Abstract
Background
Infliximab has been shown to be efficacious in acute exacerbations of ulcerative colitis (UC).
Aim
To evaluate the cost-effectiveness of infliximab treatment in patients hospitalised with acute exacerbations of UC.
Methods
A decision analysis model was constructed to simulate the progression of acute UC patients treated with infliximab induction regimen over 1 year. Infliximab treatment was compared with standard care, ciclosporin and surgery using transitions derived from infliximab and ciclosporin randomised trials. Costs and outcomes were discounted at 3.5%. Intermediate outcomes of colectomy and post-surgery complications were translated into the primary effectiveness measurement, which was quality-adjusted life years (QALYs) estimated using EQ-5D. One-way and probabilistic sensitivity analyses were performed to estimate the uncertainty around the results.
Results
The incremental cost effectiveness ratio (ICER) for infliximab was £19,545 per QALY compared to ciclosporin, which in turn dominated standard care. Sensitivity analysis indicated patient body weight, utility estimates and treatment effect of alternative treatment strategies to be the most important factors affecting cost-effectiveness.
Conclusion
Infliximab induction regimen appears to be a cost-effective treatment option for UC patients hospitalised with an acute exacerbation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a lifelong condition characterised by diffuse inflammation involving primarily the colon mucosa [1]. Patients with UC often have recurrent exacerbations of the disease resulting in hospitalisation and an increased risk of surgery. It is estimated that 20% of all UC patients will have such acute attacks at any given time [2].
For UC patients hospitalised with an acute exacerbation, the goal of treatment is to avoid the surgical procedure of colectomy and induce remission. Current standard care for these patients comprises the addition of intravenous corticosteroids (for up to 72 h) to their existing immunomodulator therapy. However, 30–40% of these patients are likely to fail intravenous (IV) steroids and require further medical intervention [2, 3].
The primary treatment options for such hospitalised UC patients are surgery or ciclosporin [4, 5]. Surgery is associated with increased risks in particular patient groups (e.g. women of child-bearing age, young males, and patients with co-morbid conditions) and may lead to significant post-surgical complications in a proportion of patients, with a negative impact upon quality of life. Ciclosporin, the other treatment alternative that is not licensed but often used, is also associated with side effects and excess mortality [6, 7].
The biological therapy infliximab (Remicade®) is an inhibitor of tumour necrosis factor α (TNF-α), a cytokine that plays a major role in the pathogenesis of UC. The efficacy of infliximab in the treatment of acute exacerbations of UC has been demonstrated in two randomised controlled trials [8, 9]. Both studies demonstrated infliximab to be a safe and efficacious treatment option in acute UC patients.
Infliximab is often perceived to be an expensive treatment option for patients with UC. A recent cost-effectiveness analysis demonstrated infliximab scheduled maintenance treatment to be cost effective in moderate-severe UC patients [10]. To date, however, no study has estimated the cost effectiveness of infliximab in acute exacerbations of UC. The present economic evaluation was performed to assess the cost-effectiveness of infliximab treatment at the licensed dose of 5 mg/kg, as compared with available alternatives, for the treatment of patients hospitalised with an acute exacerbation of UC.
Methods
Model overview
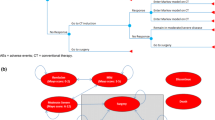
A decision analytic model was used to simulate the progression of hypothetical cohorts of patients with an exacerbation of UC receiving different treatment strategies and to track associated costs and outcomes [quality-adjusted life years (QALYs)] over 1 year. This model was developed using Microsoft Excel. A schematic representation of the model is provided in Fig. 1.
The initial model cohort consisted of acute severe UC patients not responding to 72 h of IV steroid therapy. These patients were assumed to receive one of the four treatment strategies under consideration—infliximab, ciclosporin, standard care or surgical intervention. The base case time horizon of 12 months was divided into two treatment cycles (0–3 months and 4–12 months). Further analyses were conducted over a 10-year time horizon. Treatment outcomes were characterised in the model as short-term outcomes (1st cycle; 0–3 months), medium-term outcomes (4–12 months) and long-term outcomes (12 months–10 years).
