Abstract
The increasing presence of aquatic contaminants, such as the pharmaceutical fluoxetine, has raised concerns over potentially disrupting effects on several aspects of fish reproduction. However, the effects of fluoxetine on reproductive and paternal behavior in fish remain understudied, particularly at environmentally relevant concentrations. In the current study, we therefore tested the hypothesis that waterborne fluoxetine at an environmentally relevant concentration (540 ng/l), disrupts specific reproductive and paternal behaviors in male Siamese fighting fish at distinct reproductive phases. A pre-post test design was adopted to investigate specific behavioral responses at the individual fish level in response to male conspecific intruders at two different distances from the nest across four distinct reproductive phases (before bubblenest construction, following bubblenest construction, after spawning and after hatching of the larvae). In the control specimens, the measured behaviours were not different between the spawning times and among the interactions in either distance to nest at the different reproduction phases. Our results indicate that fluoxetine specifically disrupts characteristic paternal territorial aggression behaviour only after spawning and hatching of the larvae, while male behaviour in previous reproductive phases is unaffected by fluoxetine exposure. Results of comparison between males at 1st spawning and specimens exposed to fluoxetine at 2nd spawning showed that the first reaction of the nest-holding males to the intruders, duration of fin spreading, number of bites, and 90° turn, and the frequency of sweeps were different between the spawning times after spawning or hatching of embryos. However, interaction of spawning time and reproduction phase was significant on biting behaviour. These results demonstrate that fluoxetine exposure at environmental concentrations negatively affects territorial defense behaviour in fighting fish during parental care after larval hatching, which may have possible implications on reproductive success and population dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Siamese fighting fish (Betta splendens), like many teleost fish species, exhibit a distinct reproductive and paternal care behavior (Balshine and Sloman 2011). Paternal care in Siamese fighting fish is comprised of several aspects which notably include nest-building and post-spawning territorial defense (Rainwater and Miller 1966). Energetically costly (Haller 1991; Castro et al. 2006; Alton et al. 2013), the paternal aggressive behavior in male Siamese fighting fish represents an investment to protect developing fish and enhance offspring survival (Jaroensutasinee and Jaroensutasinee 2003).
The specific behavioral sequence of reproductive behavior and subsequent paternal care is well described in Siamese fighting fish (Rainwater and Miller 1966; Bronstein 1982). Originally from Thailand, wild male fish construct isolated nests in shallow warm and oxygen-poor water adjacent to rice paddies (Jaroensutasinee and Jaroensutasinee 2001), using pharyngeal organ secretions to form bubbles (Kang and Lee 2010). Upon construction of the nest, males defend the territory against other males through displays of aggressive behaviour, an effect which becomes stronger with increasing residence time (Jaroensutasinee and Jaroensutasinee 2003). Males attract females and induce a spawning sequence to fertilize the eggs and following fertilization; eggs are collected by males and attached to the nest (Rainwater and Miller 1966). Females, as well as male intruders, are subsequently chased from the nest through aggressive displays, which increase after the spawning event has taken place (Clayton and Hinde 1968; Jaroensutasinee and Jaroensutasinee 2003). These behavioral sequences can be observed in laboratory settings (Rainwater and Miller 1966; Bronstein 1982), making the Siamese fighting fish an important model in studying fish reproductive and territorial paternal behavior (Abante 2005).
