Abstract
Background
Ulcerative colitis (UC) is the most common form of inflammatory bowel disease in the UK. Medical management aims to induce and maintain remission and to avoid complications and the necessity for surgical intervention. Colectomy removes the source of inflammation but is associated with morbidity and mortality. Newer anti-tumour necrosis factor (TNF)-α therapies may improve medical outcomes, albeit at an increased cost.
Objective
Our objective was to assess the incremental cost effectiveness of infliximab, adalimumab and golimumab versus conventional therapy and surgery from a National Health Service (NHS) and Personal Social Services (PSS) perspective over a lifetime horizon.
Methods
A Markov model was developed with health states defined according to whether the patient is alive or dead, current treatments received, history of colectomy and level of disease control. Transition probabilities were derived from network meta-analyses (NMAs) of trials of anti-TNF-α agents in the moderate-to-severe UC population. Health utilities, colectomy rates, surgical complications and resource use estimates were derived from literature. Unit costs were drawn from standard costing sources and literature and were valued at year 2013/2014 values.
Results
For patients in whom surgery is an option, colectomy is expected to dominate all medical treatment options. For patients in whom colectomy is not an option, infliximab and golimumab are expected to be ruled out due to dominance, whilst the incremental cost-effectiveness ratio (ICER) for adalimumab versus conventional treatment is expected to be approximately £50,278 per quality-adjusted life-year (QALY) gained.
Conclusions
Based on the NMAs, the ICERs for anti-TNF-α therapy versus conventional treatment or surgery are expected to be at best, in excess of £50,000 per QALY gained. The cost effectiveness of withdrawing biologic therapy upon remission and re-treating relapse is unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colectomy is expected to be more effective and less expensive than medical treatments for ulcerative colitis. |
For patients in whom colectomy is not an acceptable option, the incremental cost-effectiveness ratios (ICERs) for anti-tumour necrosis factor (TNF)-α therapy versus conventional treatment are expected to be in excess of £50,000 per quality-adjusted life-year gained. |
Using anti-TNF-α therapy to induce remission, withdrawing therapy and re-treating upon relapse may provide a more economically efficient approach compared with continuous treatment in those achieving an induction response. However, the comparative effectiveness and cost effectiveness of this approach is unclear. |
1 Introduction
Ulcerative colitis (UC) is the most common form of idiopathic inflammatory bowel disease (IBD) in the UK. An estimated 132,600 people in England and Wales have been diagnosed with UC [1]. Peak incidence is between 15 and 25 years of age, with a second peak between 55 and 65 years. The disease may be limited to the rectum (proctitis), may affect the left colon or may be extensive (pan-colitis) [2]. UC runs a relapsing and remitting course, and its symptoms can substantially impact upon patients’ health-related quality of life (HRQoL). Symptoms vary in severity and may include bloody diarrhoea, with urgency of defecation, abdominal pain and fatigue. More severe exacerbations of UC are associated with systemic effects, for example, fever, tachycardia and anaemia, and require admission to hospital for urgent monitoring and treatment [2]. The burden of UC for the National Health Service (NHS) is substantial, particularly in patients with poor disease control. Compared with quiescent IBD, disease relapse is associated with an estimated two- to threefold increase in costs for non-hospitalised cases and a 20-fold increase in costs for hospitalised cases [3]. Approximately 80 % patients with UC have mild-to-moderate disease, and approximately 20 % have severe disease. The population considered within this paper relates to patients with moderately-to-severely active UC (excluding those with acute severe UC) for whom conventional therapy has failed, who would normally be managed in an outpatient setting, and who do not require hospitalisation or the consideration of urgent surgical intervention.
Optimum treatment for patients with moderate-to-severe UC after the failure of conventional therapy is not universally agreed. Treatment typically follows an escalation approach whereby additional drugs are added to induce and maintain response. Treatment may be influenced by the severity of symptoms, the extent and location of inflammation, frequency of relapse and individual patient choice [1]. Conventional medical therapy includes aminosalicylates (5-ASAs), corticosteroids, thiopurine immunosuppression (azathioprine or mercaptopurine) and calcineurin inhibitors [4], although these do not offer cure of the disease. Given the relapsing-remitting nature of UC, treatment aims are to induce and maintain symptomatic remission, to induce and maintain repair of the colonic mucosa (“mucosal healing”), to improve HRQoL and to avoid complications, including hospitalisation and surgical intervention. Surgical treatment comprises colectomy followed by either a permanent ileostomy or an ileoanal anastomosis (“pouch”). This may be required for patients with severe UC refractory to medical therapy, or may be considered electively to restore HRQoL in patients with frequent flares or continuous symptoms associated with significant debility. However, surgery may result in complications such as infertility or pouchitis (which itself causes increased stool frequency and urgency of defecation), wound infections, wound dehiscence and small bowel obstruction as well as psychological issues such as poor body image [1]. Colectomy is also associated with a risk of peri-operative mortality [5]. Consequently, colectomy may not be considered to be an acceptable treatment option for some patients, hence a trade-off exists regarding the expected benefits and risks of surgical and medical treatment options.