Short-term outcomes
Patients treated with infliximab, ciclosporin or standard care either responded to treatment and achieved remission or failed treatment and underwent colectomy. In the absence of evidence regarding the variable course of disease severity for responders following different treatment interventions, all responders were assumed to achieve and maintain a symptom-free remission following discharge.
Medium-term outcomes
Patients achieving initial remission either maintained the remission for the rest of the 12 month period or lost response and underwent a colectomy. For patients undergoing colectomy after the first 3 months (1st cycle), no information was available on the time to colectomy. Therefore, it was assumed that, in the medium-term outcomes, colectomies occurred mid-cycle i.e. at 7.5 months.
Long-term follow-up
Long-term follow-up (up to 10 years) analysis was conducted as part of the sensitivity analysis to address the uncertainty around the choice of time horizon. In order to estimate the long-term outcomes, probability of colectomy estimated in the medium term (4–12 months) was repeated using a Markov model beyond the 1st year. Long-term follow-up analysis was conducted with a time horizon of up to 10 years.
Surgery
Patients undergoing surgery either achieved post-surgery remission and maintained it throughout the time frame of this analysis or suffered from immediate post-surgery complications. It was assumed that post-surgery complications would occur immediately following surgery and therefore in the same cycle as surgery. Patients treated for post-surgical complications are assumed to recover in the next cycle, achieve post-surgical remission and remain in remission for the rest of the analysis. Due to the shorter timeframe of this base-case analysis, long-term complications such as pouchitis and pouch failure were not considered. This is likely to favour surgery as a treatment option and adversely affect the ICERs for medical treatments such as infliximab compared to surgery.
Outcome probabilities
Intermediate outcomes of colectomy, symptom-free remission and post-surgery complications were used to derive the final outcome of QALYs.
Colectomy rate
The baseline risk of colectomy was estimated using the placebo arms of infliximab and ciclosporin clinical trials. This treatment strategy was labelled as ‘standard care.’ A meta-analysis of the placebo arms of the trials was conducted to derive a composite colectomy rate for the standard care treatment arm. The relative risk of colectomy on different treatment alternatives was determined by an indirect comparison between the clinical trials. For infliximab, the efficacy estimates were derived from the studies of Jarnerot [8] and Sands [9] whereas for ciclosporin they were derived from the studies of D’Haens [11] and Lichtiger [12].
For indirect comparison, a network meta-analysis was conducted. This allowed indirect comparison between treatment alternatives assuming that the relative treatment effects could be compared on a log-odds scale across the trials. Independent estimates for the treatment effects were made for the 0–3 and 4–12 month periods. The treatment effect estimated for the 4–12 month period was based on the probability of having a colectomy for patients still at risk after the first 3 months. The cumulative data reported in the trial was restructured to show the incremental results as illustrated in Table 1.
A Bayesian hierarchical model was used to synthesise the relative treatment effects observed within the trials. The mixed treatment comparisons (MTC) model used Markov Chain Monte Carlo Methods (MCMC) and was based on those detailed in Ades et al. [13]. The analysis was conducted using WinBUGS 1.4 [14] and the resulting colectomy rates are displayed in Table 2.
Surgical complications
A composite surgical complications rate of 23.5% per year was used. This was based on the rate of individual complications such as post-operative wound infections, post-operative rectal stump complications, post-operative bleeding, post-operative sepsis, anastomical leakage, small bowel obstruction and stoma complications derived from the United Kingdom inflammatory bowel disease (UK IBD) audit [4].
Treatment pathway and interventions
The analysis assumed a defined treatment pathway during the timeframe of this analysis for all patients hospitalised with an acute exacerbation of UC. This treatment pathway was developed based on the clinical trial evidence and in consultation with the UK clinical experts. The treatment pathway is displayed in Fig. 2.