Using this model, several abiotic and biotic factors have been shown to modulate aggressive behaviors, including aquatic contaminants, such as the mercury (de Matos Mansur et al. 2012) and the pharmaceuticals ethinyl-estradiol (Dzieweczynski and Herbert 2013; Dzieweczynski and Buckman 2013) and fluoxetine (Lynn et al. 2007; Kania et al. 2012; Kohlert et al. 2012). While studies investigating mercury and ethinyl-estradiol describe behavioural effects at environmentally relevant levels (15 ng/l), the studies investigating behavioral effects of waterborne fluoxetine exposures found behavioural effects at concentrations that are typically a magnitude higher than concentrations measured in the aquatic environment (Lynn et al. 2007; Kania et al. 2012; Kohlert et al. 2012), although studies identifying behavioural effects at environmentally relevant concentrations (540 ng/l) exist (Dzieweczynski and Herbert 2012). These apparently variable potencies of fluoxetine (Sumpter et al. 2013), even within the same species, maybe related to confounding experimental factors which have been shown to influence aggressive behavior: Examples include individual variation in aggressive displays (Hebert and Dzieweczynski 2011), the effect of the reproductive state of the animal on aggressive behavior (Jaroensutasinee and Jaroensutasinee 2003), the nature of the stimulus (Figler 1972), and the specific behaviors quantified as indices of aggression (Sumpter et al. 2013). Sumpter and colleagues suggest that is a need to reproduce critical results to better assess the aquatic toxicology of fluoxetine (Sumpter et al. 2013). The current study was designed to systematically investigate the effect of an environmentally relevant concentration of fluoxetine (540 ng/l) on several reproductive and paternal behaviors in individual male Siamese fighting fish across clearly defined reproductive phases. Behavioural endpoints in fish constitute sensitive endpoints to assess contaminant effects, as they integrate environmental stimuli and physiological processes (Scott and Sloman 2004) and affect individual fitness and population dynamics at the ecological level (Soeffker and Tyler 2012).
Fluoxetine is a widely prescribed psychoactive pharmaceutical which belongs to the class of SSRIs (selective serotonin reuptake inhibitors), and is excreted as parent compound or active nor-fluoxetine (de Vane 1999) and subsequently reaches aquatic water systems, as it is not routinely retained by WWTPs (Silva et al. 2012). SSRIs, including fluoxetine and its active metabolite nor-fluoxetine in particular, are pseudo-persistent in rivers and streams receiving wastewater from point sources (Brooks et al. 2003), and reach concentrations in the ng-µg/l range (Brooks et al. 2003; Metcalfe et al. 2010; Schultz et al. 2010). Fluoxetine has furthermore been shown to bioaccumulate in fish in the laboratory (Nakamura et al. 2008; Paterson and Metcalfe 2008) and field studies (Brooks et al. 2005; Ramirez et al. 2007; Metcalfe et al. 2010; Schultz et al. 2010), and a particularly high bioaccumulation of fluoxetine has been observed in the brain (Brooks et al. 2005; Schultz et al. 2010). This has raised concerns regarding effects on fish physiology and behavior (Kreke and Dietrich 2008) and indeed, in addition to the aforementioned behavioural studies, environmental concentrations of fluoxetine have been shown to induce disruption of reproductive physiology (Lister et al. 2009; Mennigen et al. 2010a) and metabolism (Mennigen et al. 2010b) in different fish species. However, behavioural endpoints, in spite of their apparent sensitivity to waterborne fluoxetine remain relatively understudied in fish, particularly at environmentally relevant concentrations (Mennigen et al. 2011). Recently, given the discrepancy of fluoxetine concentrations which elicit behavioral effects in various fish species, a need for replication of studies revealing has behavioural effects has been put forward (Sumpter et al. 2013).
The aim of the present study was to clarify behavioural effects of fluoxetine, which has been used as a model compound to investigate the potential adverse effects of aquatic SSRIs on aquatic organisms (Oakes et al. 2010), on characteristic paternal territorial aggression behaviors in male Siamese fighting fish at an environmentally relevant dose (540 ng/l). Specifically, we exploited the well-characterized reproductive and paternal aggressive behaviors across different reproductive phases in male Siamese fighting fish to assess potentially disruptive effects of fluoxetine on these behaviors.
Materials and methods
Fish
A total of 55 mature male Siamese fighting fish were purchased from a local distributor. Fish were transported to the laboratory and kept individually in 1 l opaque containers 2 weeks prior to experimentation to avoid priming of aggressive behavior (Dzieweczynski and Herbert 2012). The mean weight of fish was 1.71 ± 0.55 g. Fish were maintained at a water temperature of 26 ± 1 °C and under a 12L: 12D photoperiod, conditions optimal for the initiation of nest building. Physiochemical properties of the water used in the experiments were: dissolved oxygen 7.6 mg/l, pH 7.2–7.8, and total hardness 200 mg/l as CaCO3. Containers were maintained with de-chlorinated municipal water, which was changed on every third day. In addition, thirty adult females were purchased from another local distributor. Female fish were kept as groups of 15 fish in two 20 L tanks under otherwise the same conditions as the male containers. All fish were fed to satiety two times daily with 0.9 mm (diameter) commercial pellet and frozen blood worms (Mahiran firm).
