Abstract
Objective
To examine the clinical and economic impact of vedolizumab compared with infliximab, adalimumab, and golimumab in the treatment of moderately to severely active ulcerative colitis (UC) in the United Kingdom (UK).
Methods
A decision analytic model in Microsoft Excel was used to compare vedolizumab with other biologic treatments (infliximab, adalimumab, and golimumab) for the treatment of biologic-naïve patients with UC in the UK. Efficacy data were obtained from a network meta-analysis using placebo as the common comparator. Other inputs (e.g., unit costs, adverse-event disutilities, probability of surgery, mortality) were obtained from published literature. Costs were presented in 2012/2013 British pounds. Outcomes included quality-adjusted life-years (QALYs). Costs and outcomes were discounted by 3.5% per year. Incremental cost-effectiveness ratios were presented for vedolizumab compared with other biologics. Univariate and multivariate probabilistic sensitivity analyses were conducted to assess model robustness to parameter uncertainty.
Results
The model predicted that anti-tumour necrosis factor-naïve patients on vedolizumab would accrue more QALY than patients on other biologics. The incremental results suggest that vedolizumab is a cost-effective treatment compared with adalimumab (incremental cost-effectiveness ratio of £22,735/QALY) and dominant compared with infliximab and golimumab. Sensitivity analyses suggest that results are most sensitive to treatment response and transition probabilities. However, vedolizumab is cost-effective irrespective of variation in any of the input parameters.
Conclusions
Our model predicted that treatment with vedolizumab improves QALY, increases time in remission and response, and is a cost-effective treatment option compared with all other biologics for biologic-naïve patients with moderately to severely active UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disorder that affects approximately 240 per 100,000 people in the United Kingdom (UK) [1, 2]. Patients with UC have significant reductions in quality of life [3] and incur substantial economic burden [4].
Current management strategies are used to treat acute, active disease and to maintain response and prevent relapses among patients in remission [5]. Conventional therapies (e.g., aminosalicylates, steroids, immunosuppressants) are the first-line treatment option for mild-to-moderate UC and can be administered in various forms, depending on the disease severity [6]. For patients in whom conventional therapy is no longer effective, biologic therapy may be an effective alternative. Three tumor necrosis factor-alpha inhibitors (anti-TNFs) have been approved for the treatment of UC in the US and Europe: infliximab, adalimumab, and golimumab. Anti-TNFs have been shown to be more effective than conventional therapy in various clinical trials [7,8,9,10]. In February 2015, following an assessment of three anti-TNFs (infliximab, adalimumab, and golimumab), NICE approved anti-TNFs as “possible treatments for adults with moderate-to-severe UC if conventional therapy has not worked or is not suitable.” If biologic treatment fails, the only alternative left is surgery, which is extremely costly and has significant risks [11, 12].
Vedolizumab, a novel biologic therapy intended for use in the treatment of UC, is a selective antagonist that binds exclusively to the α4β7 integrin heterodimer engineered to target lymphocyte trafficking localized in the gut. It is the first gut-selective treatment for inflammatory bowel disease. As a result, its anti-inflammatory profile differs from the systemic anti-TNF treatments in that it has not demonstrated systemic immunosuppressive effects. Vedolizumab has shown positive results in a recent, multinational, blinded, placebo-controlled, randomized clinical trial (the GEMINI I trial) [13, 14]. In England and Wales, vedolizumab was the first and only biologic getting a positive reimbursement appraisal in a Single Technology Assessment (STA) process of the National Institute for Health and Care Excellence (NICE). Since then, the anti-TNFalpha have been also approved by the NICE in moderate-to-severe UC patients following a multiple technology assessment, as a drug class.
The objective of this study was to develop an economic model to estimate the cost-effectiveness of vedolizumab compared with the other biologic therapies for patients with moderately to severely active UC in the UK in order to submit it in the NICE STA of vedolizumab.