Three anti-tumour necrosis (TNF)-α antibodies—infliximab, adalimumab and golimumab—have received a marketing authorisation for the treatment of moderately-to-severely active UC in adult patients who have had an inadequate response to conventional therapy including corticosteroids and 6-mercaptopurine (6-MP) or azathioprine, or who are intolerant to or have medical contraindications to such therapies [6–8]. Infliximab is administered by intravenous infusion at a dose of 5 mg/kg followed by additional doses of 5 mg/kg at 2 and 6 weeks after the initial infusion, and every 8 weeks thereafter. Adalimumab is administered subcutaneously at 160 mg at week 0 and 80 mg at week 2 followed by 40 mg every other week (EOW), with dosing increased to 40 mg every week (EW) if clinical response is deemed insufficient. Golimumab is administered subcutaneously at an initial dose of 200 mg, followed by 100 mg at week 2; thereafter, patients with a body mass <80 kg receive 50 mg every 4 weeks, whilst those with a body mass ≥80 kg receive 100 mg every 4 weeks. The European Medicines Agency (EMA) recommends that anti-TNF-α therapy is discontinued in patients who do not achieve an induction response and in patients who experience but subsequently lose response [6–8]. Whilst their benefits have been demonstrated within several phase III randomised placebo-controlled trials (RCTs) [9–16] and their use is supported by national guidelines, the costs of these biologic agents are considerably greater than those of conventional non-biologic therapies. In the induction setting, the cost of 8 weeks of anti-TNF-α therapy ranges from £2817 (adalimumab) to £5928 (infliximab) per patient, whilst in the maintenance setting, the cost of 12 months of anti-TNF-α therapy ranges from £9187 (adalimumab 40 mg EOW dosing) to £19,905 (golimumab for patients with body mass ≥80 kg). Consequently, there is a need to assess whether the additional value of these therapies outweighs the opportunity costs associated with their use. This study presents an economic evaluation of anti-TNF-α therapies, conventional non-biologic therapies and elective colectomy for patients with moderately-to-severely active UC after the failure of conventional therapy.
2 Methods
2.1 Scope of Health Economic Analysis
A model-based cost-utility analysis was undertaken to assess the incremental cost-effectiveness of infliximab, adalimumab and golimumab versus conventional non-biologic therapy and elective surgery for patients with moderate-to-severe UC for whom at least one prior therapy has failed. Five options were included: (1) adalimumab 160/80/40 mg; (2) infliximab 5 mg/kg; (3) golimumab 200/100/100 mg (50 mg); (4) conventional non-biologic therapy (comprising a mix of 5-ASAs, immunosuppressants and corticosteroids); and (5) elective surgery. The health economic analysis was undertaken from the perspective of the UK NHS and Personal Social Services (PSS) over a lifetime horizon (60 years). Surgery is included both as a decision option and as part of the modelled treatment pathway. The cost effectiveness of each option was evaluated within a fully incremental analysis whereby each option was compared against its next best non-dominated comparator. Options that were dominated (more expensive and less effective than one or more treatment alternatives), and those that were extendedly dominated (options with an incremental cost-effectiveness ratio [ICER] higher than a more effective non-dominated option), were ruled out of the analysis. Costs and outcomes were discounted at a rate of 3.5 % per annum [17]. Costs were valued at 2013/2014 values.
2.2 Model Structure
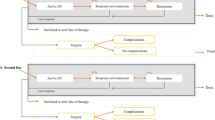
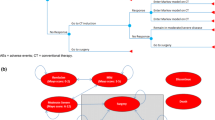
The model structure was developed based on a review of previous models, expert clinical input and consideration of the available clinical evidence for the anti-TNF-α therapies [18]. The model adopts a Markov structure with eight mutually exclusive health states (see Fig. 1). Health states were defined according to whether the patient is alive or dead, the non-surgical treatment the patient is currently receiving (biologic therapy or conventional non-biologic therapy), their prior history of colectomy and their current level of disease control (remission, response or active UC). The following health states are included: (1) on anti-TNF-α therapy—active UC; (2) on anti-TNF-α therapy—response; (3) on anti-TNF-α therapy—remission; (4) on conventional treatment—active UC; (5) on conventional treatment—response; (6) on conventional treatment—remission; (7) post-surgery (with/without complications); and (8) dead. In line with the trials of the TNF-α inhibitors [9–16], remission and response are classified according to the full Mayo score [19]. Remission is defined as a total Mayo score ≤2 with no individual subscore >1. Response is defined as a decrease from baseline in the total Mayo score of at least 3 points and at least 30 %, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore for rectal bleeding of 0 or 1. Patients without either response or remission are classified as having active UC.
