Abstract
This study isolated extracellular polysaccharides (EPS) as a powder material from cyanobacterial blooms and the powdered EPS was used to trigger colony formation of dispersed unicellular M. aeruginosa by controlling EPS concentration in culture medium. The effect of Ca2+ ions on the colony formation of M. aeruginosa was also investigated, then the interaction between EPS and Ca2+ ions on colony formation was discussed. The results showed that the addition of the powdered EPS into the medium did not cause morphological changes of M. aeruginosa, suggesting that EPS alone would not induce the colony formation of M. aeruginosa. On the other hand, a high concentration of calcium ions (1000 mg/l) caused colony formation. When EPS and Ca2+ ions in the culture medium were adjusted to 200 and 1000 mg/l, respectively, the colony density, the average cell number per colony and the particle size of M. aeruginosa showed ca. 1.7–2.0 times greater values than those in the Ca2+ added medium. Calcium ion contributed to the aggregation of M. aeruginosa via crosslinked reaction with negatively charged M. aeruginosa cells, and the addition of EPS possessing negatively charged functional groups such as carboxy groups could enhance the reaction, promoting the crosslinked reaction between EPS and Ca2+ ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial blooms caused by eutrophication have recently plagued fresh and brackish water bodies in many countries (Yang and Kong 2013), and threaten aquatic organisms with shading of sunlight, as well as spoiling the landscape (Carpenter et al. 1998; Ou et al. 2006). They also produce toxins such as microcystin and anatoxin, which could cause a health hazard to birds, livestock, and even people (Matsunaga et al. 1999; Azevedo et al. 2002; Katırcıoğlu et al. 2004). For the prevention of such issues and conservation of the water environment, it is important to suppress the appearance of cyanobacterial blooms and clarify the mechanisms by which cyanobacterial blooms dominate in the phytoplankton community.
Microcystis aeruginosa is one of the representative algal bloom-forming cyanobacteria worldwide and often forms colonies aggregating several tens and hundreds of cells in transparent mucilaginous sheath materials (Ordorika et al. 2004; Yang and Kong 2013). The colony formation of M. aeruginosa provides great advantages for these organisms to dominate over other phytoplankton species, such as the promotion of floating speed, nutrient uptake and anti-predator defense (Fogg and Walsby 1971; Bolch and Blackburn 1996; Yang et al. 2008). M. aeruginosa possesses gas vesicles (Kromkamp et al. 1988; Brookes and Ganf 2001); thereby they can enhance buoyancy by developing and expanding the colony size (Stokes’ law). There have been several attempts at on-site purification by sucking up algal blooms which have accumulated at the water surface (Kirke 2001; Hudnell et al. 2010), but only temporary effects have been obtained in most cases. It could be speculated that such negative outcomes could be due to M. aeruginosa with less ability for colony formation or buoyancy developing these abilities in the course of time and appearing as algal blooms again (Nakano et al. 2001).
It is widely recognized that, when the colony-forming M. aeruginosa isolated from cyanobacterial blooms is grown in culture medium under laboratory conditions, the colonial M. aeruginosa cells disaggregate into single cells or twin cells after multiple generations, and M. aeruginosa never forms a colony again (Stainer et al. 1971; Zhang et al. 2007). In addition, M. aeruginosa would not exhibit distinct buoyancy. In general, M. aeruginosa secretes extracellular polysaccharides (EPS) which help to maintain colonial morphology (Plude et al. 1991; Li et al. 2013), but the amount of EPS secreted by the disaggregated cells tends to decrease compared to the colonial cells (Yang et al. 2008). From these results, it might be expected that the disaggregated M. aeruginosa could form a colony if EPS production could be promoted under laboratory conditions, but ways and/or methods for artificially controlling EPS production of M. aeruginosa have not been suggested yet.
As addressed above, the disaggregation of M. aeruginosa colony into single cells or twin cells is an irreversible phenomenon under laboratory conditions, and the colony formation of M. aeruginosa never proceeds in a natural process as far as external factor such as predation by flagellate is added (Yang et al. 2008; Yang and Kong 2012). If colony formation of unicellular M. aeruginosa can be achieved through a culture experiment, the technique could be used with a wild M. aeruginosa strain (potential colony-forming strain) more effectively than the unicellular Microcystis strain. Then, M. aeruginosa cells in lake water could be collected at a high rate, and the development of such technology would contribute to establishing new measures against algal blooms including M. aeruginosa in eutrophic lakes as well as at water purification plants.