Initial treatment (day 1–3): all patients were assumed to receive 72 h of intravenous corticosteroid treatment. The corticosteroid assumed was 400 mg/day of hydrocortisone based on UK IBD audit [4].
Comparator treatment initiation (day 4–10): all patients not responding to the initial treatment were assumed to receive one of the four treatment alternatives. These included continued treatment with standard care, infliximab in addition to standard care, ciclosporin in addition to standard care or surgical intervention, as follows:
- Standard care:
-
The standard care treatment included continuation of the intravenous corticosteroid treatment of 400 mg/day hydrocortisone for an additional 7 days
- Infliximab:
-
Infliximab treatment included a first infusion of 5 mg/kg infliximab on the 4th day. These patients also received concomitant standard care comprising of intravenous corticosteroid treatment for an additional 7 days during the hospital stay. As stated above, responders to infliximab were assumed to respond within 7 days of the first infusion
- Ciclosporin:
-
Patients treated with ciclosporin received a 4 mg/kg daily dose of intravenous ciclosporin starting on the fourth day for a period of 7 days. These patients also received standard care comprising intravenous corticosteroid treatment during this period
It is assumed that all patients were hospitalised until the 10th day. Responders to medical treatments were assumed to be discharged on the 10th day and moved to an outpatient setting. Patients not responding to medical treatments on or before the 10th day were assumed to progress to surgery.
Short-term follow-up treatment (day 11–90)
Following discharge from hospital, all infliximab responders received oral corticosteroids (60 mg/day Prednisolone) and Azathioprine (2 mg/kg) for the rest of the 3-month period. In addition, responders also received the two remaining doses of infliximab (5 mg/kg) at weeks 2 and 6 following the first infusion. In the ciclosporin-treated cohort, the responders were switched to oral ciclosporin (2 mg kg−1 day−1) until the end of 3 months. In addition, these patients also received oral corticosteroids (60 mg/day Prednisolone) and Azathioprine (2 mg/kg) during this period. The responders to standard care were also switched to combination therapy comprising of oral corticosteroids (60 mg/day Prednisolone) and Azathioprine (2 mg/kg) following discharge for the rest of the 3 months.
Long-term follow-up treatment (day 91 onwards)
Patients with continued response are assumed to ‘bridge’ onto combination therapy comprising of oral corticosteroids (60 mg/day Prednisolone) and Azathioprine (2 mg/kg) and continue to receive this combination therapy for the remainder of the analysis timeframe. The base case analysis was conducted for a period of 1 year following hospitalisation for acute exacerbation of UC. A long-term analysis with a 10-year time horizon was carried out to explore uncertainty in long-term outcomes.
Surgical intervention
Surgical intervention is also included as an alternative treatment strategy to reflect a scenario where patients choose to undergo colectomy following non-response to IV steroids (by day 3). Surgical intervention is also included in the economic evaluation as a treatment outcome for patients not responding to a medical treatments (Infliximab, ciclosporin or standard care; on or before day 10). Any patient undergoing surgical intervention and achieving post-surgical remission is assumed to have a hospitalised recovery period of 7 days. Subsequently, these patients are discharged from hospital and managed in an outpatient setting. Patients suffering from post-surgical complications are assumed to require an additional 10 days of hospitalisation.
Costs
Perspective
The perspective adopted on costs was that of the National Health Service (NHS) in England and Wales using the reference year 2006–2007. Productivity costs, although significant, were omitted due to this choice of the perspective.
Infliximab acquisition and administration
The total cost associated with infliximab treatment was broken down into its acquisition cost (£419.62 per 100 mg vial) and the cost of administering an IV infusion. Assuming a mean body weight of 80 kg for an adult UC patient in England and Wales, the drug acquisition cost was £1,678.48. The administration cost was assumed to be £62.66 per infusion resulting in a total cost per infusion of £1,741.14 [15].