The model time horizon is divided into two phases: (1) induction and (2) maintenance. The model adopts an 8-week cycle length for the induction phase and a 26-week cycle length for the maintenance phase. During the induction phase, patients receiving anti-TNF-α therapy who achieve response or remission are assumed to continue receiving the same agent as maintenance therapy. Patients who do not respond to anti-TNF-α induction therapy discontinue and subsequently receive conventional non-biologic therapy. Patients receiving conventional therapy are assumed to continue receiving treatment irrespective of their induction response. Patients in the colectomy group are assumed to undergo surgery during the induction phase and subsequently remain in the post-surgery state. During the maintenance phase, patients receiving anti-TNF-α therapy are assumed to continue receiving the same anti-TNF-α agent for as long as they maintain response/remission. If patients receiving anti-TNF-α therapy lose response, they are assumed to transit to the active UC state and subsequently receive conventional therapy. Patients in the conventional therapy group, and those who have previously achieved but lost response to anti-TNF-α therapy, continue receiving conventional therapy irrespective of whether they achieve response or remission. A time-independent probability of undergoing surgery is applied to patients with active UC who are receiving conventional therapy. Patients in the colectomy group, and those who have undergone surgery after receiving prior biologic/non-biologic drug therapy, remain in the post-surgery state until death. All patients have a probability of dying from other causes during each cycle. A proportion of patients undergoing surgery are assigned an additional risk of peri-operative death. HRQoL is assumed to be determined by the patient’s level of disease control, whether they have previously undergone colectomy and the incidence of post-surgical complications. For patients undergoing surgery, a disutility is assigned to patients who develop chronic pouchitis. Other surgical complications are assumed to be transient and do not have a long-term impact upon HRQoL. Resource costs include those associated with drug acquisition, drug administration (infliximab only), surgery and related complications, and UC health state costs (endoscopy, blood tests, consultant visits and hospitalisations).
The model employs the following key structural assumptions:
-
The induction phase is assumed to be 8 weeks in duration; this reflects the design of the trials used to inform efficacy parameters within the model, excluding the PURSUIT-SC trial, which assessed patients’ induction response at the earlier timepoint of 6 weeks.
-
Patients continue receiving anti-TNF-α therapy provided they are still obtaining benefit from it. At the beginning of the maintenance phase, the decision to continue anti-TNF-α therapy is determined by whether the patient achieves response/remission at the end of the induction cycle. During each subsequent maintenance cycle, the decision to continue anti-TNF-α therapy is determined by whether the patient maintains response/remission at the end of the previous maintenance cycle.
-
Patients receiving anti-TNF-α therapy concurrently receive conventional non-biologic therapies. Usage of these therapies is the same for all medical treatment groups.
-
Patients who discontinue anti-TNF-α therapy subsequently receive conventional therapy only.
-
Patients with active UC receiving conventional treatment may undergo colectomy during any cycle; patients receiving anti-TNF-α therapy will receive at least one cycle of conventional therapy prior to undergoing surgery.
-
Excluding chronic pouchitis, surgical complications are transient and can be resolved through further surgery or medical management. Complications are assumed to occur during the first model cycle following surgery.
-
Chronic pouchitis following surgery results in additional treatment costs and a decrement in HRQoL.
2.3 Evidence Used to Inform the Model Parameters
Transition probabilities for the model were derived from a series of network meta-analyses (NMAs) undertaken alongside a systematic review of RCTs of infliximab, adalimumab and golimumab (see Table 1) [18]. All other model parameters were derived from the literature, standard costing sources and expert opinion (see Table 2).
2.3.1 Patient Characteristics
Patient characteristics were based on the trials included in the NMAs [9–14]. Patients are assumed to enter the model aged 40 years, and 43 % of patients are assumed to be female. Patients are assumed to have a mean body mass of 77 kg, and 32 % of patients are assumed to have a body mass >80 kg [12, 13].
2.3.2 Transition Probabilities for Anti-Tumour Necrosis Factor (TNF)-α Agents and Conventional Non-Biologic Therapies
The probabilities of achieving and maintaining response or remission were estimated via NMAs of six placebo-controlled trials of TNF-α inhibitors (ACT1, ACT2, ULTRA1, ULTRA 2, PURSUIT-SC and PURSUIT-M) [18], undertaken using a Bayesian approach [29]. A further trial reported by Suzuki et al. [14] was excluded from the base-case analysis but was considered separately within the sensitivity analysis. In all trials, patients also received concurrent non-biologic treatments as background therapy. None of the trials included colectomy as a treatment arm. Data relating to clinical response and remission, as defined by the complete Mayo score, for the anti-TNF-α agents and placebo in the induction setting were extracted directly from the RCT publications [9, 10, 12]. Data relating to response and remission for the anti-TNF-α agents and placebo in the maintenance setting (conditional on outcomes at previous timepoints) were obtained directly from the manufacturers of the biologics. Since remission is a subset of response, the available data were ordered, categorical in nature and assumed to arise from a multinomial distribution. Relative treatment effects were estimated on the probit scale using random effects NMAs of clinical response and remission, with conventional treatment as the reference group. A random-effects NMA was also undertaken to estimate the absolute treatment effect for the conventional therapy group. The probabilities of transiting between response, remission and no response during induction (assessed at 6–8 weeks), during the first 6 months of maintenance therapy (from 6–8 to 30–32 weeks) and during the second 6 months of maintenance therapy (from 30–32 to 52–54 weeks) for all anti-TNF-α agents were derived by combining the estimated relative treatment effects for the anti-TNF-α agents and the absolute treatment effects for conventional treatment. Owing to the absence of feedback loops in the network, it was not possible to formally check for inconsistency between direct and indirect evidence. All analyses were conducted using the software package OpenBUGS [30]. The joint distributions for the absolute effects estimated from the NMAs (CODA samples drawn from the posterior distribution) were used as direct inputs into the health economic model. Further details of the statistical models used can be found in Appendix 1 in the Electronic Supplementary Material (ESM).