The objectives in this study were to isolate EPS as a powder material from cyanobacterial blooms, composed mainly of the colony-forming M. aeruginosa in eutrophic lakes, and then use the powdered EPS to trigger colony formation of dispersed unicellular M. aeruginosa by controlling EPS concentration in the culture medium. As reported by Zhao et al. (2011), colony formation of M. aeruginosa would also be related to bivalent cation ions, such as calcium ions, and a high concentration of Ca2+ (100 mg/l) could contribute to the aggregation of M. aeruginosa due to the bridging effect of Ca2+ ions between M. aeruginosa cells and adherent EPS on the cells (Wang et al. 2011). Based on the studies mentioned above, we also examined the effect of Ca2+ concentration on colony formation of the dispersed unicellular M. aeruginosa. The interaction between EPS and Ca2+ ions on colony formation of M. aeruginosa is discussed. We expected that if it was possible to form colonies from dispersed unicellular M. aeruginosa, the technique could be effectively used with actual algal blooms. This study also has valuable and essential information that helps us to understand M. aeruginosa dominance in the phytoplankton community, and/or appearance of algal blooms in eutrophic lakes.
Materials and methods
Unicellular M. aeruginosa
Unicellular M. aeruginosa strain (UTEX LB 2061) was obtained from the Culture Collection of Algae of the University of Texas, USA. This strain exists as single cells or twin cells, and highly dispersed in the medium. Colony formation of this strain was investigated in this study.
M. aeruginosa was transferred to Wright’s cryptophytes (WC) medium (Guillard and Lorenzen 1972), containing nitrogen and phosphorus concentrations of 14 mg/l and 1.55 mg/l, respectively, and cultured at 25 °C and 27 µmol photons/(m2 s) under static conditions. The solution pH was adjusted to 8.0 by 0.5 M HCl. The cultivation was continued for about 2 weeks, then an aliquot of the medium was transferred to fresh WC medium to subculture the unicellular M. aeruginosa. All media and glassware used for cultivation were autoclaved at 121 °C for 30 min. Each flask was manually stirred several times a day, and randomly replaced to make sure that light irradiance was uniform. All procedures were conducted on a clean bench to minimize bacterial contamination.
Cyanobacterial blooms
The cyanobacterial bloom sample was obtained from Lake Senba, Japan (36°22′N, 140°27′E) in September, 2014. The dense cyanobacterial bloom sample was collected from the top 5 cm of the water column. The sample was immediately transferred to the laboratory, and stored at 4 °C in dark conditions before use. Optical microscopic observation confirmed that the main species in the cyanobacterial bloom was a colony-forming species of the genus Microcystis. EPS was extracted from this sample.
Extraction of EPS from cyanobacterial blooms
Extraction of EPS from cyanobacterial blooms was performed according to the method of Amemiya and Nakayama (1984), and Nishikawa and Kuriyama (1974) with some modifications. EPS on the cell surface of cyanobacterial blooms was solubilized with 2 % EDTA and 0.25 M NaOH and settled for 1 h at 25 °C. The bulk solution obtained in this process was centrifuged at 3000 rpm for 15 min, and the supernatant was collected. Approximately 1.5 times the volume of ice-cold ethanol was added to supernatant, then the mixture was stored overnight at −20 °C to precipitate EPS. After that, EPS was collected after centrifugation at 3000 rpm for 15 min and freeze-dried at −0.1 MPa, −20 °C for 3 h. The dried EPS was ground into a uniform powder and stored in a desiccator before use. As described below, the EPS sample possessed functional groups such as carboxy groups, which contribute to colony formation in M. aeruginosa. Thus, it is inferred that the activity and/or functionality of the EPS survives the chemical extraction processes used in this study.
Colony formation of unicellular M. aeruginosa
The unicellular M. aeruginosa was precultured in 350 ml WC medium (N = 14 mg/l and P = 1.55 mg/l) in a 500-ml Erlenmeyer flask at a light irradiance of 135 µmol photons/(m2 s) with a light and dark cycle of 14:10 h, respectively, at 25 °C. The solution pH was adjusted to 8.0 by 0.5 M HCl. Each flask was manually stirred several times a day, and randomly replaced to make sure there was uniform light irradiance. The preculture was conducted for 7 days, during which M. aeruginosa reached an exponential growth phase. After preculture, the culture medium was centrifuged at 3000 rpm for 15 min and the supernatant was removed. The residual cells were dispersed in deionized water and centrifuged at 3000 rpm for 15 min to wash the cells. The washing process was repeated 3 times to remove ions from the medium.