Methods
Model structure
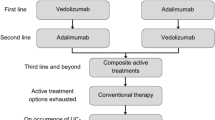
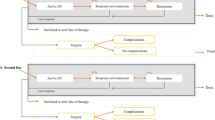
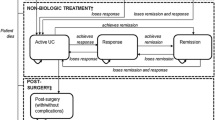
We developed a hybrid decision tree/Markov model (Fig. 1) to estimate the costs and outcomes associated with biologic therapy. The model design is similar to that presented by Tsai and colleagues [15]. In this design, the induction phase is modeled via a decision tree (Fig. 1a), and the maintenance phase is modeled via a Markov structure (Fig. 1b). The health states in the model were remission (Mayo score 0–2), mild disease (Mayo score 3–5), moderate-to-severe disease (Mayo score 6–12), surgery, postsurgical remission, and postsurgical complications.
Patients enter the model with moderate-to-severe disease. These patients are assigned to a biologic and monitored for induction response. Patients who respond during the induction period and do not discontinue due to adverse events remain on treatment in the maintenance phase (Markov model). These patients enter the Markov model in remission, mild disease, or moderate-to-severe disease, depending upon their level of response in induction (Fig. 1b). These patients remain on treatment and transition among these three health states, or they may eventually lose response. Patients who do not respond during the induction phase, who lose response in the maintenance phase, or who discontinue due to adverse events switch to induction with conventional therapy.
Patients initiating induction with conventional therapy follow a similar treatment pattern as patients on biologic treatment. However, patients who do not respond to conventional therapy either switch to another conventional therapy combination and remain in moderate-to-severe disease or transition to surgery.
Once patients transition to surgery, they discontinue pharmacotherapy and enter the surgery portion of the Markov model (Fig. 1b). These patients may remain complication-free (postsurgery remission), experience complications (postsurgery complications), or require a subsequent surgery (surgery). Patients may transition to death from any health state in the model.
The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services. The time horizon of the model was set to a patient’s lifetime. Costs and benefits were discounted annually at 3.5% annually, as specified by NICE [2].
Patient population
Patients in this analysis are individuals with a diagnosis of moderately to severely active UC (i.e., Mayo score of 6 or greater) who have had an inadequate response with, lost response to, or are intolerant to a conventional therapy such that they have switched to a treatment with a biologic (anti-TNF). The patient population (1) was anti-TNF–naïve, (2) had an average age of 40.36 years, (3) was 58% male, and (4) had an average weight of 76.29 kg. These data were estimated based on the pooled patient populations of trials included in a mixed-treatment comparison [16].
Treatments
In this analysis, patients may be treated with one of the following:
-
Vedolizumab: 300 mg intravenous infusions at weeks 0, 2, and 6, and every 8 weeks thereafter.
-
Infliximab: 5 mg/kg intravenous infusions at weeks 0, 2, and 6, and every 8 weeks thereafter.
-
Adalimumab: 160 mg subcutaneous injection at week 0, 80 mg injection at week 2, and 40 mg injection every 2 weeks thereafter.
-
Golimumab: 100 mg subcutaneous injection at week 0, 50 mg injection at week 2 and 6, and 50 mg injection every 4 weeks thereafter.
Patients could also receive conventional therapy, as consistent with the clinical trials. Patients responding to biologics were treated for up to 3 years. After 3 years, these patients were assumed to be treated with conventional therapy alone.
Model inputs
Clinical efficacy
Treatment efficacy is presented as the probability of response and remission during the induction phase and the probability of remaining in remission or mild disease at the end of the maintenance phase (at 1 year). Response was defined as a decrease in Mayo score of 3 or more (30% or more) from baseline. A Mayo score of 2 or less and no individual subscore of more than 1 was used to identify patients in remission.
Due to the lack of head-to-head clinical trial data, we used the results of a network meta-analysis based on each drug’s pivotal placebo-controlled clinical trials [7,8,9,10, 13, 17]. The estimates for response and remission for each treatment from the meta-analysis are presented in Table 1. Because it was possible to achieve response without reaching the mild or remission health states, we assumed that 10.1% of responders on any treatment remained in the moderate-to-severe health state based on data observed in the pooled patient population of the GEMINI I trial.