The mean transition probabilities and associated credible intervals (CrIs) derived from the NMAs are presented in Table 1. In the induction setting, all treatments were associated with statistically significant beneficial effects relative to placebo, with the greatest effect being associated with infliximab. For patients classified as responders at the end of induction, treatment effects were not statistically significant, although the greatest effect at 8–32 weeks was associated with golimumab 100 mg. At 32–52 weeks, only infliximab and golimumab 50 mg were associated with beneficial, albeit non-significant, effects on clinical response. For patients classified as being in remission at the end of induction, all treatments except for adalimumab were associated with beneficial treatment effects relative to placebo, with the greatest effect being associated with golimumab 50 mg and 100 mg, although the effects were not statistically significant at 8–32 weeks. At 32–52 weeks, all treatments except golimumab 50 mg were associated with beneficial treatment effects relative to placebo, with the greatest effect being associated with adalimumab (the only treatment with a statistically significant benefit). Adalimumab was associated with the highest probability of maintaining remission [18].
As longer-term follow-up data were missing from the RCTs, the model assumes that the transition matrix derived from the second maintenance cycle NMA (32–52 weeks) applies indefinitely to all subsequent maintenance cycles.
2.3.3 Surgery Rate
Given the short duration of the RCTs of the anti-TNF-α therapies, the colectomy rate for patients with moderate-to-severe UC was instead based on a focussed literature review [18]. Studies were considered potentially relevant if they reported on long-term colectomy rates and if they either related to the moderate-to-severe UC population as a collective patient population or reported on colectomy rates in moderate and severe UC populations separately. Most of the identified studies reported surgery rates in patients experiencing UC flare and were thus likely to overestimate the true colectomy rate within the population represented by the model. The study reported by Solberg et al. [22] was selected for use in the model as it was based on a large population (423 patients completed 10-year follow-up) and did not specifically relate to patients who had experienced UC flare. A constant 6-month colectomy rate of 0.0051 was applied within the model. Given the uncertainty in this parameter, this rate was tested in the sensitivity analyses.
2.3.4 Mortality
Peri-operative mortality rates for patients undergoing colectomy were taken from the 2012 UK IBD Audit [5]. A surgical mortality rate of 0.03 was assumed, based on 28 deaths reported amongst 807 elective and emergency surgical episodes in adult UC patients. Other-cause mortality was modelled according to age- and sex-specific life tables from the Office for National Statistics (ONS) [31].
2.3.5 Probability of Surgery-Related Complications
The trials of the TNF-α inhibitors do not report data on surgery-related complications. The probability of experiencing transient and chronic surgery-related complications and the use of medical/surgical approaches for the treatment of these were based on Arai et al. [23]. With the exception of chronic pouchitis, which is assumed to persist indefinitely, surgical complications are assumed to be transient. The model assumes that 47.3 % of patients experience transient complications and that a further 5 % will develop chronic pouchitis. The model assumes that 19 % of complications require further surgery, whilst the remaining 81 % require medical treatment only.
2.3.6 Health-Related Quality of Life (QoL)
None of the trials included in the NMAs included the use of a preference-based measure of HRQoL. Instead, utility estimates were derived from a systematic review of EQ-5D studies undertaken in UC populations [18]. Studies were considered potentially relevant if they reported EQ-5D estimates according to level of disease control or reported valuations of post-surgery states. Of the ten identified studies, two (Woehl et al. [20] and Swinburn et al. [32]) were UK based and included large numbers of patients (n = 180 and n = 230, respectively). These studies also included the greatest coverage of the health states in the model. Woehl et al. [20] was selected for the base-case analysis as the valuation for the surgery state (utility 0.71–0.72) was most similar to, yet still lower than, the values reported in the post-surgery EQ-5D studies (range 0.85–0.90) [33–35]. The valuation of the post-colectomy state within the Swinburn et al. [32] study was considerably lower (utility 0.59). As neither Woehl et al. [20] nor Swinburn et al. [32] report on the impact of surgical complications on HRQoL, the disutility associated with chronic pouchitis was taken from Arseneau et al. [21]. The base-case analysis uses the following utility values: utility remission 0.87 (95 % CrI 0.82–0.91); disutility for loss of remission (difference between remission and response) = 0.11 (95 % CrI 0.08–0.14); disutility for loss of response (difference between response and no response) = 0.35 (95 % CrI 0.26–0.45); utility post-surgery = 0.71 (95 % CrI 0.61–0.80); and disutility for post-surgical complications = 0.17 (95 % CrI 0.13–0.21).