The washed unicellular M. aeruginosa cells were inoculated into 50 ml deionized water in a 100-ml Erlenmeyer flask to give an initial cell density of 106 cells/ml. The deionized water medium was used in the experiment to eliminate the effect of dissolved ions contained in the WC medium. Calcium chloride (CaCl2) was used to adjust the Ca2+ ion concentration and directly added to flasks, whereas the powdered EPS was first dissolved in deionized water and adjusted to 5000 mg/l, then the solution was filtered using Whatman GF/C filter paper. After that, the filtrate was added to each flask. A flask with no addition of EPS or Ca2+ ions was made and run as a control. Concentrations of EPS and Ca2+ ions in the medium were determined in preliminary experiments. Briefly, EPS concentrations from 0.1 to 500 mg/l, or calcium concentrations from 0 to 750 mg/l did not affect the colony formation of M. aeruginosa. It was also revealed that a calcium concentration of 1000 mg/l led to colony formation, and that the EPS concentration inducing the promotion of colony formation was above 200 mg/l when the calcium concentration was adjusted to 1000 mg/l. From these results, three kinds of experimental medium were prepared to investigate the effects of EPS and calcium ions on colony formation: EPS (200 mg/l) added medium, Ca2+ (1000 mg/l) added medium and EPS (200 mg/l) + Ca2+ (1000 mg/l) added medium. The solution pH was adjusted to 6.0 ± 0.1 by 0.5 M NaOH, because colony formation did not occur under higher pH conditions (data not shown). Each flask was cultured in an incubator at 25 °C, light irradiance of 135 µmol photons/(m2 s), light and dark cycle of 14:10 h, respectively, and cultivated for 24 h under static conditions. After cultivation, each flask was agitated just for 3 s by a touch mixer (PMM-21, Yamato Scientific, Japan) to avoid colonial aggregation by sedimentation in the bottom of the flask. An aliquot of the culture medium was taken to measure cell and colony densities. The aggregation of more than 3 cells was defined as a colony in this study. Each sample was filtered through a Whatman GF/C filter paper and the filtrate was used for the determination of dissolved ions. The experiment was conducted in triplicate (n = 3) and the results were expressed as the mean value with a standard deviation (SD).
Analysis
Colony density, total cell density, and cell density except colonies, were measured by direct counting using an optical microscope with a digital camera system (AM-4023X, Dino-lite, Taiwan) and plankton counting chamber (MPC-200, Matsunami Glass, Japan). The average cell number per colony (ACN) was calculated by the following equation:
where C t is the total cell density, C e is the cell density except colonies, and c is the colony density.
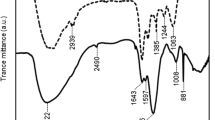
The zeta potential (ζ potential) of M. aeruginosa cells was determined by a zeta potential analyzer (ZC-3000, Microtec, Japan). Cell and colony size distribution were also determined by zeta potential analyzer, although particles larger than 30 µm could not be detected. In the particle size measurement, a particle diameter above 10 µm was regarded as a colony because twin cells of M. aeruginosa were approximately 8–10 µm. In each experiment 300 particles were measured and the frequency distribution and frequency percentage were calculated. The solution pH was measured by pH meter (D-51, Horiba, Japan). Total sugar content was determined by the phenol–sulfuric acid method with glucose as the standard (Dubois et al. 1956). Nitrogen and phosphorus concentrations were analyzed by ion chromatography (ICS-1100, Dionex, USA) and molybdenum blue method (Murphy and Riley 1962) using a UV–Vis spectrophotometer (UV-2550, Shimadzu, Japan), respectively. Fourier transformed infrared (FT-IR) spectroscopy analysis was performed to characterize major functional groups on the EPS using an IR Affinity-1 instrument (Shimadzu, Japan). The powdered EPS was mixed with potassium bromide (KBr) in the ratio of 1:100 and a pellet with a diameter of 3 mm was prepared for the FT-IR analysis by pressing the mixture. The FT-IR spectra were recorded between 4000 and 400 cm−1 with a 4-cm−1 resolution in transmittance mode.