To estimate disease progression following induction, we derived transition probabilities to minimize the sum of squared errors of the modeled percentage of patients in remission and mild disease at 1 year relative to that estimated based on the observed trial data. We assumed that the transition probabilities beyond the first year on treatment were the same as those estimated for the first year on treatment. These transition probabilities are presented in Appendix 1.
For patients who experienced surgery, we derived the postsurgery transition probabilities from a targeted review of the available published literature. These transition probabilities are presented in Appendix 1.
Clinical safety
Patients on biologic therapy may discontinue due to lack of efficacy at the end of the induction phase or at the end of the maintenance phase. Additionally, these patients may discontinue due to adverse events. The data for discontinuations in the induction and maintenance phases were obtained from the clinical trial data [7,8,9,10, 13] (Table 2). Patients who switched to conventional therapy were assumed to continue receiving conventional therapy until the end of the model’s time horizon or until the patient transitions to surgery.
Mortality
Patients with UC have not shown to have an increased mortality rate over patients without UC, and as such UC-specific mortality has not been included in previously published models [15, 18]. As a result, mortality was assumed similar to the general population, where mortality increases over time as patients age [19].
Utility
Utility weights range from 0.0 to 1.0, where a utility value of 1.0 represents perfect health and a value of 0.0 represents death. These utility values are used to estimate quality-adjusted life-years (QALYs) by multiplying the number of life-years within a particular health state by that health state’s utility weight.
Utility weights are presented by health state and were obtained from an analysis of the GEMINI I trial. Health state utilities are further decremented due to adverse events. Utility values used in the model are presented in Table 2.
Costs
Costs considered in this analysis include acquisition and administration of drugs, direct health care costs associated with each health state, and the cost of managing adverse events (Table 2). The daily cost of conventional therapy was estimated as a mix of treatments that compose conventional therapy based on interviews with clinician experts [20]. For patients still on biologic therapy, the cost of concomitant conventional therapy was assumed half that of conventional therapy alone.
Nonsurgical health state costs were estimated by applying NHS reference costs to resource use reported by health state by Tsai et al. [15] (Table 2). All costs were converted to 8-week costs and reported in 2012/2013 British pounds. Costs for surgery were estimated from Buchanan et al. [12]. Adverse-event costs were estimated as weighted averages using the relevant health care resource group codes in the NHS reference cost schedule [21] and the assumption that all patients are hospitalized with these adverse events (Table 2).
Model calculations
The main outcome measure was the incremental cost per QALY gained. For each comparison examined, we derived the incremental cost per QALY by calculating the difference between the cost of vedolizumab and the cost of the other biologics, which was then divided by the difference in QALYs between each treatment.
Sensitivity analyses
To test the robustness of the model assumptions and parameters, we examined the effects of changing parameters in one-way sensitivity analyses. Analyzed parameters included percentage of patients responding to therapy or in remission during induction and maintenance periods, percentage of patients at end of induction who remain in moderate-to-severe disease, percentage of patients experiencing surgery, transition probabilities, adverse-event incidence, percentage of patients discontinuing due to adverse events, relative risk of mortality, health state utilities and adverse-event disutilities, and health state and adverse-event costs. Effects of varying individual parameters were examined using plausible ranges of values from the literature, 95% confidence intervals, or by varying estimates by ±20%. Sensitivity analysis results for each input were ranked from most sensitive to least sensitive and plotted on a tornado diagram.
In addition to the one-way sensitivity analysis, a scenario analysis was also conducted in which we varied the maximum duration of time on biologic therapy from 1 to 5 years. This assumption was tested to account for uncertainty of the long-term effectiveness of the drug and the impact of applying a discontinuation rule in the model.
Finally, we also performed a probabilistic sensitivity analysis examining the impact of varying all parameters at the same time according to prespecified distributions. A second-order Monte Carlo simulation was conducted in which variability was examined over 3000 iterations.