2.3.7 Resources and Costs
Drug acquisition costs were derived from the British National Formulary (BNF) [24]. The costs of adalimumab were adjusted to account for the proportion of patients in the ULTRA trials whose dose was escalated to the EW regimen. The costs of conventional therapies were assumed to be the same for all treatment groups. Health state costs relating to the use of elective and emergency endoscopy, hospitalisations, consultant visits and blood tests were drawn from a previous modelling study reported by Tsai et al. [25]. The relative risks of hospitalisation for infliximab and adalimumab were derived from an NMA reported by the manufacturer of infliximab and golimumab [26]; as no evidence was available on hospitalisation rates for golimumab, the relative risk of hospitalisation was assumed to be the same as that for adalimumab. Unit costs associated with health state resource use were taken from NHS Reference Costs 2013 [27]. The costs of surgery and stoma care were drawn from a published costing study [28]. Cost estimates were uplifted to current prices using Hospital and Community Health Services (HCHS) inflation indices [36].
2.4 Methods for Model Evaluation
Estimates of mean QALY gains and costs for each option were derived probabilistically using Monte Carlo sampling over 10,000 iterations. Decision uncertainty was represented using cost-effectiveness acceptability curves (CEACs). Further sensitivity analyses were undertaken to explore the extent to which individual model parameters impact upon the model results. These included the inclusion/exclusion of data from Suzuki et al. [14] in the NMA, the inclusion of intention-to-treat (ITT) data from ULTRA2 [11] in the NMA, “within-trial” comparisons using only direct trial data for each anti-TNF-α agent, and varying assumptions regarding the relevant time horizon, the starting age of the population, the source of health utility estimates, the colectomy rate and assumptions regarding resource costs. In addition, given uncertainty surrounding the long-term transition probabilities, two additional highly optimistic scenarios are presented whereby from week 60 onwards: (1) all patients receiving anti-TNF-α therapy remain in their last observed health state, and (2) all patients transit to and remain in the remission health state. To account for clinical and patient preferences for surgical treatment of UC, results for all analyses are presented separately for patients for whom colectomy is a potentially acceptable option and for those for whom it is not; the analyses of these two populations differ only in terms of the inclusion of immediate elective colectomy as a potential treatment option—all other model inputs and assumptions remain unchanged.
2.5 Methods for Model Verification and Validation
A number of measures were taken to ensure the credibility of the model. These included internal and external peer review by clinical and methodological experts, double programming of the deterministic version of the model, scrutiny of implemented model coding and formulae, checking the accuracy of all model inputs against sources, investigating potentially discrepant or unexpected results identified through black box testing and comparison of the model results against those generated using other UC models.
3 Results
3.1 Central Estimates of Cost Effectiveness
Table 3 presents the central estimates of cost effectiveness for infliximab, adalimumab, golimumab, colectomy and conventional therapy. For the population in whom colectomy is an acceptable option, colectomy is expected to produce 14.71 QALYs at a cost of approximately £56,268 over the average patient's remaining lifetime. All medical options are expected to produce substantially fewer QALYs at a greater cost than elective surgery, hence colectomy is expected to dominate infliximab, adalimumab, golimumab and conventional non-biologic treatments. Assuming a willingness-to-pay (WTP) threshold of £30,000 per QALY gained or less, the probability that colectomy produces the greatest amount of expected net benefit is approximately 1.0 (see Fig. 2). The probability that any of the anti-TNF-α agents produce the greatest expected net benefit at this threshold is approximately zero. For the population in whom colectomy is not an acceptable option, infliximab is expected to be dominated by adalimumab (although the difference in expected QALYs between these options is very small), whereas golimumab is expected to be ruled out due to extended dominance (by adalimumab and conventional non-biologic therapy). The ICER for adalimumab versus conventional therapy is expected to be £50,278 per QALY gained. Assuming a WTP threshold of £30,000 per QALY gained, the probability that conventional treatment produces the greatest expected net benefit is approximately 0.98 (see Fig. 2).
3.2 Sensitivity Analysis Results
The sensitivity analyses suggest that for the population in whom colectomy is an option, colectomy remains dominant in most of the scenarios considered (see Table 4). For time horizons of 10 years or less, colectomy is expected to be more effective and more costly than conventional therapy, yielding ICERs for colectomy versus conventional therapy of less than £9,000 per QALY gained; the anti-TNF-α agents remain dominated in these scenarios. The sensitivity analyses indicate that the model results are particularly sensitive to assumptions regarding HRQoL. The use of alternative utility estimates from Swinburn et al. [32] results in a situation whereby colectomy moves from being the most effective option to the least effective option; this is largely driven by the lower post-colectomy utility value in this study. Under this scenario, the ICER for adalimumab versus conventional non-biologic therapy (the next best non-dominated option) is estimated to be £79,714 per QALY gained, whereas the ICER for infliximab versus adalimumab is estimated to be £178,982 per QALY gained; golimumab is ruled out of the analysis due to simple dominance. Within the analyses in which patients receiving anti-TNF-α therapy remain in their last health state or remain in the remission state from week 60 onwards, colectomy remains dominant.