All experimental data were represented by the mean ± standard deviation (SD). All data were subjected to one-way analysis of variance (ANOVA) followed by Tukey tests to examine significant differences between individual mean values. All statistical tests were carried out using Microsoft Excel Statistics 2008 (Social Survey Research Information Co., Ltd., Japan). Differences were considered statistically significant at p < 0.05.
Results
Optical microscopic observation
After 24 h of cultivation, M. aeruginosa was present as single or twin cells and highly dispersed in the culture medium in the control (Fig. 1a). The same result was obtained in the medium with EPS addition, which exhibited only single and twin cells, but the medium also contained sheath-like substances, which did not exist in the control (Fig. 1b). In contrast, several small colonies as well as single and twin cells were found in the Ca2+ added medium (Fig. 1c). This trend was similarly observed in the EPS + Ca2+ added medium, but this medium also allowed M. aeruginosa to form much larger colonies than in the Ca2+ added medium (Fig. 1d).
Colony and cell densities of M. aeruginosa
Although there were no colonies in the control and the EPS added medium, M. aeruginosa formed colonies in the Ca2+ added medium and the EPS + Ca2+ added medium (Table 1). The colony densities of the Ca2+ added medium and the EPS + Ca2+ added medium were 3.44 × 103 ± 2.88 × 102 and 6.05 × 103 ± 2.72 × 102 colonies/ml, respectively, and the density for the latter medium was significantly higher than that for the former medium (p < 0.05).
The total cell density of the control was decreased from 1.00 × 106 to 9.00 × 105 ± 6.00 × 104 cells/ml after the cultivation time of 24 h (Table 1), whereas a significant increase in the total cell density compared with the control was observed for the EPS added medium (p < 0.05) (1.12 × 106 ± 1.37 × 105 cells/ml). There was no remarkable difference in the total cell density between the control and the Ca2+ added medium (p > 0.05) (9.80 × 105 ± 1.73 × 104 cells/ml), although ca. 1.4 times higher cell density of 1.39 × 106 ± 2.65 × 104 cells/ml than that for the control (p < 0.05) was obtained for the EPS + Ca2+ medium. The cell density except colonies showed the opposite trend against colony density, indicating that the density for the Ca2+ added medium (5.67 × 105 ± 1.94 × 104 cells/ml) and the EPS + Ca2+ added medium (1.25 × 105 ± 3.49 × 103 cells/ml) were much lower than the control, and a significant difference in the density was also observed for the two media (p < 0.05) (Table 1).
The mean cell number per colony, calculated from values of the colony density, the total cell density and the cell density except colonies, for the Ca2+ added medium was 1.20 × 102 ± 1.05 × 101 cells/colony (Table 1), while a much higher value of cell numbers (2.09 × 102 ± 1.36 × 101 cells/colony) was obtained for the EPS + Ca2+ added medium (p < 0.05).
Cell and colony size distribution
The cell and/or colony size distribution in the control medium showed that ca. 98 ± 58 cells (33 ± 19 %) and 202 ± 58 cells (67 ± 19 %) of M. aeruginosa were in the range in 0–5 µm and 5–10 µm, respectively, and cells larger than 10 µm were not found in the medium (Table 2). In the EPS added medium, most of the cells had a particle diameter of less than 10 μm. On the other hand, the Ca2+ added medium included larger particles possessing 10- to 15- and 15- to 20-µm diameters with the proportions of 21 ± 3 and 5 ± 4 %, respectively. In the EPS + Ca2+ medium, particles with all ranges of diameters were present in the medium, and diameters ranging from 20 to 30 µm appeared only in this medium, at a proportion of ca. 4 ± 2 % of the total cell density. For both Ca2+ added and EPS + Ca2+ added me dium, particles in the range 0–5 µm were apparently fewer than in the EPS added medium (p < 0.05).
Change in zeta potential
As shown in Fig. 2, the ζ potential for the control medium was −32.6 ± 0.8 mV, which was similar to the value of −31.0 ± 1.8 mV obtained for the EPS added medium. In the Ca2+ added medium, the ζ potential indicated a significantly lower value (−22.9 ± 4.1 mV) than that for the control (p < 0.05). A decrease in ζ potential was also observed for the EPS + Ca2+ added medium: this value of −17.0 ± 7.9 mV was the lowest in all media used in this study and was remarkably lower than that in the control medium (p < 0.05).