Model results
Base-case analyses
Table 3 presents the base-case results. Patients on vedolizumab were expected to gain more QALYs than patients treated with any other therapy. Patients on vedolizumab spent more time in remission, had a slight improvement in overall survival, and experienced fewer surgeries. At a willingness-to-pay threshold of £30,000 per QALY, treatment with vedolizumab appears to be a cost-effective and potentially dominant strategy (i.e., more effective and less costly) compared with the other biologics. Over a lifetime horizon, vedolizumab was dominant compared with each anti-TNF.
One-way sensitivity analysis
Figure 2a–c illustrates one-way sensitivity analyses on the incremental cost per QALY gained for vedolizumab compared with infliximab, adalimumab, and golimumab, respectively, to variations in model parameters. Results were most sensitive to changes in the percentage of patients responding during induction period and the probability of transitioning to remission and health state costs. However, vedolizumab remained cost-effective in all individual parameter variations, as the incremental cost-effectiveness ratio did not exceed £30,000 per QALY in any case. In the scenario analysis for discontinuation, vedolizumab remained more effective than all other treatments with a 5-year discontinuation rule. Vedolizumab was dominant compared with infliximab, highly cost-effective (ICER = £10,618) compared with golimumab. Vedolizumab was not cost-effective compared with adalimumab (ICER = £59,466). With a 1-year discontinuation rule, vedolizumab was dominant regardless of comparator.
a One-way sensitivity analysis results: vedolizumab vs. infliximab. AE adverse event, CI confidence interval, CT conventional therapy, IFX infliximab, QALY quality-adjusted life-year, VDZ vedolizumab. b One-way sensitivity analysis results: vedolizumab vs. adalimumab. ADA adalimumab, CI confidence interval, CT conventional therapy, QALY quality-adjusted life-year, VDZ vedolizumab. c One-way sensitivity analysis results: vedolizumab vs. golimumab. AE adverse event, CI confidence interval, CT conventional therapy, GOL golimumab, QALY quality-adjusted life-year, VDZ vedolizumab
Multiway probabilistic sensitivity analysis
Assuming a £30,000 per QALY threshold, vedolizumab was dominant (more effective and less costly) compared to infliximab in 97.6 of cases, cost-effective compared to golimumab in 92.2 of cases (with dominance occurring in 61.7 of cases), and cost-effective in 59.6% of cases in comparison with adalimumab.
Vedolizumab was the more effective treatment in 99.6, 95.0, and 98.0% of cases compared with infliximab, adalimumab, and golimumab, respectively. The results of these probabilistic sensitivity analyses are presented in Fig. 3.
Discussion
In this study, we compared vedolizumab with anti-TNF treatments infliximab, adalimumab, and golimumab in anti-TNF–naïve patients with moderately to severely active UC to determine the cost-effectiveness of vedolizumab in this population. Compared with the other biologics, vedolizumab was the more effective treatment. When considering costs and a lifetime time horizon, vedolizumab was dominant (more effective and less costly) when compared with all other biologics.
Our findings are consistent with previously published analyses [15]. Tsai and colleagues [15] found that anti-TNF–naïve patients on infliximab incurred 4.591 QALYs over a 10-year period. When our model is set to 10 years, uses the utility weights presented in by Tsai and colleagues [15], and sets mortality to be independent of UC health state, we estimate 4.650 QALYs for patients on infliximab. These similarities provide validity to the estimates from our analysis.
Two recent publications of economic models have considered vedolizumab in UC. Essat and colleagues [32] published a CEA model and a critical analysis of the Takeda initial CEA model of vedolizumab in moderate-to-severe UC patients, submitted during the NICE process. The co-authors were part of the Evidence Review Group selected by the National Institute of Clinical and health Excellence (NICE) who did the single technology assessment of vedolizumab in UC. Our model integrated the feedback from the NICE appraisal. It is worth noting that NICE accepted the final Takeda’s model and required modifications in Essat’s model as well, which drastically changed the ICER in favor of vedolizumab. Based on Essat’s modified model, the Takeda’s model presented here, the clinical evidence submitted, as well as the level of unmet need, NICE concluded that vedolizumab was a cost-effective treatment option compared to CT, to other biologics, and to surgery.