Comparator and concomitant treatment cost
The costs of comparator treatments and concomitant medications used in the analysis were calculated based on the average doses used in the clinical trials and was costed based on pack sizes in the British National Formulary (BNF; September 2007; http://bnf.org/bnf/). Table 3 outlines the drug costs used in the model.
Surgery, hospitalisation and other assessments
The resource use costs for hospitalisation and other assessments were estimated based on expert opinion of UK gastroenterologists. A Delphi panel of five experts estimated the resource use of UC patients during and after hospitalisation. These estimates were then used to cost the resource use for each health state.
Consultant visits
While estimating the number of consultant visits, it was assumed that responders were to visit a consultant on day 30 and day 90 from the day of hospitalisation. Patients achieving remission (medical or surgical) were assumed to have one consultant visit every 3 months in the follow-up period. Patients suffering from surgical complications were assumed to have no additional consultant visits during the period they were hospitalised for their complications.
Hospital episodes
All patients were assumed to have 10 days of hospitalisation during initial treatment period. This included the first 3 days of IV steroid treatment and a 7-day recovery period on rescue treatment following steroid failure. Patients achieving and maintaining remission were assumed not to have any subsequent hospitalisation. Patients suffering post-surgery complications were assumed to have 10 days of hospital stay in addition to the stay due to their surgical procedure.
Surgical procedures
The surgical procedure included colectomy, which is comprised primarily of ileal pouch anal anastomosis (IPAA) and illeostomy. Clinical expert opinion suggested that all patients undergoing colectomy for UC would first undergo an illeostomy comprising of two separate procedures approximately 3 months apart. Therefore the cost an illeostomy was estimated to be twice as much as a ‘complex procedure in gastroenterology’. It was also assumed that a small proportion of illeostomy patients would undergo a third procedure called IPAA approximately 3–6 months after the illeostomy. The cost of IPAA therefore included an additional cost of a ‘major procedure in gastroenterology.’ The total cost of surgery was calculated using a weighted average based on the prevalence of these surgical techniques (29% IPAA, 71% illeostomy) [4].
Diagnostic procedures
Two separate types of endoscopy costs were estimated and used in the analysis. It was assumed that all patients hospitalised with an acute exacerbation would initially undergo an inpatient endoscopy to confirm presence and severity of UC. Patients suffering from post-surgery complications who also were hospitalised were assumed to have an additional endoscopy to confirm the type and extent of their complication. In contrast, responders to medical or surgical treatment were assumed to have two additional day case endoscopies at day 30 and day 90 to confirm their remission status. Following the initial treatment period (0–3 months), all patients were assumed to have a diagnostic endoscopy once every 3 months as displayed in Table 3.
Outcomes
The primary effectiveness measure used in these analyses was the QALY. The intermediate treatment outcomes of colectomy, symptom-free remission and surgical complications were translated into the final outcome of QALYs using the health state preferences obtained from a UC patient survey carried out in Cardiff Hospital using the EQ-5D [16] and valued using UK tariffs [17].
The derived utility estimates were classified into individual pre-surgery health states by indexing them with a simple clinical colitis activity index (SCAI) [18]. Patients were classified into remission (SCAI: 0–2) and active UC (SCAI: 3 and above) [18]. Separate sets of utilities were available for IPAA and illeostomy. Therefore, a weighted average based on the prevalence of these surgical techniques (29% IPAA, 71% Illeostomy) was used as the utility for post-surgery remission [4]. The Woehl study [16] did not capture utilities associated with post-surgery complications. Therefore, these were assumed to be the same as that of active UC. A separate set of utilities were also available from the Arseneau study [19] and were used in the sensitivity analysis. Table 4 provides a summary of all health state preference estimates employed in the economic evaluation.