Total sugar content and nutrient concentration in each medium
The total sugar contents for the control and the Ca2+ added media were not detected either before (t = 0 h) or after (t = 24 h) the experiment (Fig. 3). When the powdered EPS was added into the medium, the total sugar contents for both EPS added and EPS + Ca2+ added media showed ca. 1.57 ± 0.71 and 1.63 ± 0.41 mg/l at t = 0 h, and 1.80 ± 0.70 and 1.83 ± 0.42 mg/l at t = 24 h, respectively. Although total sugar contents were slightly increased during the experiment, a significant change in total sugar content before and after the experiment was not observed for either media (p > 0.05).
During the culture experiment of M. aeruginosa, a nitrogen concentration was not detected in any of the media, whereas the opposite result with phosphorus was observed when the powdered EPS was added to the medium (Fig. 4). Phosphorus concentrations for the EPS added and the EPS + Ca2+ added media ranged from 3.01 ± 0.05 to 3.25 ± 0.02 mg-P/l during the experiment. Although phosphorus concentration for the EPS added medium was remarkably decreased after 24 h (p < 0.05), little difference in the concentration before and after the experiment was observed for the EPS + Ca2+ added medium (p > 0.05).
Changes in phosphorus concentration before and after culture experiment in each medium. Values are represented as mean ± standard deviation (n = 3). Asterisk (*) indicates a significant difference compared with control (p < 0.05). Double dagger (‡) represents a significant difference between media at t = 0 and 24 h
FT-IR analysis of the EPS extracted from cyanobacterial blooms
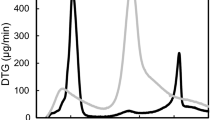
The results of the FT-IR spectra of the EPS extracted by cyanobacterial blooms are presented in Fig. 5. The results showed that the EPS had a broad band at 3429 cm−1, implying the presence of hydroxyl groups (Xu et al. 2013). The band around 1647 cm−1 could be attributed to the C=O adsorption of uronic acids (Chakraborty et al. 2015). The presence of C=O and C–O bonds from carboxylate might be exhibited by the adsorption at 1456 cm−1 (Bramhachari et al. 2007). A peak at 1020 cm−1 was attributed to the C–O in alcohols, suggesting the hydroxyl groups in the EPS (Abd-El-Haleem et al. 2008). The linkage between monosaccharides was observed as the band at 820 cm−1 (Ismail 2013).
Discussion
It is widely recognized that the colony-forming wild strain of M. aeruginosa loses its colony formation ability and becomes single cells and/or twin cells by cultivation under laboratory conditions. Although a reasonable explanation has not been suggested, several studies imply that colony formation could be induced by the presence of zooplankton (Burkert et al. 2001; Yang et al. 2008), infochemicals secreted by Daphnia (Lampert et al. 1994), extracellular microcystins produced by M. aeruginosa (Sedmak and Eleršek 2005), and fluid motion (Li et al. 2013). Among these parameters, one of the influential factors causing colony formation in Microcystis is extracellular polysaccharides (EPS). In this study, we investigated colony formation in M. aeruginosa by using powdered EPS to control the EPS concentration in the culture medium. Since EPS strongly interacts with cationic ions (Iijima et al. 2002; Wang et al. 2011), colony formation may be affected by the interaction of these factors. The effects of EPS and calcium concentration as well as nutrients (nitrogen and phosphorus) and total EPS sugar content on colony formation are discussed in detail below.