Within the population in whom colectomy is not an option, golimumab is ruled out of most of the sensitivity analyses due to dominance. It is noteworthy that the analyses based on point estimates of parameters differ from the probabilistic results due to a change in the rank ordering of treatments between these analyses. Within the deterministic version of the model, infliximab becomes the most effective treatment; the ICER for infliximab versus adalimumab is £150,576 per QALY gained, whereas the ICER for adalimumab versus conventional non-biologic therapy is £68,606 per QALY gained. Where colectomy is not an option, the choice of studies and population data for inclusion in the NMA influences the ICERs for adalimumab and infliximab; however, irrespective of the studies included in the NMAs, the most favourable ICER for these products remains in excess of £54,000 per QALY gained. The analysis based only on direct trial data from ACT1/2 [9] indicates that the ICER for infliximab versus conventional therapy is £96,403 per QALY gained. The analysis based only on direct trial data from ULTRA1/2 [10, 11] indicates that the ICER for adalimumab versus conventional therapy is £69,782 per QALY gained. The analysis based only on direct trial data from PURSUIT [12, 13] indicates that the ICER for golimumab versus conventional therapy is £90,413 per QALY gained. However, these direct analyses do not consider the totality of the evidence base and are therefore of limited relevance to decision making. The use of utilities from Swinburn et al. [32] produces ICERs for adalimumab and infliximab that are less favourable than those generated using Woehl et al. [20]; in this analysis, golimumab remains dominated. Within the analyses in which patients receiving anti-TNF-α therapy remain in their last health state or remain in the remission state from week 60 onwards, the cost effectiveness of infliximab and adalimumab is improved considerably. Within the latter scenario, the ICER for adalimumab versus conventional non-biologic therapy is reduced to slightly below £30,000 per QALY gained. However, this is an extremely optimistic analysis that is not supported by empirical evidence.
4 Discussion
4.1 Summary of Headline Cost-Effectiveness Results
The clinically optimal treatment for moderately active or refractory UC unresponsive to conventional medical therapy remains unclear, although a number of options exist. These include the use of anti-TNF-α agents, the continued use of conventional therapy, or surgery, depending on patient and clinician preferences, duration and frequency of flares, and the impact of the disease, drug treatment and monitoring on an individual’s HRQoL. Given the nature of a surgical intervention in this situation, it is recognised that patients may not be suitable for surgery for a number of reasons, including their own preferences and perceptions of their post-surgical HRQoL, body image and risks of complications, including effects on fertility. As a result, the health economic analysis has been presented including and excluding colectomy as a comparator.
On the basis of this study, within an adult UC population in whom elective surgical intervention is an option, colectomy is expected to dominate infliximab, adalimumab, golimumab and conventional non-biologic therapy. For patients in whom elective colectomy is not an acceptable option, infliximab and golimumab are expected to be ruled out of the analyses, whilst the ICER for adalimumab versus conventional non-biologic therapy is expected to be approximately £50,278 per QALY gained. The sensitivity analyses highlight that the model results are particularly sensitive to the utility values assumed within the model, most notably, the post-colectomy utility score. This parameter has the potential to dramatically change the conclusions drawn from the model regarding the clinical value of elective colectomy as a treatment option. Within the utility studies reported by Woehl et al. [20] and Swinburn et al. [32], both of which were published only as abstracts, only a small number of patients underwent surgery, thus making existing estimates very uncertain. Despite this uncertainty, the conclusions regarding the cost effectiveness of the TNF-α inhibitors remain largely unchanged irrespective of the source of the utility data used or whether elective surgery should be considered as a potential treatment option—across most analyses considered, the most favourable ICER for any of the TNF-α inhibitors remains in excess of £50,000 per QALY gained. The ICER for adalimumab versus standard care was below £30,000 per QALY gained in one scenario (whereby all patients are assumed to achieve and maintain remission); however, it is important to note that this analysis represents an optimistic hypothetical scenario that is not supported by results of the NMAs.
The cost effectiveness of anti-TNF-α therapies versus standard care in patients with moderate-to-severe active UC following the failure of conventional therapy has previously been assessed within one UK study [25] and one Canadian study [37]. Xie et al. [37] included adalimumab as a subsequent-line therapy for non-responders to infliximab, whilst Tsai et al. [25] compared infliximab only against standard care. Both studies used a Markov approach to model costs and health gains associated with response and remission and included surgery as a consequence of ineffective medical treatment. Based on a 10-year time horizon, Tsai et al. [25] reported an ICER for infliximab of £27,424 per QALY gained assuming that responders continue maintenance therapy. Assuming a remission-only continuation rule, Tsai et al. [25] report an ICER for infliximab versus standard care of £19,696 per QALY gained (year 2006/2007 values). Xie et al. [37] reported considerably less favourable estimates for the cost effectiveness of infliximab versus usual care of greater than $CAN350,000 per QALY gained (year 2008 values). This contrasting finding may in part be explained by the use of a shorter 5-year time horizon and pessimistic assumptions whereby the utility associated with response is the same as that for active UC. Importantly, neither of these studies include all relevant biologic treatment options or colectomy as comparators and both studies adopted short time horizons.
4.2 Strengths and Limitations of the Analysis
This is the first study to assess the cost effectiveness of all relevant treatment options in the moderate-to-severe UC population over a lifetime horizon. The key strength of the analysis is that treatment efficacy estimates are drawn from a series of NMAs derived from trial data obtained from the manufacturers of the TNF-α inhibitors. The model structure and selection of model inputs has been informed by considerable expert opinion. In addition, sensitivity analyses have been extensively applied to explore the impact of uncertainty on the cost effectiveness of competing treatment options. Despite these strengths, decision makers should consider the applicability and relevance of the analysis in the context of their own local healthcare setting. The results of the health economic analysis should also be interpreted in light of the limitations of the evidence used to inform the model.