Cost-effectiveness analyses
The results of the cost-effectiveness analysis are reported here in the form of incremental cost per QALY gained. Costs and outcomes were calculated separately for each treatment alternative and were discounted at 3.5% per annum, in accordance with National Institute of Health and Clinical Excellence (NICE) guidelines [20]. Multiple one-way sensitivity analyses were conducted varying the parameters such as treatment effect, time horizon, patient weight, utility estimates, infliximab administration costs, failure rate for infliximab non-responders and hospitalisation period to assess the variability surrounding the model results.
The uncertainty surrounding other important variables such as outcome probabilities, costs of healthcare resources and health state utilities was explored using probabilistic sensitivity analyses (PSA) with 10,000 simulations. In PSA, the transition probabilities and utility estimates were explored using beta distributions, while costs was varied using normal distribution. The means and standard deviations derived from data sources were used to estimate the distribution parameters.
Results
Cost-effectiveness analyses
The costs and benefits associated with each treatment and the resulting incremental analysis are displayed in Table 5.
Sensitivity analyses
The results of one-way sensitivity analysis are displayed in Table 6. The results of the PSA suggested infliximab to be cost effective, with a willingness to pay as low as £16,000, as displayed in Figs. 3 and 4.
Discussion
Infliximab is an effective rescue strategy for patients with an acute exacerbation of UC [8, 9]. The objective of this analysis was to assess the cost-effectiveness of infliximab treatment at the licensed dose of 5 mg/kg, as a rescue therapy for UC patients hospitalised with an acute exacerbation.
It is important to note that, in the current analysis, we used the full induction dose of infliximab (infusions at week 0, 2 and 6) in patients treated with infliximab to estimate its cost effectiveness. This was based on the licence holders’ (Centocor Inc., http://www.centocor.com/) understanding of infliximab’s licence for UC, which requires a full induction dose to be administered to all responders followed by further treatment at the discretion of the physician even though all the existing trial evidence suggests clinical benefit with a single infusion of infliximab. Therefore, in the analyses we assumed that the effectiveness of a full induction dose of infliximab to be at least as effective as a single infusion of infliximab but with the full cost of three infusions. There is some evidence that a full induction dose of infliximab is likely to be significantly more efficacious compared to a single infusion [21]. On this basis, we argue that the efficacy estimates for infliximab used in these analyses are likely to be conservative.
The adverse effects of the treatment alternatives were excluded from the analyses. There was no information available on the immediate side effects of standard care, and infliximab trials in UC patients (ACT I and II) have indicated a non-significant side effect profile for infliximab. In addition, it was also unclear whether patients receiving just the induction dose of infliximab would suffer side effects to the same extent as patients on scheduled maintenance treatment as in ACT I and II. Ciclosporin has several side effects reported in the literature, and its side effect profile was a major concern for its use in this setting. However, there was no information available on the quality of life impact of these side effects. Therefore, rather than assigning an arbitrary decrement in utility, we selected to exclude the impact of ciclosporin side effects. This may have lead to a significantly conservative incremental quality of life benefit for infliximab and standard care compared to ciclosporin, thus resulting in a conservative ICER.
The results of the cost effectiveness analysis indicated patient weight to be one of the most important parameters affecting ICER. The average patient weight in the health outcomes data repository (HODaR) database for UC patients 6 months following discharge was 73 kg [16]. Therefore, we used a patient weight of 80 kg in our base case analysis. However, the feedback received from clinicians suggested that patients hospitalised with an acute exacerbation tend to weigh significantly less than moderate-to-severe UC patients in an outpatient setting. The above results indicate that with a significant proportion of patients weighing less than 70 kg the cost effectiveness of infliximab can be further improved.