The addition of powdered EPS to the culture medium did not cause morphological changes in M. aeruginosa, suggesting that EPS alone would not induce colony formation in M. aeruginosa. The phosphorus concentration in the media with EPS addition was measured to be about 3.0 mg-P/l, although nitrogen was not detected in any media. As shown in Table 1, the total cell density in the EPS added medium increased after the 24-h cultivation compared with the control (p < 0.05), while a significant decrease in the phosphorus concentration during the experiment was also observed (p < 0.05) (Fig. 4), indicating that the increase in M. aeruginosa cells correlated with the presence of phosphorus. The same trend of increase in total cell density was obtained in the EPS + Ca2+ added medium, although the phosphorus concentration in the medium was not largely reduced after 24 h (p > 0.05). Shi et al. (2013) examined the effect of calcium ions on the growth of M. aeruginosa and revealed that a calcium concentration of 60 mg/l increased the growth of M. aeruginosa. In this study, the total cell density in the EPS + Ca2+ medium remarkably increased compared with the control (p < 0.05), but such trends were not obtained in the Ca2+ added medium. These results imply that the higher cell density in the EPS + Ca2+ medium than in the same medium without EPS addition could not be attributed to calcium ions or phosphorus. Amemiya and Nakayama (1984) investigated metal content in the EPS obtained from algal blooms (mainly the genus Microcystis) and indicated that EPS contained several metals (Na,K,Ca,Mg,Fe,Mn,Zn,Ni,Cu), and the highest content of the metals was Fe (ca. 25 % in total metal contents). As suggested by Nagai et al. (2007), iron limitation significantly affects the growth of M. aeruginosa. From these results, it is suggested that metals, especially Fe, contained in EPS could affect the total cell density of M. aeruginosa in the media with EPS addition in this study. Despite the higher phosphorus concentration, which was ca. 3 times greater than the phosphorus level (1.2 mg/l) in an extremely hypereutrophic lake (Xu et al. 2003), M. aeruginosa never formed colonies in the EPS added medium, implying that phosphorus was not involved in the development of M. aeruginosa colony formation.
The total sugar content in the media with EPS addition was approximately 1.5–2.0 mg/l larger than those without EPS addition at the beginning of the experiment (p < 0.05), and the content tended to increase after 24 h (Fig. 3), although a significant difference before and after the experiment was not observed (p > 0.05). Several studies examined the effects of nutrient concentration (nitrogen and phosphorus) on the amount of EPS production for M. aeruginosa, and indicated that M. aeruginosa enhanced EPS synthesis when the concentration in the medium was limited (Wang et al. 2010a, b). It has also been suggested that EPS secretion by strains of the cyanobacterium Nostoc was promoted under nitrogen limitation (Otero and Vincenzini 2003). In this study, nitrogen was not present in all media, and phosphorus was not detected in the media without EPS addition, but the EPS was not observed. The results in this study clearly indicate that the EPS production of M. aeruginosa was not related to nutriment limitation. It is also implied that the increase in phosphorus concentration or other substances such as trace metal ions accompanying the addition of the powered EPS may contribute to EPS production, although a significant difference between the media at the beginning and the end of the experiment was not observed.
The values of ζ potential in the Ca2+ added media were ca. 10–20 mV lower than those without calcium addition. Qu et al. (2015) examined the effect of calcium ions on the ζ potential of colonies and cells of M. aeruginosa in an EPS solution originating from M. aeruginosa and clarified that the ζ potential value was gradually lowered by increasing the amount of calcium ions added. In the present study, the addition of calcium ions to each medium also led to a decrease in the ζ potential value of M. aeruginosa cells/colonies, implying that the reduction of negative surface charge of EPS may promote the aggregation of cells and colonies.
Despite EPS (200 mg/l) alone having no effect on colony formation in M. aeruginosa, calcium ions (1000 mg/l) did contribute to the morphological change. Wang et al. (2011) investigated the effects of calcium and magnesium concentrations on the colony formation of Microcystis. Their results clearly indicated that colony formation was concerned only with calcium ions, and that the concentration inducing colonies was required to be above 100 mg/l. In our preliminary experiments, calcium concentrations from 0 mg/l to 750 mg/l at pH 6.0 in aqueous solution did not cause colony formation in M. aeruginosa (data not shown). This disagreement was due to the differences in M. aeruginosa strain, the presence of nutrients/other trace ions as well as culture conditions such as light and temperature. When calcium ions and EPS concentrations were simultaneously adjusted to 1000 and 200 mg/l, respectively, colony formation was promoted more than in the Ca2+ added medium. Previous studies clarified that EPS contained large portions of protein and polysaccharide, and the latter consisted of amino acids and several monosaccharides possessing surface functional groups such as carboxy groups (Amemiya and Nakayama 1984, Plude et al. 1991). According to the study of Amemiya and Nakayama (1984), EPS was composed of 12.2–14.8 % uronic acids. Plude et al. (1991) revealed the presence of pectin as uronic polymers in the EPS of M. flos-aque. In the present study, carboxy groups on the EPS surface were detected by FT-IR analysis (Fig. 5), which implies the presence of uronic acids including pectin. Although we were concerned that the chemical processes for the extraction of EPS from cyanobacterial blooms in this study may alter the properties of EPS, the addition of the powdered EPS as well as Ca2+ ions induced colony formation in M. aeruginosa. This indicates that the powdered EPS may be different from that in situ, but the activity or functionality of the EPS was fundamentally kept after the chemical processes. Iijima et al. (2002) indicated that carboxy groups on uronic acid were crosslinked by polyvalent metal cations such as calcium ions. Wang et al. (2011) also suggested the possibility that divalent cations crosslinked with negatively charged functional groups within bacterial EPS could contribute to cell–cell aggregation. In the present study, a slight amount of EPS surrounding M. aeruginosa cells allowed them to aggregate with each other via crosslinked reactions induced by the addition of Ca2+ ions, causing colony formation in the Ca2+ added medium. Similarly, M. aeruginosa colonies in the Ca2+ + EPS added medium were formed in the same way as the Ca2+ added medium, but due to the presence of a large amount of EPS added, the crosslink reaction between EPS and Ca2+ ions was promoted. This would be the mechanism by which the large colonies of M. aeruginosa formed in the Ca2+ + EPS added medium.