4.2.1 Limited Follow-Up Data Within the Trials of the Biologics
The anti-TNF-α trials had a maximum follow-up of 54 weeks following randomisation, hence there is considerable uncertainty with respect to the long-term effectiveness of these products. In particular, the model assumes that the second maintenance cycle (from around 32 to 52 weeks) applies indefinitely to each subsequent maintenance cycle. This is a strong assumption that cannot be substantiated empirically. The sensitivity analysis indicates that, under highly optimistic assumptions of long-term treatment benefits, the cost effectiveness of the anti-TNF-α therapies could be improved, although these assumptions are not supported by the available evidence. Further studies are required to determine the long-term benefits of these therapies in patients with moderate-to-severe UC.
4.2.2 Use of Placebo Group Data to Inform Baseline Response Rates
Baseline response rates for the conventional management group were based on the placebo group arms of the trials included in the NMAs. This could reflect a limitation of the analysis if there is a substantial difference between response rates observed in the trial placebo arms and those expected in clinical practice. The health economic model, which draws together the induction and maintenance phase NMAs, suggests that the probability that patients receiving conventional non-biologic therapies achieve and maintain response at 60 weeks is approximately 0.21. This appears to be consistent with what would typically be expected in usual clinical practice. Whilst not currently available, long-term audit data may provide an alternative source of baseline response rates in future economic analyses of UC therapies.
4.2.3 Absence of Preference-Based HRQoL Measures within Clinical Trials
None of the studies included in the NMAs included the use of a preference-based measure of HRQoL. As a consequence, the model draws on HRQoL estimates reported within the literature. These studies included only a small number of patients who have undergone prior colectomy.
4.2.4 Adherence to TNF-α Inhibitors Based on Observed Trial Data
The model assumes that adherence to biologic therapy, and the relationship between adherence and treatment efficacy, will reflect the rates observed in the trials included in the NMAs. The impact of lower adherence levels on the cost effectiveness of the TNF-α inhibitors is unclear.
4.2.5 Absence of Head-to-Head Trial Data for the TNF-α Inhibitors and Surgery
All six trials included in the NMAs compare anti-TNF-α therapies against placebo. The systematic review did not identify any head-to-head studies comparing the anti-TNF-α agents against each other or against surgery [18]. Given the inter-individual differences in suitability for and acceptability of surgery, or indeed drug treatment, and their potential side effects, designing such a study may be difficult, but not impossible.
4.2.6 Availability of Biosimilars for Infliximab
At the time of the analysis, two biosimilars for infliximab were about to enter the market; however, a UK list price for these products was not available. Based on the modelled population of patients in whom colectomy is an acceptable option, surgery would remain dominant irrespective of the price of an infliximab biosimilar. In the population in whom colectomy is not an option, the price of an infliximab biosimilar would need to be approximately 70 % lower than proprietary infliximab to achieve an ICER of £30,000 per QALY gained.
4.2.7 Optimal Treatment Strategies
The model assumes that patients will continue to receive anti-TNF-α therapy for as long as they are receiving benefit from it, that is, if they achieve and maintain response or remission. This assumption was made based on the marketing authorisations for infliximab, adalimumab and golimumab [6–8]. The model does not include switching between biologic therapies or treatment interruptions as this is unlikely, at least in primary non-responders. However, in reality, there may be more efficient means of using these therapies, for example, withdrawing anti-TNF-α therapy for those patients who achieve deep remission, subsequently reverting back to less expensive conventional non-biologic treatments and reintroducing anti-TNF-α therapy on relapse. Importantly, there are currently no randomised data to demonstrate the comparative effectiveness of such a treatment approach.
5 Conclusions
The ICERs for anti-TNF-α therapies versus conventional therapy or surgery are expected to be, at best, in excess of £50,000 per QALY gained. The cost effectiveness of alternative strategies involving the withdrawal of anti-TNF-α therapy upon remission and re-treating relapse is unknown.
References
National Institute for Health and Care Excellence. Ulcerative colitis: Management in adults, children and young people. NICE Clinical Guideline Number 166. London: NICE; 2013, pp 1–37.
Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. Br Med J 2013; 346. http://www.bmj.com/content/346/bmj.f432.long. Accessed 01 May 2014.
Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53(10):1471–8.
Bayless TM, Hanauer SB. Advanced therapy in inflammatory bowel disease: volume 1—IBD and ulcerative colitis. 3rd ed. Shelton: Connecticut: People’s Medical Publishing House—USA; 2011.
Royal College of Physicians. Report of the results for the national clinical audit of adult inflammatory bowel disease inpatient care in the UK. 1-67. London: RCP; 2012, p. 1–68.
European Medicines Agency. Summary of product characteristics—infliximab. London: EMA; 2009. p. 1–56.
European Medicines Agency. Summary of product characteristics—adalimumab. London: EMA; 2009. p. 1–292.