Fluoxetine exposure
From the fish stock, 9 and 14 males with bubblenests were selected separately for future spawnings. All of them were initially tested across different reproductive stages without fluoxetine exposure (6d). Subsequently, 9 males were again tested across the same reproductive stages without fluoxetine exposure (‘control’) and the remaining fish (n = 14) tested under fluoxetine-exposure (‘exposed’) for another 6d (nominal concentration of 540 ng/l fluoxetine in spawning test tanks). It should be noted that control tests run recently after the tests of 14 individuals. Fluoxetine was obtained from Pharmaceutical Company of Dr. Abidi; Tehran, Iran. Following the baseline behavioural characterization of fighting fish under control conditions, we achieved a nominal fluoxetine concentration of 540 ng/l as follows: 12.15 µg fluoxetine were added to each tank containing a water volume of 22.5 l by pipetting 100 µl of a stock solution of fluoxetine (1.22 mg fluoxetine in 10 ml of distilled water). We choose this concentration because it has been found in wastewater effluent and therefore represents an environmentally relevant concentration (Fent et al. 2006). Also, the use of only a single exposure of other contaminants has been reported in previous studies (e.g. Monteiro et al. 2009). A pre-post repeated measurement experimental design was chosen in order to test behavioral changes within specific individuals, as previous studies highlighted inter-individual variability in behavioural responses in male Siamese fighting fish and specifically formulated the need to investigate individual level responses in fighting fish exposed to fluoxetine (Dzieweczynski and Herbert 2012). In contrast to previous studies (Lynn et al. 2007; Dzieweczynski and Herbert 2012; Kania et al. 2012; Kohlert et al. 2012), male–male interaction between live conspecifics were considered, as these encounters have been shown to especially elicit the highest degree of territorial aggression in domesticated B. splendens (Robertson and Sale 1975; Bols 1977; Verbeek et al. 2007).
Spawning tank set-up
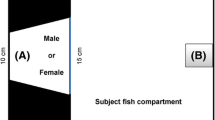
We used seven rectangular glass tanks with dimensions of 45 × 30 × 35 cm for the behavioural studies (Fig. 1). The water height was 20 cm and the bottom of the aquarium was clear. The tanks were equipped with an aquarium heater to keep the temperature at 26 °C, and contained a clay pot to provide a hiding space for females. Two glass sheets (25 × 12 cm) were embedded vertically at the two ends of the tank (designated A and B in Fig. 1) in order to place intruders at different distances to an 8 × 8 cm Styrofoam nest situated slightly under the water surface in the corner of the aquarium (designated C in Fig. 1). This arrangement allowed for the investigation of behavioural responses to intruders at close proximity to the nest (A) as well as to intruders at a larger distance relative to the nest (B), since territorial aggressive behavior in male B. splendens qualitatively depends on distance of the intruder (Bronstein 1982).
Reproductive phases and behaviors
Aggressive behaviors were tested at 4 distinct reproductive phases described hereafter. The same males were tested at these four reproductive phases to allow for detection of intra-individual responses. The first behavioural measurements (after 5–7 h following the male´s introduction to the tank) were performed before the construction of the bubblenest (BB). The second behavioural measurements were made after male fish had constructed their initial bubblenest (AB). The third set of behavioural measurements were made following spawning (AS). To induce spawning activity, a female ready for spawning was introduced to the tank following the second measurements. Female spawning capacity was determined by the presence of a big belly and the appearance of white spots (ovipositors) on their vent. In most cases, the female spawned 1 day after it was paired with a male. After spawning, the female was removed from the tank and a third measurement of behaviors was conducted 1 h after female removal. The final behavioural measurements were performed after hatching of the larvae (AH). For each set of behavioural measurements, the duration of the interaction was 5 min with 10 min intervals between measurements. The conspecific male intruders were first added to box B to simulate an intrusion further away from the nest. Following this, intruders were inserted to box A to measure the male´s behavioral responses towards an intruder near to his nest. This setup was chosen since territorial and aggressive behaviors towards an intruder are inversely correlated to distance (Bronstein 1982). To prevent pseudoreplication of behavioral responses between conspecifics, each intruder was tested only for one interaction (Box B), and a novel intruder was introduced when measuring the response closer to the nest (Box A). However, the same intruders were used at different reproductive phases (first, second, third and fourth measurements). Specifically, the measured behaviors consisted of (1) The response time before the first reaction against intruder, (2) the duration of gill flaring, (3) duration of fin spreading, (4) the number of biting behaviours, (5) the frequency of sweeps to the nest, and (6) the number of 90° turns, all of which have been described as aggressive and territorial behaviors in Siamese fighting fish (Clayton and Hinde 1968; Simpson 1968; Lynn et al. 2007).