References
Abd-El-Haleem DA, Al-Thani RF, Al-Mokemy T, Al-Marii S, Hassan F (2008) Isolation and characterization of extracellular bioflocculants produced by bacteria isolated from Qatari ecosystems. Pol J Microbiol 57:231–239
Amemiya Y, Nakayama O (1984) The chemical composition and metal adsorption capacity of the sheath materials isolated from Microcystis cyanobacteria. Jap J Limnol 45:187–193
Azevedo SMOF, Carmichael WW, Jochimsen EM, Rinehart KL, Lau S, Shaw GR, Eaglesham GK (2002) Human intoxication by microcystins during renal dialysis treatment in Caruaru—Brazil. Toxicology 181–182:441–446
Bolch CJS, Blackburn SI (1996) Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kutz. J Appl Phycol 8:5–13
Bramhachari PV, Kishor PK, Ramadevi R, Kumar R, Rao BR, Dubey SK (2007) Isolation and characterization of mucous exopolysaccharide (EPS) produced by Vibrio furnissii strain VB0S3. J Microbiol Biotechnol 17:44–51
Brookes JD, Ganf GG (2001) Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light. J Plankton Res 23:1399–1411
Burkert U, Hyenstrand P, Drakare S, Blomqvist P (2001) Effects of the mixotrophic flagellate Ochromonas sp. on colony formation in Microcystis aeruginosa. Aquat Ecol 35:11–17
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Chakraborty T, Sen AK, Pal R (2015) Stress induced enhancement in exo-polysaccharide production in Spirulina subsalsa and its chemical characterization. J Algal Biomass Utln 6:24–38
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fogg GE, Walsby AE (1971) Buoyancy regulation and the growth of planktonic blue-green algae. Mitt Int Verein Limnol 19:182–188
Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14
Hudnell HK, Jones C, Labisi B, Lucero V, Hill DR, Eilers J (2010) Freshwater harmful algal bloom (FHAB) suppression with solar powered circulation (SPC). Harmful Algae 9:208–217
Iijima M, Hatakeyama T, Nakamura K, Hatakeyama H (2002) Effect of annealing on calcium pectin gel formation by thermomechanical analysis. J Therm Anal Calorim 70:815–824
Ismail SA (2013) Extracellular polysaccharides produced by the newly discovered source Scopularis spp. Egypt Pharmaceut J 12:36–39
Katırcıoğlu H, Akın BS, Atıcı T (2004) Microalgal toxin(s): characteristics and importance. Afr J Biotechnol 3:667–674
Kirke BK (2001) Pumping downwards to prevent algal blooms. In: Poster presentation, IWA 2nd World Water Congress, Berlin, Germany, pp 1–6
Kromkamp J, Botterweg J, Mur LR (1988) Buoyancy regulation in Microcystis aeruginosa grown at different temperatures. FEMS Microbiol Ecol 53:231–237
Lampert W, Rothhaupt KO, Vonelert E (1994) Chemical induction of colony formation in a green alga (Scenedesmus acutus) by grazers (Daphnia). Limnol Oceanogr 39:1543–1550
Li L, Zhu W, Wang T, Luo Y, Chen F, Tan X (2013) Effect of fluid motion on colony formation in Microcystis aeruginosa. Water Sci Eng 6:106–116
Matsunaga H, Harada KI, Senma M, Ito Y, Yasuda N, Ushida S, Kimura Y (1999) Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: sudden appearance of toxic cyanobacteria. Nat Toxins 7:81–84
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–34
Nagai T, Imai A, Matsushige K, Fukushima T (2007) Growth characteristics and growth modeling of Microcystis aeruginosa and Planktothrix agardhii under iron limitation. Limnology 8:261–270
Nakano K, Lee TJ, Matsumura M (2001) In situ algal bloom control by the integration of ultrasonic radiation and jet circulation to flushing. Environ Sci Tech 35:4941–4946
Nishikawa S, Kuriyama M (1974) Nucleic acid as a component of mucilage in activated sludge. J Ferment Technol 52:335–338