The base case analysis was conducted for a period of 1 year. We selected this timeframe to capture the medium-term (4–12 months) surgery risks as observed in clinical studies. In practice, the acute exacerbation episode is usually finished within the first 3 months following hospitalisation, and therefore we explored the impact of a shorter time horizon in sensitivity analysis by reducing the time horizon to 3 months. Another important parameter affecting ICERs was long-term treatment effects. The sensitivity analysis demonstrated that, even with a constant treatment effect, the ICERs were marginally above the acceptable threshold. It is important to note that this extrapolation is based on a very small sample size in placebo (n = 19), infliximab (n = 17) and ciclosporin (n = 11) treatment arms and therefore the results are subject to a high degree of uncertainty. The long-term follow-up (up to 2 years) to the Jarnerot study also demonstrated that patients avoiding colectomy and achieving remission were likely to maintain remission over the longer term. Therefore, from the sensitivity analysis in clinical practice the true ICERs for long-term follow-up are likely to fall somewhere between constant treatment effect estimates and maximum treatment effect estimates.
The administration cost of infliximab used in the current analysis was £94. This was based on the cost of “consultant-led face-to-face adult follow-up” attendance data in medical gastroenterology, which was considered as an aggregate incorporating all tests, assessments and staffing costs associated with the infusion [15]. In the current analysis, it was assumed that the first infliximab infusion would not incur any additional administration cost as the patient was already hospitalised. Therefore, the administration cost of two additional infusions (£188) was spread over the entire induction dose, resulting in a mean administration cost of £62.66 per infusion. A previous NICE appraisal [22] has used administration costs ranging from £65.02 to £124. Cost variation in this range resulted in ICERs that were well within the acceptable threshold. The other important parameter affecting ICERs was the hospitalisation period following initiation of therapy. We used a mean hospital stay of 7 days based on clinical trial information, UK IBD audit data and clinical expert opinion. The sensitivity analysis suggested that, even with a change of 50% in the estimated hospital stay, infliximab remains cost effective compared to the alternatives. Other parameters such as utility estimates and the complications rate had a much smaller impact on resulting ICERs.
The current analyses have several strengths. Baseline disease progression as well as treatment efficacy was determined using trial evidence. Utilities and costs were estimated using the most appropriate methodologies accepted by the technology appraisal bodies across the world, and uncertainty around them was addressed using multiple sets of estimates derived from the literature as well as by conducting PSAs. All assumptions related to the clinical pathway and treatment alternatives were developed in consultation with a panel of UK gastroenterologists. Where data was not available, all assumptions used were conservative and adversely affect infliximab’s case against alternative treatments.
However, the current analyses also have several limitations. The analysis was based on trial evidence with small sample sizes. This introduces significant uncertainty in the resultant ICERs. We conducted extensive PSA to explore the uncertainty around the results. However, some uncertainty arising out of data limitations could not be addressed. The results therefore are exploratory in nature and should be interpreted with caution. Current and planned trials may be able to address this issue in future [23]. There also was a lack of formal measures of variance and likely distributions of certain model parameters, such as post-surgery complications, to inform PSA. Therefore, not all variables were subjected to PSA. Due to unavailability of resource use estimates for UC patients in the literature, the estimates used in the model were based on expert opinion, which may have introduced bias in the analysis.
In conclusion, infliximab is an effective rescue treatment for UC patients hospitalised with an acute exacerbation and provides significant clinical benefit over treatment alternatives including surgery. This economic analysis demonstrated that the incremental costs associated with achieving these clinical benefits are reasonable, and that induction therapy with infliximab represents a cost-effective treatment option.
