Abstract
The cell carbohydrate content of cyanobacteria can alter buoyancy, and the ability to regulate the buoyancy is one of the most important mechanisms of cyanobacterial blooms. The net accumulation of carbohydrate in cell is affected by photosynthesis, respiration, synthesis of proteins, and other metabolisms, which are connected to the growth. The aim of this work is to seek the relationship between growth rate and intracellular carbohydrate content. The cell carbohydrate content in Microcystis aeruginosa cultures with different growth characteristics was investigated, and the relationship between growth rate and accumulated carbohydrate in cyanobacterial cells was analyzed. The result showed that the specific growth rate was inversely proportional to cell carbohydrate content. The growth rate was relatively high when the cell carbohydrate content was low. It can be indicated that high growth occurs when cells are buoyant, which favors blooms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major factors that affect the growth of cyanobacteria are light, temperature, and the chemical composition of the medium. The rate of photosynthetic carbon fixation is essentially governed by the light intensity to which the cell is exposed (Reynolds et al. 1987), and photosynthetically fixed carbon is accumulated in cyanobacterial cells in the form of carbohydrate (density 1,500 kg m−3) which adds to the excess density of the cells (Reynolds et al. 1987; Sunda and Huntsman 2004).
The ability to regulate the buoyancy is one of the most important mechanisms of cyanobacterial blooms (Reynolds et al. 1987; Wallace and Hamilton 2000). Carbohydrate changes rapidly in response to light and can alter buoyancy (Brookes and Ganf 2001). Subsequent studies showed that the accumulation of carbohydrate “ballast” provides the main mechanism of density regulation in Microcystis (Ibelings et al. 1991; Kromkamp and Mur 1984; Thomas and Walsby 1985).
The net accumulation of carbohydrate in cell interacts with nutrient availability and other ambient factors, the key control being the relationship between carbon fixation and utilization (Reynolds et al. 1987; Spilling and Markager 2008). As photosynthesis proceeds, carbohydrate is produced which, with a density of 1,500 kg m−3, will increase the overall density of the cyanobacteria. However, the stored carbohydrate is used to support respiration, synthesis of proteins and other biopolymers, and to fuel growth (Brookes and Ganf 2001; Cuhel et al. 1984; Howard 1994). Oliver (1994) found that when nutrients are abundant, buoyancy is promoted; however, buoyancy decreases when the major nutrients, nitrogen and phosphorus, are limiting. It was also reported that respiration was not only governing growth but was also itself a function of the growth rate (Langdon 1993; Laws and Chalup 1990; Spilling and Markager 2008).
The aim of this work is to seek the relationship between growth rate and intracellular carbohydrate content. In this study, the cell carbohydrate contents in Microcystis aeruginosa cultures with different growth characteristics were investigated, and the relationship between growth rate and accumulated carbohydrate in cyanobacterial cells was analyzed.
Materials and methods
The bloom-forming M. aeruginosa (FACHB 905), provided by Freshwater Algae Culture Collection, Institute of Hydrobiology, Chinese Academy of Sciences, was used for the experiments. The culture was grown in BG-11 medium at a constant temperature of 25 ± 2°C and an irradiance of 25 μmol photons m−2 s−1 on a 14:10 light:dark cycle, without aeration but with daily stirring. All chemicals used were of analytical grade.
Prior to inoculation, the unialgal M. aeruginosa culture was starved in phosphorus-free BG-11 medium to zero-growth. Then, the starved cells were inoculated at an initial cell density of ~2 × 105 cells mL−1 into capped sterilized Erlenmeyer flasks containing BG-11 medium of different phosphorus concentrations. The cultures were illuminated with cool fluorescent white lamps (CDMTP150W942, Philips). In order to obtain cultures with different growth characteristics, experiments were carried out at the irradiances of 10, 20, 40, 70, 100, and 150 μmol photons m−2 s−1 on a 14:10 light:dark cycle at the temperature of 25 ± 2°C. At each irradiance, four gradients of total phosphors (TP) concentrations of 0.01, 0.1, 1, and 10 mg P L−1 were employed. The cultures were stirred four times everyday.