Yokomizo and colleagues [33] developed a cost consequence model for vedolizumab in UC based on mucosal healing (MH). Our model is instead a cost-utility model, integrating the Mayo score clinical efficacy with quality of life. Our model also differs in that it takes a long-term perspective whereas the Yokomizo model was a 1-year analysis. Yokomizo’s did not use an indirect treatment comparison to account for differences in study design or population characteristics between trials. Thus the efficacy measures used in their model might not be accurate. Finally, this publication used US cost, and therefore any firm conclusions cannot be done for the British healthcare setting, given that costs of care in the USA are much higher than in the UK. As such, our model is not comparable to the Yokomizo model.
This analysis was for the anti-TNF–naïve population. It is worth noting that many patients fail anti-TNF therapy and, as such, additional lines of pharmacotherapy are administered. As a result, it would be valuable to compare the economic value of biologic therapies in an anti-TNF–failure population as well. However, since limited data exist for the anti-TNF treatments (golimumab and infliximab trials included only anti-TNF–naïve patients), such a comparison is not feasible at this time. It will be important to study these drugs in different populations once such data are available to better understand each drug’s economic value in all relevant populations.
The model does not consider infliximab biosimilars for two reasons: at the time of the model design, biosimilars were not yet available in the UK; and because biosimilars are not required to conduct randomized clinical trials, the assumption of equivalent efficacy may be tenuous. However, the model does allow for a simple scenario consideration. If we were to assume equivalent efficacy and a price 70% of branded infliximab price for the biosimilars, vedolizumab would remain dominant. For vedolizumab to not be cost-effective, the biosimilar price would have to be less than 25% of that of branded infliximab.
This analysis is subject to a few limitations with regard to the published clinical trial data. Most notably, the lack of head-to-head clinical data makes comparisons of efficacy among the biologic treatments difficult. Thus, a commonly used and accepted approach to indirect comparisons was used to estimate efficacy relative to a common comparator. With regard to the available clinical data, we have relied on published clinical data from multinational clinical trials. Additionally, due to the lack of data on anti-TNF–failure patients, we did not consider treatment switching in the model. It is unclear what effect this would have on results, as it is unclear what the efficacy of subsequent biologic treatment would be (and whether or not such treatment would be cost-effective compared with conventional therapy). It will be important to consider collecting real-world data in patients who have failed one (or more) biologic treatments to gain a better understanding of the economic implications of biologic pathways.
Finally, we note the limitation of the difference in the trial designs for the placebo-controlled trials for adalimumab from those for vedolizumab. Most notably, the adalimumab maintenance trials only included those patients who achieved remission during the induction phase, whereas the GEMINI I trial allowed all responders to continue. As such, one would expect patients in the adalimumab maintenance trials to better maintain their response than patients in the GEMINI I trial because only those who showed the highest level of response continued in the adalimumab maintenance trials. We conservatively make no attempt to address this design issue in the model and assume that the maintenance transition probabilities for adalimumab (which are based on induction remitters only) apply to both induction remitters and nonremitting responders. As such, the results vs. adalimumab likely underestimate vedolizumab’s cost-effectiveness. In addition, we attribute the change in cost-effectiveness results in the discontinuation stopping rule scenario analysis to overestimating adalimumab’s maintenance efficacy.
While the one-way sensitivity analyses uniformly found vedolizumab to be cost-effective, this was not quite the case for the probabilistic sensitivity analyses. For example, vedolizumab was cost-effective compared with adalimumab in 59.6% of simulations. When vedolizumab was not cost-effective, it was typically due to drug costs, but vedolizumab was still more effective. In over 98% of cases compared with infliximab and golimumab, and in more than 95% of cases compared with adalimumab, vedolizumab was more effective. So while we cannot say so with 100% certainty, the results strongly suggest that vedolizumab is the cost-effective strategy.
Conclusions
In summary, vedolizumab appears to be more efficacious than infliximab, adalimumab, and golimumab in an anti-TNF–naïve UC population. Based on the results of this analysis, vedolizumab is likely to be cost-effective and very possibly dominant when compared with these biologic treatments. As such, vedolizumab provides a valuable treatment alternative for biologic-naive UK patients with moderately to severely active UC who have previously failed or are intolerant to conventional therapy.