Statistics
Data was tested for normality using the Kolmogorov–Smirnov test. Two independent repeated measures analysis of variance (ANOVA) with the Greenhouse-Geisser test were used, one for control treatment and the other for fluoxetine-exposed treatment, by a within subjects factor of the spawning time, 1st spawn and 2nd spawn, for the 6 behavioural changes at the 4 reproductive phases (BB, AB, AS and AH) against conspecific intruders at 2 distances of the nest, away from nest or close to it. Then, behaviour changes against intruders at the same distance to the nest at these 4 reproductive phases among two spawnings were analysed by paired samples t tests. All statistical analyses were conducted using SPSS, ver. 19.
Results
Behaviour changes of the nest-holding males in the control treatment
Insignificant differences were found between 1st and 2nd spawnings for each of the seven types of aggressive acts (Table 1). For the interactions i.e. spawning time × reproduction phase, spawning time × intruder’s distances to the nest, and spawning time × reproduction phase × intruder’s distances to the nest, these had no significant effect on different behaviour changes (Table 1). In addition, paired samples t test showed insignificant effect between 1st and 2nd spawnings at the same intruder’s distances to the nest at the 4 reproduction phases (Fig. 2a–f).
Changes of the 6 territorial behaviours for the same male fighting fish of 9 individuals in the control treatment during the 4 reproductive phases, before bubblenest (BB), after bubblenest (AB), after spawning (AS) and after hatching (AH) against conspecific intruders at two different distances to the nest, away from the nest or close to it, after two spawning activities
Behaviour changes of the nest-holding males after exposure to fluoxetine
Spawning time had significant effect on the first reaction of the nest-holding males to the intruders, duration of fin spreading, biting, sweeping, and 90° turn frequency (Table 2). After exposure to the fluoxetine in the 2nd spawning, gill flaring was only the behaviour which has not been affected. Also, interaction of spawning time × reproduction phase was only significant for the frequency of biting. No significant differences were observed for the other interactions (Table 2). For the paired comparisons of the behaviours between 1st and 2nd spawnings at the same distances of the intruders to the nest, significant differences was found for the gill flaring, fin spreading, frequency of biting, and 90° turn at the late phases of reproduction i.e. after spawning (AS), and after hatching (AH) (Fig. 3a–f). Gill flaring of the nest-holding males against intruders close to the nest at the AS decreased after exposure to fluoxetine (t = 2.284, p = 0.4; Fig. 3b). At the AH close to the nest, the frequency of fin spreading was significantly decreased after fluoxetine exposure (t = 4.098, p = 0.001; Fig. 3c). At the second spawning after exposure to fluoxetine, biting frequency of the nest-holding males significantly decreased at the AS and AH in the both intruder’s distances to the nest (AS-away: t = 2.386, p = 0.033; AS-near: t = 4.087, p = 0.001; AH-away: t = 2.532, p = 0.25; AH-near: t = 2.673, p = 0.19; Fig. 3d). Also, significant reduction was observed at the AS in the near distance to the nest for the frequency of 90° turn behaviour (t = 2.211, p = 0.046; Fig. 3f).