Growth of M. aeruginosa was determined by taking 0.1 mL culture subsamples from the different treatments on the third day, and every day thereafter for growth evaluation. The cells were counted by using a blood-cell counting chamber.
The specific growth rate (μ; d−1) during the logarithmic growth phase was calculated by Eq. (1):
where N 0 is the initial cell density (cells mL−1), and N t is the cell density (cells mL−1) after incubation for t days.
For the determination of cell chemical content, cyanobacterial cells were filtered through 0.45-μm pore size cellulose acetate membrane filters and then washed with BG-11 medium. The carbohydrate was spectrophotometrically determined at 620 nm by Anthrone Reagent (Herbert et al. 1971).
Results and discussion
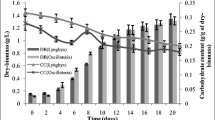
The cell carbohydrate content variation at different irradiances and ambient P concentrations is shown in Fig. 1. It is obvious that at a constant irradiance the cell carbohydrate content of M. aeruginosa is inversely related to ambient P concentration. The cell carbohydrate content goes up when the ambient P concentration decreases. This indicates that the available P concentration affects the utilization and accumulation of carbohydrate in cyanobacterial cells, which agrees with the observations of Oliver (1994). The effect of irradiance on cell carbohydrate content is not as obvious as ambient P concentration. Although it is reported that photosynthesis (synthesis of carbohydrate) has regular relationship to irradiance (Behrenfeld and Falkowski 1997; Jassby and Platt 1976; Markager 1994), the processes of respiration and synthesis of protein and other polymers are affected by various factors. Therefore, the relationship between accumulation of carbohydrate and irradiance is complex. The relationship between accumulation of carbohydrate and one of the environmental parameters may be indefinite, however, the cell carbohydrate content reflects the combined effects of ambient factors on cyanobacterial cell.
The correlation between the specific growth rate (μ) and the cell carbohydrate content (Q C) is shown in Fig. 2. The Pearson’s correlation coefficient (ρ = −0.503, P = 0.012) shows that the specific growth rate is inversely proportional to cell carbohydrate content. The growth rate is high when the cell carbohydrate content is low. Laws and Chalup (1990) inferred from a microalgal growth model that the correlation between growth rate and Chl a:C is positive under nutrient-limited conditions, which indicated a negative relationship between growth rate and cellular carbon.
The key control of the net accumulation of carbohydrate is the relationship between carbon fixation and utilization (Reynolds et al. 1987). The rate of photosynthetic carbon fixation is essentially governed by the light intensity and the ambient CO2 concentration. On the other hand, the fixed carbohydrate is used to support respiration, synthesis of proteins and other biopolymers, and to fuel growth (Brookes and Ganf 2001; Cuhel et al. 1984; Howard 1994).
There is usually a positive linear relationship between growth rate and respiration at high temperature (~20°C; Langdon 1993; Verity 1982). According to this observation, cells with higher growth rate are accompanied by higher respiration rate, utilizing relatively more carbohydrate. A higher division rate should require more synthesis of proteins, which is also consuming carbohydrate. Both of the ways above should contribute to the inverse correlation between growth rate and cell carbohydrate content.
Subsequent studies showed that the accumulation of carbohydrate “ballast” provides the main mechanism of buoyancy regulation in Microcystis (Ibelings et al. 1991; Kromkamp and Mur 1984; Thomas and Walsby 1985). Thus, at high growth rate, the low cell carbohydrate content may lead to high buoyancy (Wallace and Hamilton 2000). This indicates that high growth occurs when cells are buoyant, which favors blooms.
