD, Torgersen CE, Baxter CV, Li HW (2002) Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes: a continuous view of the river is needed to understand how processes interacting among scales set the context for stream fishes and their habitat. Bioscience 52:483–498
Ferrier S, Manion G, Elith J, Richardson K (2007) Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers Distrib 13:252–264
Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish 17:581–613
Froese R, Pauly D (2011) FishBase. https://www.fishbase.de/home.htm. Accessed 20 Aug 2020
Gavioli A, Milardi M, Castaldelli G, Fano EA, Soininen J (2019) Diversity patterns of native and exotic fish species suggest homogenization processes, but partly fail to highlight extinction threats. Divers Distrib 25:983–994
Goswami C, Mukhopadhyay D, Poddar BC (2012a) Tectonic control on the drainage system in a piedmont region in tectonically active eastern Himalayas. Front Earth Sci 6:29–38
Goswami UC, Basistha SK, Bora D, Shyamkumar K, Saikia B, Changsan K (2012b) Fish diversity of North East India, inclusive of the Himalayan and Indo Burma biodiversity hotspots zones: a checklist on their taxonomic status, economic importance, geographical distribution, present status and prevailing threats. Int J Biodiv Conserv 4:592–613
Goswami CC, Jana P, Weber JC (2019) Evolution of landscape in a piedmont section of Eastern Himalayan foothills along India-Bhutan border: a tectono-geomorphic perspective. J Mt Sci 16:2828–2843
Griffith DA, Peres-Neto PR (2006) Spatial modeling in ecology: the flexibility of eigenfunction spatial analyses. Ecology 87:2603–2613
Grönroos M, Heino J, Siqueira T, Landeiro VL, Kotanen J, Bini LM (2013) Metacommunity structuring in stream networks: roles of dispersal mode, distance type, and regional environmental context. Ecol Evol 3:4473–4487
Guégan J-F, Lek S, Oberdorff T (1998) Energy availability and habitat heterogeneity predict global riverine fish diversity. Nature 391:382–384
Guha D, Bardhan S, Basir SR, De AK, Sarkar A (2007) Imprints of Himalayan thrust tectonics on the Quaternary piedmont sediments of the Neora-Jaldhaka valley, Darjeeling-Sikkim Sub-Himalayas, India. J Asian Earth Sci 30:464–473
Hanski I (2001) Spatially realistic theory of metapopulation ecology. Naturwissenschaften 88:372–381
Haynes D, Jokela A, Manson S (2018) IPUMS-Terra: integrated big heterogeneous spatiotemporal data analysis system. J Geogr Syst 20:343–361
Heino J (2011) A macroecological perspective of diversity patterns in the freshwater realm. Freshwat Biol 56:1703–1722
Heino J, Grönroos M (2017) Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia 183:151–160
Heino J, Mykrä H, Kotanen J, Muotka T (2007) Ecological filters and variability in stream macroinvertebrate communities: do taxonomic and functional structure follow the same path? Ecography 30:217–230
Heino J, Virkkala R, Toivonen H (2009) Climate change and freshwater biodiversity: detected patterns, future trends and adaptations in northern regions. Biol Rev 84:39–54
Heino J, Melo AS, Bini LM, Altermatt F, Al-Shami SA, Angeler DG, Bonada N, Brand C, Callisto M, Cottenie K (2015a) A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecol Evol 5:1235–1248
Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015b) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwat Biol 60:845–869
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography (MPB-32). Princeton University Press, Princeton
Huntington JL, Hegewisch KC, Daudert B, Morton CG, Abatzoglou JT, McEvoy DJ, Erickson T (2017) Climate engine: Cloud computing and visualization of climate and remote sensing data for advanced natural resource monitoring and process understanding. Bull Am Meteor Soc 98:2397–2410
IUCN (2020) The IUCN Red List of Threatened Species. https://www.iucnredlist.org. Accessed 20 Aug 2020