Changes of the 6 territorial behaviours for the same male fighting fish of 14 individuals in the fluoxetine-exposed treatment during the 4 reproductive phases, before bubblenest (BB), after bubblenest (AB), after spawning (AS) and after hatching (AH) against conspecific intruders at two different distances from the nest, away from the nest or close to it, after two spawning activities. *p < 0.05; **p < 0.01
Discussion
The pharmaceutical fluoxetine has emerged as an aquatic contaminant of concern, and several studies have shown effects on fish reproduction, especially with respect to physiology (Mennigen et al. 2011). Our study provides evidence that fluoxetine can disrupt aggressive paternal care behaviour in individual fighting fish at specific reproductive phases at an environmentally relevant concentration of 540 ng/l. Our study found that nest-holding male fighting fish display an increase in aggressive behaviour following the spawning event, evident in terms of increased biting events towards an intruder, irrespective of the intruders distance to the nest. This observation confirms previous observations (Clayton and Hinde 1968; Jaroensutasinee and Jaroensutasinee 2003). Conversely, sweeping behaviour was more frequently observed when intruders were farther to the nest, suggesting that fighting fish can discriminate between intruders close to the nest and further away from the nest.
In our study, fluoxetine affected some measures of stereotyped aggression but not all measures. Following exposure of fluoxetine at 540 ng/l, the aggressive behaviour was suppressed after spawning and after hatching, but not in other measured reproductive phases. The most evident behaviour was the biting behaviour, which did not increase from baseline levels following spawning when fish were exposed to fluoxetine. Similarly, a reduction of gill flaring, fin spreading and 90 degree turns was observed following spawning after exposure to fluoxetine. Gill flaring and fin spreading are the main aggressive behaviours in fighting fish (Dzieweczynski and Leopard 2010). In contrast to the biting behaviour, these responses were suppressed by fluoxetine only when the intruder was placed closer to the nest, suggesting a specific suppression of nest territorial defense behaviour following spawning.
Our study confirms recent studies suggesting disruption of male aggressive behaviour during the paternal care period in B. splendens species at environmentally relevant concentrations of fluoxetine. In a previous study, it was reported that fluoxetine generally decreased aggressive behaviour of Thalassoma bifasciatum (Perreault et al. 2003). Similarly in fighting fish, fluoxetine has been shown to reduce aggressive behaviour when injected i.p. at a dose of 40 µg per day for the duration of 2 weeks (Kania et al. 2012). In waterborne exposures, concentrations of 350 and 705 µg/l fluoxetine decreased aggressive and locomotor behaviours, an effect which persisted 13d after removal of fluoxetine (Kohlert et al. 2012). In a short term waterborne fluoxetine exposure of fighting fish, inhibition of 90° turns and time spent in broadside were observed at a concentration of 3 ug/l fluoxetine (Lynn et al. 2007). In addition, previous study by Clotfelter and Paolino (2003) showed when a male fighting fish that allowed observing aggressive contests between pairs of male conspecific (‘bystander’) perform more aggression than a male observed an empty tank. Therefore in the present study, use of the control treatment allowed us to compare behavioural differences between nest-holding males after exposure to fluoxetine and the males without fluoxetine exposure at the 2nd spawning.
Evidence suggests that aggressive and reproductive behaviour in fish is modulated by neuroendocrine mechanisms, including the neurotransmitter serotonin (Somoza and Peter 1991), the neuropeptides vasotocin and isotocin (Foran and Bass 1999), and the sex steroid testosterone (Smith 1969). As in other teleost fish, the telencephalon and hypothalamic area appear to mediate aggressive behavior in B. splendens (Santangelo and Bass 2006). Injection of 5-HT or the 5HTR1A agonist OH-DPAT decreased aggression in fighting fish (Clotfelter et al. 2007). Indeed, fluoxetine and other SSRIs pharmacologically act to increase 5-HT in the synaptic cleft by inhibiting the selective serotonin reuptake. However, future studies should measure 5-HT turnover in the brain, especially since lower, waterborne concentrations of fluoxetine resulted in a paradoxical decrease in 5-HT in hybrid striped bass, that was correlated with the disruption of prey catching behaviour (Gaworecki and Klaine 2008; Clotfelter et al. 2007). An additional neuroendocrine target associated with a role in aggressive and reproductive behaviours are the neuropeptides vasotocin and isotocin. A higher abundance of vasotocin in the preoptic area has been associated with increased aggressive behaviour in several fish, including the highly territorial damselfish Stegastes leucostictus (Santangelo and Bass 2006). Isotocin has been shown to be involved in submissive behaviour in Neolamprologus pulcher, a cichlid fish that establishes dominant-subordinate relationships (Reddon et al. 2012), facilitating social approach behaviour in goldfish (Thompson and Walton 2004) and in promoting paternal care in Amatitlania nigrofasciata, a monogamous cichlid fish (O’Connell et al. 2012). Interestingly, isotocin and vasotocin have been shown to be modulated by fluoxetine in different fish species. Pharmacological injections of fluoxetine reduced vasotocin expression in the preoptic area of male wrasses Thalassoma bifasciatum, which correlated with a decrease in aggressive behaviour (Semsar et al. 2004). Similarly, injections of fluoxetine reduced the expression of isotocin in hypothalamus and telencephalon of female goldfish (Mennigen et al. 2008). Therefore, especially given the diversity of neuropeptide effects in the class of teleost species (Godwin and Thompson 2012), future studies should investigate the potential involvement of neuropeptides in the observed behavioural effects of during paternal care in fighting fish and their disruption by fluoxetine.