References
Baumgart, D.C., Sandborn, W.J.: Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369(9573), 1641–1657 (2007)
National Institute for Health and Care Excellence (NICE). Clinical guidelines CG166: ulcerative colitis. https://www.nice.org.uk/guidance/cg166/chapter/introduction (2010). Accessed 29 August 2013
Rubin, D.T., Dubinsky, M.C., Panaccione, R., Siegel, C.A., Binion, D.G., Kane, S.V., et al.: The impact of ulcerative colitis on patients’ lives compared to other chronic diseases: a patient survey. Dig. Dis. Sci. 55(4), 1044–1052 (2010)
Cohen, R.D., Yu, A.P., Wu, E.Q., Xie, J., Mulani, P.M., Chao, J.: Systematic review: the costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 31(7), 693–707 (2010)
Kornbluth, A., Sachar, D.B.: Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. J. Gastroenterol. 105(3), 501–523 (2010)
National Institutes of Health. Ulcerative colitis. http://www.nlm.nih.gov/medlineplus/ulcerativecolitis.html (2010). Accessed 4 Sept 2012
Rutgeerts, P., Sandborn, W.J., Feagan, B.G., Reinisch, W., Olson, A., Johanns, J., et al.: Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353(23), 2462–2476 (2005)
Reinisch, W., Sandborn, W.J., Hommes, D.W., D’Haens, G., Hanauer, S., Schreiber, S., et al.: Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 60(6), 780–787 (2011)
Sandborn, W.J., Feagan, B.G., Marano, C., Zhang, H., Strauss, R., Johanns, J., et al.: Subcutaneous golimumab induces clinical response and remission in patients with moderate to severe ulcerative colitis. Gastroenterology 146(1), 85–95 (2014)
Sandborn, W.J., Van, A.G., Reinisch, W., Colombel, J.F., D’Haens, G., Wolf, D.C., et al.: Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142(2), 257–265 (2012)
Loftus, E.V., Delgado, D.J., Friedman, H.S., Sandborn, W.J.: Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the US. Am. J. Gastroenterol. 103, 1737–1745 (2008)
Buchanan, J., Wordsworth, S., Ahmad, T., Perrin, A., Vermeire, S., Sans, M., et al.: Managing the long term care of inflammatory bowel disease patients: the cost to European health care providers. J. Crohns Colitis. 5(4), 301–316 (2011)
Feagan, B.G., Rutgeerts, P., Sands, B.E., Hanauer, S., Colombel, J.F., Sandborn, W.J., et al.: Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369(8), 699–710 (2013)
Sandborn, W., Sands, B., Rutgeerts, P., Sankoh, S., Rosario, M., Milch, C., et al.: Sustained therapeutic benefit of vedolizumab throughout one year in ulcerative colitis in GEMINI I, a randomized, placebo-controlled, double-blind, multicenter trial. J. Crohns Colitis. 7, S138–S139 (2013)
Tsai, H.H., Punekar, Y.S., Morris, J., Fortun, P.: A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment. Pharmacol. Ther. 28(10), 1230–1239 (2008)
Ling, C., Colosia, A., Vickers, A., Ainsworth, C.: Systematic literature review and network meta-analysis in ulcerative colitis. Revised final report. (Takeda data on file, 31 Jan 2014)
Suzuki, Y., Motoya, S., Hanai, H., Matsumoto, T., Hibi, T., Robinson, A.M., et al.: Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J. Gastroenterol. 49(2), 283–294 (2014)
Office for National Statistics: Death registrations summary tables, England and Wales 2010. http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-227638 (2011). Accessed 12 Feb 2012
Jess, T., Gamborg, M., Munkholm, P., Sorensen, T.I.A.: Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am. J. Gastroenterol. 102(3), 609–617 (2007)
Royal College of Physicians: National clinical audit of biological therapies: UK inflammatory bowel disease (IBD) audit. http://www.rcplondon.ac.uk/sites/default/files/national_clinical_audit_report_of_biological_therapies_-_adult_report._29_august_2013.pdf (2013). Accessed 29 Aug 2013
Department of Health: NHS reference costs 2011–12. https://www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (2013). Accessed April 2016