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170
Jafari A, Khademi H, Finke PA, Van de Wauw J, Ayoubi S (2014) Spatial prediction of soil great groups by boosted regression trees using a limited point dataset in an arid region, southeastern Iran. Geoderma 232:148–163
Jayaram K, Singh K (1977) On a collection of fish from North Bengal. Rec Zool Surv India 72:243–275
Kandel P, Gurung J, Chettri N, Ning W, Sharma E (2016) Biodiversity research trends and gap analysis from a transboundary landscape, Eastern Himalayas. J Asia-Pacific Biodiv 9:1–10
Karger DN, Zimmermann NE (2019) Climatologies at High Resolution for the Earth Land Surface Areas CHELSA V1. 2: Technical Specification. Springer Nature, London
Karmakar M (2011) Ecotourism and its impact on the regional economy-A study of North Bengal (India). Tourismos 6:251–270
Kozel SJ, Hubert WA (1989) Factors influencing the abundance of brook trout (Salvelinus fonfinalis) in forested mountain streams. J Freshwat Ecol 5:113–122
Kuemmerlen M, Schmalz B, Guse B, Cai Q, Fohrer N, Jähnig SC (2014) Integrating catchment properties in small scale species distribution models of stream macroinvertebrates. Ecol Model 277:77–86
Kundu S, Rath S, Laishram K, Pakrashi A, Das U, Tyagi K, Kumar V, Chandra K (2019) DNA barcoding identified selected ornamental fishes in Murti river of East India. Mitochondrial DNA Part B 4:594–598
Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol Lett 16:951–963
Legendre P, Legendre L (2012) Numerical ecology, 3 English. Springer, Heidelberg
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Leonidas V, Eleni K, Evangelia S, Alcibiades EN, Nikolaos ST, Drosos K, Charalampos D, Datry T (2020) Spatial factors control the structure of fish metacommunity in a Mediterranean intermittent river. Ecohydrol Hydrobiol 20:346–356
Leprieur F, Olden JD, Lek S, Brosse S (2009) Contrasting patterns and mechanisms of spatial turnover for native and exotic freshwater fish in Europe. J Biogeogr 36:1899–1912
Leprieur F, Tedesco PA, Hugueny B, Beauchard O, Dürr HH, Brosse S, Oberdorff T (2011) Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecol Lett 14:325–334
Leroy B, Dias MS, Giraud E, Hugueny B, Jézéquel C, Leprieur F, Oberdorff T, Tedesco PA (2019) Global biogeographical regions of freshwater fish species. J Biogeogr 46:2407–2419
López-Delgado EO, Winemiller KO, Villa-Navarro FA (2019) Do metacommunity theories explain spatial variation in fish assemblage structure in a pristine tropical river? Freshwat Biol 64:367–379
Margules CR, Pressey RL (2000) Systematic conservation planning. Nature 405:243
McKnight MW, White PS, McDonald RI, Lamoreux JF, Sechrest W, Ridgely RS, Stuart SN (2007) Putting beta-diversity on the map: broad-scale congruence and coincidence in the extremes. PLoS Biol 5:e272
Melchior LG, Rossa-Feres DdC, da Silva FR (2017) Evaluating multiple spatial scales to understand the distribution of anuran beta diversity in the Brazilian Atlantic Forest. Ecol Evol 7:2403–2413
Menon AGK (1999) Check list-fresh water fishes of India. Zoological Survey of India, Kolkata
Milardi M, Aschonitis V, Gavioli A, Lanzoni M, Fano EA, Castaldelli G (2018) Run to the hills: exotic fish invasions and water quality degradation drive native fish to higher altitudes. Sci Total Environ 624:1325–1335
Milardi M, Gavioli A, Soininen J, Castaldelli G (2019) Exotic species invasions undermine regional functional diversity of freshwater fish. Sci Rep 9:1–10
Nagar K, Sharma M, Tripathi A, Sansi R (2012) Electron microscopic study of adhesive organ of Garra lamta (Ham.). Int Res J Biol Sci 1:43–48
Naha D, Sathyakumar S, Dash S, Chettri A, Rawat G (2019) Assessment and prediction of spatial patterns of human-elephant conflicts in changing land cover scenarios of a human-dominated landscape in North Bengal. PLoS ONE 14:e0210580