Ordorika LV, Fastner J, Kurmayer R, Hisbergues M, Dittmann E, Komarek J (2004) Distribution of microcystin-producing and non-microcystin-producing Microcystis sp. in European freshwater bodies: detection of microcystins and microcystin genes in individual colonies. Syst Appl Microbiol 27:592–602
Otero A, Vincenzini M (2003) Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J Biotechnol 102:143–152
Ou MM, Wang Y, Zhou BX, Cai WM (2006) Effects of iron and phosphorus on Microcystis physiological reactions. Biochem Environ Sci 19:399–404
Plude JL, Parker DL, Schommer OJ (1991) Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flos-aquae C3-40. Appl Environ Micro Biol 57:1696–1700
Qu F, Du X, Liu B, He J, Ren N, Li G, Liang H (2015) Control of ultrafiltration membrane fouling caused by Microcystis cells with permanganate preoxidation: significance of in situ formed manganese dioxide. Chem Eng J 279:56–65
Sedmak B, Eleršek T (2005) Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb Ecol 50:298–305
Shi J, Wu Z, Song L (2013) Physiological and molecular responses to calcium supplementation in Microcystis aeruginosa (Cyanobacteria). N Z J Mar Freshw Res 47:51–61
Stainer RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Wang C, Kong HN, He SB, Zheng XY, Li CJ (2010a) The inverse correlation between growth rate and cell carbohydrate content of Microcystis aeruginosa. J Appl Phycol 22:105–107
Wang W, Liu Y, Yang Z (2010b) Combined effects of nitrogen content in media and Ochromonas sp. grazing on colony formation of cultured Microcystis aeruginosa. J Limnol 69:193–198
Wang YW, Zhao J, Li JH, Li SS, Zhang LH, Wu M (2011) Effects of calcium levels on colonial aggregation and buoyancy of Microcystis aeruginosa. Curr Microbiol 62:679–683
Xu FL, Tao S, Dawson RW, Li BG (2003) A GIS-based method of lake eutrophication assessment. Ecol Model 144:231–244
Xu H, Yu G, Jiang H (2013) Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 93:75–81
Yang Z, Kong F (2012) Formation of large colonies: a defense mechanism of Microcystis aeruginosa under continuous grazing pressure by flagellate Ochromonas sp. J Limnol 71:61–66
Yang Z, Kong F (2013) Abiotic factors in colony formation: effects of nutrition and light on extracellular polysaccharide production and cell aggregates of Microcystis aeruginosa. Chin J Oceanol Limnol 31:796–802
Yang Z, Kong F, Shi X, Zhang M, Xing P, Cao H (2008) Changes in the morphology and polysaccharide content of Microcystis aeruginosa (Cyanobacteria) during flagellate grazing. J Phycol 44:716–720
Zhang M, Kong F, Tan X, Yang Z, Cao H, Xing P (2007) Biochemical, morphological, and genetic variations in Microcystis aeruginosa due to colony disaggregation. World J Microbiol Biotechnol 23:663–670
Zhao L, Lu L, Li M, Xu Z, Zhu W (2011) Effects of Ca and Mg levels on colony formation and EPS content of cultured M. aeruginosa. Procedia Environ Sci 10:1452–1458
Acknowledgments
This work was supported by JSPS KAKENHI grant number 15K18142. The authors thank the anonymous reviewers for their fruitful comments and advice on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Qinglong Wu.
Rights and permissions
About this article
Cite this article
Sato, M., Amano, Y., Machida, M. et al. Colony formation of highly dispersed Microcystis aeruginosa by controlling extracellular polysaccharides and calcium ion concentrations in aquatic solution. Limnology 18, 111–119 (2017). https://doi.org/10.1007/s10201-016-0494-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-016-0494-7