In conclusion, the results of the present demonstrate that environmentally relevant concentration of fluoxetine can disrupt reproductive and aggressive behaviors in male fighting fish during the parental care period following the spawning event. This is of importance, since disruption of reproductive behaviour is ecologically relevant in that it contributes to species survival. Given the detection of fluoxetine and SSRIs in aquatic systems at concentrations of up to 3 ug/l, comparative studies on fish behaviour are warranted. Determination of neuroendocrine mechanism and targeted dose response studies of behavior and neuroendocrine endpoints on the one hand are warranted to determine the molecular mode of action. Field studies and population studies are warranted to determine ecological meaning of these behavioural effects.
References
Abante ME (2005) Using a popular pet fish species to study territorial behaviour. J Biol Educ 39(2):81–86
Alton LA, Portugal SJ, White CR (2013) Balancing the competing requirements of air-breathing and display behaviour during male–male interactions in siamese fighting fish Betta splendens. Comp Biochem Physiol A 164:363–367
Balshine S, Sloman K (2011) Parental care in fishes. In: Farrell AP (ed) Encyclopedia of fish physiology: from genome to environment, vol 1. Academic Press, San Diego, pp 670–677
Bols RJ (1977) Display reinforcement in the Siamese fighting fish, Betta splendens: aggressive motivation or curiosity? J Comp Physiol Psychol 91(2):233–244
Bronstein PM (1982) Breeding, paternal behaviour, and their interruption in Betta splendens. Anim Learn Behav 10(2):145–151
Brooks BW, Foran CM, Richards SM, Weston J, Turner PK, Stanley JK, Solomon KR, Slattery M, La Point TW (2003) Aquatic ecotoxicology of fluoxetine. Toxicol Lett 142:169–183
Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ (2005) Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem 24(2):464–469
Castro N, Ros AFH, Becker K, Oliveira RF (2006) Metabolic costs of aggressive behaviour in the Siamese fighting fish, Betta splendens. Aggr Behav 32:474–480
Clayton FL, Hinde RA (1968) The habituation and recovery of aggressive display in Betta splendens. Behaviour 30:96–106
Clotfelter ED, Paolino AD (2003) Bystanders to contests between conspecifics are primed for increased aggression in male fighting fish. Anim Behav 66(2):343–347
Clotfelter ED, O’Hare EP, McNitt MM, Carpenter RE, Summers CH (2007) Serotonin decreases aggression via 5-HT1A receptors in the fighting fish, Betta splendens. Pharmacol Biochem Behav 87:222–231
De Matos Mansur B, Calvavcante S, Rodriguez dos Santos B, Gouveia A (2012) Effects of mercury chloride (HgCl2) on Betta splendens aggressive display. Spanish J Psychol 15(1):442–450
de Vane CL (1999) Metabolism and pharmacokinetics of selective serotonin reuptake inhibitors. Cell Mol Neurobiol 19(4):443–466
Dzieweczynski TL, Buckman CM (2013) Acute exposure to 17α-ethinylestradiol disrupts audience effects on male–male interactions in Siamese fighting fish, Betta splendens. Horm behave 63:497–502
Dzieweczynski TL, Herbert OL (2012) Fluoxetine alters behavioural consistency of aggression and courtship in male Siamese fighting fish, Betta splendens. Physiol Behav 107:92–97
Dzieweczynski TL, Herbert OL (2013) The effects of short-term exposure to an endocrine disruptor on behavioural consistency in male juvenile and adult Siamese fighting fish. Arch Environ Contam Toxicol 64(2):316–326
Dzieweczynski TL, Leopard AK (2010) The effects of stimulus type on consistency of responses to conflicting stimuli in Siamese fighting fish. Behav Proc 85(2):83–89