Nieto K, Mélin F (2017) Variability of chlorophyll-a concentration in the Gulf of Guinea and its relation to physical oceanographic variables. Prog Oceanogr 151:97–115
Oberdorff T, Lek S, Gu F, Gan JF (1999) Patterns of endemism in riverine fish of the Northern Hemisphere. Ecol Lett 2:75–81
Panja S, Podder A, Homechaudhuri S (2020) Evaluation of Aquatic Ecological Systems through dynamics of Ichthyofaunal diversity in a Himalayan torrential river. Murti Limnologica. https://doi.org/10.1016/j.limno.2020.125779
Panja S, Chakrabarty M, Podder A, Roy A, Biswas M, Homechaudhuri S (2021a) Comparative assessment of piscine beta diversity profile and key determinant environmental factors in two freshwater rivers of variable spatial scale in Dooars, West Bengal, India. Trop Ecol. https://doi.org/10.1007/s42965-021-00171-4
Panja S, Podder A, Homechaudhuri S (2021b) Understanding the impact of future climatic scenarios upon key environmental factors that determine piscine assemblage of a torrential upland river of Eastern Himalayas, India. Curr Sci 120:1471–1481. https://doi.org/10.18520/cs/v120/i9/1471-1481
Paul M, Gupta S, Banerjee S (2009) Fish fauna of major rivers of Darjeeling district, with special reference to their conservation status. Rec Zool Survey India 109:15–23
Pelletier J, Broxton P, Hazenberg P, Zeng X, Troch P, Niu G, Williams Z, Brunke M, Gochis D (2016) Global 1-km gridded thickness of soil, regolith, and sedimentary deposit layers. https://daac.ornl.gov/. Accessed 20 Aug 2020
Perez Rocha M, Bini LM, Domisch S, Tolonen KT, Jyrkänkallio-Mikkola J, Soininen J, Hjort J, Heino J (2018) Local environment and space drive multiple facets of stream macroinvertebrate beta diversity. J Biogeogr 45:2744–2754
Planque B, Loots C, Petitgas P, Lindstrøm U, Vaz S (2011) Understanding what controls the spatial distribution of fish populations using a multi-model approach. Fish Oceanogr 20:1–17
Platts WS (1979) Relationships among stream order, fish populations, and aquatic geomorphology in an Idaho river drainage. Fisheries 4:5–9
Reyjol Y, Hugueny B, Pont D, Bianco PG, Beier U, Caiola N, Casals F, Cowx I, Economou A, Ferreira T (2007) Patterns in species richness and endemism of European freshwater fish. Global Ecol Biogeogr 16:65–75
Ricotta C, Marignani M (2007) Computing β-diversity with Rao’s quadratic entropy: A change of perspective. Divers Distrib 13:237–241
Rudra K (2018) Rivers of the tarai-doors and barind tract. In: Rudra K (ed) Rivers of the Ganga-Brahmaputra-Meghna Delta. Springer, Cham, pp 27–47
Sam K, Chakma N (2019) An exposition into the changing climate of Bengal Duars through the analysis of more than 100 years’ trend and climatic oscillations. J Earth Syst Sci 128:1–12
Sarkar T, Pal J (2018) Exotic fish diversity in the river Teesta, West Bengal, India. IJABFP 9:1–14
Sharma S, Legendre P, De Cáceres M, Boisclair D (2011) The role of environmental and spatial processes in structuring native and non-native fish communities across thousands of lakes. Ecography 34:762–771
Shaw G (1938) The fishes of northern Bengal. J Asiatic Soc Bengal 3:6
Shurin JB, Cottenie K, Hillebrand H (2009) Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159:151–159
Singh RB (2015) Urban development challenges, risks and resilience in asian mega cities. Springer, Japan
Sor R, Legendre P, Lek S (2018) Uniqueness of sampling site contributions to the total variance of macroinvertebrate communities in the Lower Mekong Basin. Ecol Indicators 84:425–432
Talwar P, Jhingran A (1991) Inland fishes of India and adjacent countries, vol 2. Oxford & IBH Publishing Co, New Delhi
Team R (2015) RStudio: integrated development for R. RStudio Inc, Boston
Team RC (2013) R: A language and environment for statistical computing. https://cran.r-project.org/. Accessed 20 Aug 2020
Thompson R, Townsend C (2006) A truce with neutral theory: local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. J Anim Ecol 75:476–484