Guven and Howard (2006) reviewed several key models of cyanobacteria, most of which were process based and relied on environmental parameters. Compared with the existing models, this work tried to link growth rate to intracellular carbohydrate content—one of the characteristics of the cells themselves. The net accumulation of carbohydrate is the sum of synthesis by photosynthesis and consumption by respiration and other metabolic processes, reflecting the combined effect of light, nutrients, temperature and other environmental factors on the cyanobacterial cell. We suggest it will be useful in studying the effect of environmental factors on cyanobacterial cell and predicting the coming variation trend of the physiology of cyanobacteria.
References
Behrenfeld MJ, Falkowski PG (1997) A consumer’s guide to phytoplankton primary productivity models. Limnol Oceanogr 42:1479–1491
Brookes JD, Ganf GG (2001) Variations in the buoyancy response of Microcystis aeruginosa to nitrogen, phosphorus and light. J Plankton Res 23(12):1399–1411. doi:10.1093/plankt/23.12.1399
Cuhel RL, Ortner PB, Lean DRS (1984) Night synthesis of protein by algae. Limnol Oceanogr 29:731–744
Guven B, Howard A (2006) A review and classification of the existing models of cyanobacteria. Prog Phys Geogr 30:1–24. doi:10.1191/0309133306pp464ra
Herbert D, Phipps PJ, Strange RE (1971) Chemical Analysis of Microbial Cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology. Vol. 5, Part 2. Academic, London, pp 209–344
Howard A (1994) Problem cyanobacterial blooms: explanation and simulation modelling. T I Br Geogr 19(2):213–224. doi:10.2307/622755
Ibelings BW, Mur LR, Walsby AE (1991) Diurnal changes in buoyancy and vertical distribution in populations of Microcystis in two shallow lakes. J Plankton Res 13:419–436. doi:10.1093/plankt/13.2.419
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Kromkamp JC, Mur LR (1984) Buoyant density changes in the cyanobacterium Microcystis aeruginosa due to changes in the cellular carbohydrate content. FEMS Microbiol Lett 25:105–109. doi:10.1111/j.1574-6968.1984.tb01384.x
Langdon C (1993) The significance of respiration in production measurements based on oxygen. ICES Mar Sci Symp 197:69–78
Laws EA, Chalup MS (1990) A microalgal growth model. Limnol Oceanogr 35:597–608
Markager S (1994) Open-water measurement of areal photosynthesis in a dense phytoplankton community. Arch Hydrobiol 129:405–424
Oliver RL (1994) Floating and sinking in gas-vacuolate cyanobacteria. J Phycol 30:161–173. doi:10.1111/j.0022-3646.1994.00161.x
Reynolds CS, Oliver RL, Walsby AE (1987) Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. N Z J Mar Freshw Res 21:379–390
Spilling K, Markager S (2008) Ecophysiological growth characteristics and modeling of the onset of the spring bloom in the Baltic Sea. J Mar Syst 73:323–337. doi:10.1016/j.jmarsys.2006.10.012
Sunda WG, Huntsman SA (2004) Relationships among photoperiod, carbon fixation, growth, chlorophyll a, and cellular iron and zinc in a coastal diatom. Limnol Oceanogr 49:1742–1753
Thomas RH, Walsby AE (1985) Buoyancy regulation in a strain of Microcystis. J Gen Microbiol 131:799–809
Verity PG (1982) Effects of temperature, irradiance, and daylength on the marine diatom Leptocylindrus danicus Cleve. IV. Growth. J Exp Mar Biol Ecol 60:209–222. doi:10.1016/0022-0981(82)90160-5
Wallace BB, Hamilton DP (2000) Simulation of water-bloom formation in the cyanobacterium Microcystis aeruginosa. J Plant Res 22:1127–1138. doi:10.1093/plankt/22.6.1127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Kong, Hn., He, Sb. et al. The inverse correlation between growth rate and cell carbohydrate content of Microcystis aeruginosa . J Appl Phycol 22, 105–107 (2010). https://doi.org/10.1007/s10811-009-9421-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9421-1