Brown, R.E., Hutton, J., Burrell, A.: Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 19(11), 1091–1102 (2001)
Porco, T.C., Lewis, B., Marseille, E., Grinsdale, J., Flood, J., Royce, S.E.: Cost-effectiveness of tuberculosis evaluation and treatment of newly-arrived immigrants. BMC Public Health 6, 157 (2006)
Beusterien, K.M., Davies, J., Leach, M., Meiklejohn, D., Grinspan, J., O’Toole, A., et al.: Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual. Life Outcomes 8, 50 (2010)
Beusterien, K.M., Szabo, S.M., Kotapati, S., Mukherjee, J., Hoos, A., Hersey, P., et al.: Societal preference values for advanced melanoma health states in the UK and Australia. Br. J. Cancer 101(3), 387–389 (2009)
Final Clinical Study Report C13006: A phase 3, randomized, placebo-controlled, blinded, multicenter study of the induction and maintenance of clinical response and remission by vedolizumab (MLN0002) in patients with moderate to severe ulcerative colitis (September 2012)
Punekar, Y., Hawkins, N.: Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. Eur. J. Health Econ. 11(1), 67–76 (2010)
British National Formulary. No. 66. 2013. http://www.bnf.org/bnf/go?bnf/current/December (2013). Accessed April 2016
Curtis, L.: Unit costs of health and social care 2012. http://www.pssru.ac.uk/archive/pdf/uc/uc2012/full-with-covers.pdf. Accessed 16 Dec 2013
Frolkis, A.D., Dykeman, J., Negrón, M.E., deBruyn, J., Jette, N., Fiest, K.M., et al.: Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 145(5), 996–1006 (2013)
Xie, F., Blackhouse, G., Assai, N., Gaebel, K., Rovertson, D., Goeree, R.: Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost. Eff. Resour. Alloc. 7, 20 (2009)
Essat, M., Tappenden, P., Ren, S., Bessey, A., Archer, R., Wong, R., Lobo, A., Hoque, S.: Vedolizumab for the treatment of adults with moderate-to-severe active ulcerative colitis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 34(3), 245–257 (2016)
Yokomizo, L., Limketkai, B., Park, K.T.: Cost-effectiveness of adalimumab, infliximab, or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastro. 3(1), e000093 (2016)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The research presented in this manuscript was funded by Takeda Pharmaceuticals, AG. Ms. Bergman, Ms. Chevrou-Severac, Mr. Selby, and Dr. Smyth are employees of Takeda. Dr. Kerrigan is an employee of PHMR Associates, a contract research organization that received funding from Takeda to conduct the analyses. Mr. Wilson is an employee of RTI Health Solutions, a contract research organization that received funding from Takeda to conduct the analyses.
Source of funding
The research presented in this manuscript was funded by Takeda Pharmaceuticals, AG.
Appendix 1: Transition probabilities
Appendix 1: Transition probabilities
To ensure that the Mayo-based health state transition matrices for the maintenance phase generate patient flows that closely depict outcomes based on response and remission data, we performed a calibration of the Markov transition probabilities. Specifically, we used the efficacy data for the induction and maintenance phases for each treatment from a network meta-analysis of placebo-controlled trials to derive transition probabilities. We then calibrated these transition probabilities such that the percentages of patients in remission and mild disease at 54 weeks most closely mirrored the expected percentages based on the efficacy data from the meta-analyses. The transition probabilities estimated for each treatment are presented in Table 4.
Transition probabilities for the surgery and postsurgical health states were derived from the published literature, as given in Table 5.
Rights and permissions
About this article
Cite this article
Wilson, M.R., Bergman, A., Chevrou-Severac, H. et al. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ 19, 229–240 (2018). https://doi.org/10.1007/s10198-017-0879-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-017-0879-5