Fent K, Westron AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76:122–159
Figler MH (1972) The relation between eliciting stimulus strength and habituation of the threat display in male Siamese fighting fish, Betta splendens. Behaviour 42:63–96
Foran CM, Bass AH (1999) Preoptic GnRH and AVT: axes for sexual plasticity in teleost fish. Gen Comp Endocrinol 116(2):141–152
Gaworecki KM, Klaine SJ (2008) Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat Toxicol 88(4):207–213
Godwin J, Thompson R (2012) Nonapeptides and social behavior in fishes. Horm Behave 61(3):230–238
Haller J (1991) Biochemical cost of a fight in fed and fasted Betta splendens. Physiol Behav 49:79–82
Hebert OL, Dzieweczynski TL (2011) Influence of prior exposure to females on behavioral consistency in male Siamese fighting fish. Behaviour 148(14):1473–1489
Jaroensutasinee M, Jaroensutasinee K (2001) Bubble nest habitat characteristics of wild Siamese fighting fish. J Fish Biol 58(5):1311–1319
Jaroensutasinee M, Jaroensutasinee K (2003) Type of intruder and reproductive phase influence male territorial defence in wild-caught Siamese fighting fish. Behav Proc 64(1):23–29
Kang CK, Lee TH (2010) The pharyngeal organ in the buccal cavity of the male Siamese fighting fish, Betta splendens, supplies mucus for building bubble nests. Zool Sci 27(11):861–866
Kania BF, Gralak MA, Wielgosz M (2012) Four week fluoxetine (SSRI) exposure diminishes aggressive behaviour of male Siamese fighting fish (Betta splendens). J Behav Brain Sci 2:185–190
Kohlert JG, Mangan BP, Kodra C, Drako L, Long E, Simpson H (2012) Decreased aggressive and locomotor behaviours in Betta splendens after exposure to fluoxetine. Psychol Rep 112(1):51–62
Kreke N, Dietrich DR (2008) Physiological endpoints for potential SSRI interactions in fish. Crit Rev Toxicol 38(3):215–247
Lister A, Regan C, Van Zwol J, Van Der Kraak G (2009) Inhibition of egg production in zebra fish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat Toxicol 95:320–329
Lynn SE, Egar JM, Walker BG, Sperry TS, Ramenofsky M (2007) Fish on Prozac: a simple, noninvasive physiology laboratory investigating the mechanisms of aggressive behavior in Betta splendens. Adv Physiol Educ 31:353–363
Mennigen JA, Martyniuk CJ, Crump K, Xiong H, Zhao E, Popesku J, Anisman H, Cossins AR, Xia X, Trudeau VL (2008) Effects of fluoxetine on the reproductive axis of female goldfish (Carassius auratus). Physiol Genom 35(3):273–282
Mennigen JA, Lado WE, Zamora JM, Duarte-Guterman P, Langlois VS, Metcalfe CD, Chang JP, Moon TW, Trudeau VL (2010a) Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus. Aquat Toxicol 100(4):354–364
Mennigen JA, Sassine J, Trudeau VL, Moon TW (2010b) Effects of waterborne fluoxetine on food intake, weight and energy metabolism parameters in goldfish, Carassius auratus. Aquat Toxicol 100:128–137
Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL (2011) Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac. J Toxicol Environ Health Part B 14(5–7):387–412
Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, Andrews DM (2010) Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ Toxicol Chem 29(1):79–89
Monteiro DA, Rantin FT, Kalinin AL (2009) The effects of selenium on oxidative stress biomarkers in the freshwater characid fish matrinxã, Brycon cephalus (Günther, 1869) exposed to organophosphate insecticide Folisuper 600 BR® (methyl parathion). Compar Biochem Physiol Part C 149(1):40–49