Thuiller W, Georges D, Engler R (2014) Ensemble platform for species distribution modeling, Package Version, 3-1. http://cran.r-project.org/web/packages/biomod2/biomod2.pdf
Trabucco A, Zomer R (2019) Global aridity index and potential evapotranspiration (ET0) climate database v2. Accessed 20 Aug 2020
Viana DS, Figuerola J, Schwenk K, Manca M, Hobæk A, Mjelde M, Preston C, Gornall R, Croft J, King R (2016) Assembly mechanisms determining high species turnover in aquatic communities over regional and continental scales. Ecography 39:281–288
Villéger S, Grenouillet G, Brosse S (2013) Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Global Ecol Biogeogr 22:671–681
Vilmi A, Karjalainen SM, Heino J (2017) Ecological uniqueness of stream and lake diatom communities shows different macroecological patterns. Divers Distrib 23:1042–1053
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wiersma YF, Urban DL (2005) Beta diversity and nature reserve system design in the Yukon, Canada. Conserv Biol 19:1262–1272
Yamazaki D, Ikeshima D, Sosa J, Bates PD, Allen GH, Pavelsky TM (2019) MERIT Hydro: a high-resolution global hydrography map based on latest topography dataset. Water Resour Res 55:5053–5073
Yao J, Huang J, Ding Y, Xu Y, Xu H, Zang R (2020) Ecological uniqueness of species assemblages and their determinants in forest communities. Divers Distrib 7:454–462
Zhang Y, Ling C (2018) A strategy to apply machine learning to small datasets in materials science. NPJ Comput Mater 4:1–8
Acknowledgements
The authors of this study sincerely acknowledge DST INSPIRE-AORC for the financial support of this research (Sanction No. DST/INSPIRE Fellowship/2016/IF160059). The authors also acknowledge the Department of Zoology, University of Calcutta for providing the facilities and frameworks, and a sincere gratitude to those locals and anglers of Sikkim, North Bengal, Dooars region for their concern, help, and immense supports. We highly appreciate the reviewers for giving valuable inputs, which has improved the content and readability of the manuscript.
Funding
The authors of this manuscript sincerely acknowledge DST INSPIRE-AORC for the financial support of this research (Sanction No. DST/INSPIRE Fellowship/2016/IF160059).
Author information
Authors and Affiliations
Contributions
SP has formulated, generated, identified the samples, analyzed the data, and prepared the manuscript primarily. AP has generated the data, participated in the analysis, and drafting the manuscript secondarily. MC has generated data and identified the samples. SH has supervised the study and edited the manuscript before final submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors are declaring here no conflict of interest.
Ethical approval
This study has been conducted by following the ethical guidelines endorsed by the University of Calcutta, University Grant Commission, and Govt. of India. No vertebrate animals have been sampled, which are already forbidden to be captured from the wild. No surveys and sampling procedures were extended to the protected areas and the water bodies within. The authors are now declaring the fulfillment of all ethical commitments subjected to this research work.
Informed consent
All the authors have given their full consent for the publication of this manuscript in this journal.
Additional information
Handling Editor: Akira Terui.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panja, S., Podder, A., Chakrabarty, M. et al. Spatial pattern of freshwater habitats and their prioritization using additive partitions of beta diversity of inhabitant piscine assemblages in the Terai–Dooars ecoregion of Eastern Himalayas. Limnology 23, 57–72 (2022). https://doi.org/10.1007/s10201-021-00666-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-021-00666-y