European Medicines Agency. Summary of product characteristics—golimumab. London: EMA; 2009. p. 1–199.
Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olsen A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–76.
Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer SB, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7.
Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257–65.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):85–95.
Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96–109.
Suzuki Y, Motoya S, Hanai H, Matsumoto T, Hibi T, Robinson AM, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014;49(2):283–94.
Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400.
Probert CS, Hearing SD, Schreiber S, Kühbacher T, Ghosh S, Arnott ID, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut. 2003;52(7):998–1002.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London: NICE; 2013. p. 1–102.
Archer R, Tappenden P, Ren S, Martyn St-James M, Harvey R, Basarir H et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy: Clinical effectiveness systematic review and economic model. Final report to the National Institute for Health and Care Excellence. Sheffield: University of Sheffield 2014, p. 1–438.
Cooney RM, Warren BF, Altman DG, Abreu MT, Travis SP. Outcome measurement in clinical trials for ulcerative colitis: towards standardisation. Trials 2007;8(17). http://www.trialsjournal.com/content/8/1/17. Accessed 01 June 2014).
Woehl A, Hawthorne AB, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis. Gut. 2008;57(Suppl1):A153.
Arseneau KO, Sultan S, Provenzale DT, Onken J, Bickston SJ, Foley E, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol. 2006;4(9):1135–42.
Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44(4):431–40.
Arai K, Koganei K, Kimura H, Akatani M, Kitoh F, Sugita A, et al. Incidence and outcome of complications following restorative proctocolectomy. Am J Surg. 2005;190(1):39–42.
BMJ Group and the Royal Pharmaceutical Society of Great Britain. Br Natl Formul. 2014. https://www.medicinescomplete.com/mc/bnf/current/. Accessed 01 May 2014.
Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Therapeut. 2008;28(10):1230–9.
Merck, Sharp, Dohme. Manufacturer’s submission of evidence to the National Institute for Health and Care Excellence: Infliximab. Hertfordshire: MSD; 2014.
Department of Health. NHS Reference Costs 2012/13. London: DH. https://www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013. Accessed 04 May 2014.
Buchanan J, Wordsworth S, Ahmad T, Perrin A, Vermeire S, Sans M, et al. Managing the long term care of inflammatory bowel disease patients: The cost to European health care providers. J Crohn Colitis. 2011;5(4):301–16.
Dias S, Welton N, Sutton A, Ades A. NICE Decision Support Unit Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Sheffield: University of Sheffield; 2011. p. 1–98.
Thomas A, O’Hara B, Ligges U, Sturtz S. Making BUGS open. R News. 2015;6:12–7.
Office for National Statistics. Interim life tables 2009–2011. 2013. http://www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2009-2011/stb-2009-2011.html. Accessed 05 May 2014.
Swinburn P, Elwick H, Bean K, Curry A, Patel S, Bodger K, et al. The impact of surgery on health related quality of life in ulcerative colitis. Gut. 2012;61(Suppl2):A237.
Van der Valk ME, Mangen MJ, Dijkstra G, Bodegraven AA, Fiddler H, De Jong DJ et al. Is there a difference in quality of life and costs between ulcerative colitis patients with a pouch or an ileostomy? Dig Dis Wk; 2012.
Richards DM, Hughes SA, Irving MH, Scott NA. Patient quality of life after successful restorative proctocolectomy is normal. Colorectal Dis. 2001;3(4):223–6.
Kuruvilla K, Osler T, Hyman NH. A comparison of the quality of life of ulcerative colitis patients after IPAA vs ileostomy. Dis Colon Rectum. 2012;55(11):1131–7.
Curtis L. Unit costs of health and social care 2014. 1–294. Personal Social Services Research Unit: Kent; 2014.
Xie F, Blackhouse G, Assasi N, Gaebel K, Robertson D, Goeree R. Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost Eff Resour Alloc. 2009;7:20.
Acknowledgments
The authors would like to thank MSD and AbbVie for providing additional data over the course of the National Institute for Health and Care Excellence (NICE) appraisal for which the model was developed.
Author Contributions
Paul Tappenden and Hasan Basarir developed the health economic model. Rachel Archer and Marrissa Martyn-St James undertook the systematic review of clinical effectiveness evidence. Shijie Ren, Rebecca Harvey and John Stevens undertook the network meta-analyses. Sami Hoque and Alan Lobo provided ongoing clinical advice during the model development. All authors contributed to the preparation of this manuscript. Paul Tappenden will act as the overall guarantor for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme to inform the NICE Technology Appraisal Programme (Project Number 12/51/01). Alan Lobo has received money for attending an advisory board for Takeda Pharmaceuticals. Paul Tappenden, Shijie Ren, Rachel Archer, Rebecca Harvey, Marrissa Martyn-St James, Hasan Basarir, John Stevens and Sami Hoque do not have any conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tappenden, P., Ren, S., Archer, R. et al. A Model-Based Economic Evaluation of Biologic and Non-Biologic Options for the Treatment of Adults with Moderately-to-Severely Active Ulcerative Colitis after the Failure of Conventional Therapy. PharmacoEconomics 34, 1023–1038 (2016). https://doi.org/10.1007/s40273-016-0409-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0409-9