References
Carter, M.J., Lobo, A.J., Travis, S.P.: Guidelines for the management of inflammatory bowel disease in adults. Gut 53(5), V1–V16 (2004)
Jarnerot, G., Rolny, P., Sandberg-Gertzen, H.: Intensive intravenous treatment of ulcerative colitis. Gastroenterology 89(5), 1005–1013 (1985)
Truelove, S.C., Jewell, D.P.: Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet 1(7866), 1067–1070 (1974)
Leiper, K., Lowe, D., Driscoll, R., et al.: UK IBD audit 2006: National results for the organisation and process of IBD care in the UK. Published 2007; available at http://www.rcplondon.ac.uk/college/ceeu/ceeu_uk_ibd_audit_2006.pdf
Travis, S., Trange, E., Lemann, A., et al.: European evidence based consensus on the diagnosis and management of ulcerative colitis: current management. J. Crohn’s Colitis 2, 24–62 (2008)
Arts, J., D’Haens, G., Zeegers, M., et al.: Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflam. Bowel Dis. 10, 73–78 (2004)
Sandborn, W.J.: A critical review of cyclosporine therapy in inflammatory bowel disease. Inflam. Bowel Dis. 1, 48–63 (1995)
Jarnerot, G., Hertervig, E., Friis-liby, I., et al.: Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized placebo controlled study. Gastroenterology 128, 1805–1811 (2005)
Sands, B.E., Tremaine, W.J., Sandborn, W.J., Rutgeerts, P.J., Hanauer, S.B., Mayer, L., Targan, S.R., Podolsky, D.K.: Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflam. Bowel Dis. 7(2), 83–88 (2001)
Tsai, H.H., Punekar, Y.S., Morris, J., et al.: A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment. Pharmacol. Ther. 15;28(10), 1230–1239 (2008)
D’Haens, G., Lemmens, L., Geboes, K., et al.: Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology 120(6), 1323–1329 (2001)
Lichtiger, S., Present, D.H., Kornbluth, A., et al.: Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 330(26), 1841–1845 (1994)
Ades, A., Welton, N., Caldwell, D., et al.: Multiparameter evidence synthesis in epidemiology and medical decision-making. J. Health Serv. Res. Policy 13(Suppl 3), 12–22 (2008)
Lunn, D.J., Thomas, A., Best, N., Spiegelhalter, D.: WinBUGS–a Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 10, 325–337 (2000)
Reference costs 2006/07: National schedule of reference costs. Department of Health. January 2008
Woehl, A., Hawthorne, B., Morgan, C., Punekar, Y., McEwan, P.: The epidemiology and healthcare resource use in patients with Crohn’s disease: a population based UK study. Value Health 10(6), A355 (2007)
Dolan, P.: Modeling valuations for EuroQol health states. Med. Care 35(11), 1095–1108 (1997)
Walmsley, R.S., Ayres, R.C.S., Pounder, R.E., Allan, R.N.: A simple clinical colitis activity index. Gut 43(10), 29–32 (1998)
Arseneau, K., Sultan, S., Provenzale, D., et al.: Do patient preferences influence decisions on treatment for patients with steroid refractory ulcerative colitis? Clin. Gastroenterol. Hepatol. 4(9), 1135–1142 (2006)
National Institute of Health and Clinical Excellence (NICE): Guide to the Methods of Technology Appraisal (reference NO515). NICE, London, (2004), http://www.nice.org.uk/niceMedia/pdf/TAP_Methods.pdf
Kohn, A., Daperno, M., Armuzzi, A., et al.: Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment. Pharmacol. Ther. 26(5), 747–756 (2007)
National Institute of Health and Clinical Excellence (NICE). Guidance on the Use of Infliximab for Psoriasis. London: Technology Appraisal Guidance 134, January (2008), http://www.nelm.nhs.uk/en/NeLM-Area/Evidence/Guidelines/NICE-guidance-on-the-use-of-infliximab-for-the-treatment-of-psoriasis/
Laharie, D.: A randomized, multicenter open label study comparing cyclosporine with infliximab in steroid-refractory severe attacks of ulcerative colitis (CYSIF). Clinicaltrials.gov identifier–NCT00542152, http://clinicaltrials.gov/ct2/show/NCT00542152
Acknowledgements
This study was funded in full by Schering-Plough Ltd, and the writing of this paper was funded in full by Schering-Plough Ltd. Writing support was provided by Global Health Solutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Punekar, Y.S., Hawkins, N. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis. Eur J Health Econ 11, 67–76 (2010). https://doi.org/10.1007/s10198-009-0199-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-009-0199-5