Nakamura Y, Yamamoto H, Sekizawa J, Kondo T, Hirai N, Tatarazako N (2008) The effects of pH on fluoxetine in Japanese medaka (Oryzias latipes): acute toxicity in fish larvae and bioaccumulation in juvenile fish. Chemosphere 70(5):865–873
Oakes KD, Coors A, Escher BI, Fenner K, Garric J, Gust M, Knacker T, Kuster A, Kussatz C, Metcalfe CD, Monteiro S, Moon TW, Mennigen JA, Parrot J, Pery A, Ramil M, Roennefahrt I, Tarazona JW, Sanchez-Arguello P, Ternes TA, Trudeau VL, Boucard T, Van Der Kraak GJ, Servos MR (2010) Environmental risk assessment for the serotonin re-uptake inhibitor fluoxetine: case study using the European risk assessment framework. Integr Environ Assess Manag 6:524–539
O’Connell LA, Matthews BJ, Hofmann HA (2012) Isotocin regulates paternal care in a monogamous cichlid fish. Hormon behave 61(5):725–733
Paterson G, Metcalfe CD (2008) Uptake and depuration of the anti-depressant fluoxetine by the Japanese medaka (Oryzias latipes). Chemosphere 74(1):125–130
Perreault HA, Semsar K, Godwin J (2003) Fluoxetine treatment decreases territorial aggression in a coral reef fish. Physiol Behav 79(4):719–724
Rainwater FL, Miller RJ (1966) Courtship and reproductive behavior of the Siamese fighting fish, Betta splendens Regan (Pisces, Belontiidae). Proc Okla Acad Sci For 98–114
Ramirez AJ, Mottaleb MA, Brooks BW, Chambliss CK (2007) Analysis of pharmaceuticals in fish using liquid chromatography-tandem mass spectrometry. Anal Chem 79(8):3155–3163
Reddon AR, O’Connor CM, Marsh-Rollo SE, Balshine S (2012) Effects of isotocin on social responses in a cooperatively breeding fish. Anim Behav 84:753–760
Robertson CM, Sale PF (1975) Sexual discrimination in the Siamese fighting fish (Betta splendens Regan). Behaviour 54:1–25
Santangelo N, Bass AH (2006) New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proc R Soc B 273(1605):3085–3092
Schultz MM, Furlong ET, Kolpin DW, Werner SL, Schoenfuss HL, Barber LB, Blazer VS, Norris DO, Vajda AM (2010) Antidepressant pharmaceuticals in two US effluent-impacted streams: occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ Sci Technol 44:1918–1925
Scott GR, Sloman KA (2004) The effect of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68(4):369–392
Semsar K, Perreault HA, Godwin J (2004) Fluoxetine-treated male wrasses exhibit low AVT expression. Brain Res 1029(2):141–147
Silva LJ, Lino CM, Meisel LM, Pena A (2012) Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: an ecopharmacovigilance approach. Sci Total Environ 437:185–195
Simpson MJA (1968) The display of the Siamese fighting fish, Betta splendens. Anim Behav Monog 1:1
Smith RJF (1969) Control of pre-spawning behaviour of sunfish (Lepomis gibbosus and L. megalotis) I. Gonadal androgens. Anim Behav 17:279–285
Soeffker M, Tyler C (2012) Endocrinre disrupting chemicals and sexual behaviours in fish- a critical review on effects and possible consequences. Crit Rev Toxicol 42(8):653–668
Somoza GM, Peter RE (1991) Effects of serotonin on gonadotropin and growth hormone release from in vitro perifused goldfish pituitary fragments. Gen Comp Endocrinol 82(1):103–110
Sumpter JP, Donnachie RL, Johnson AC (2013) The apparently very variable potency of the anti-depressant fluoxetine. Aquat Toxicol 151:57–60
Thompson RR, Walton JC (2004) Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav Neuro 118:620–626
Verbeek P, Iwamoto T, Murakami N (2007) Differences in aggression between wild-type and domesticated fighting fish are context dependent. Anim Behav 73(1):75–83
Acknowledgments
We are grateful to aquaculture Department of University of Tehran for providing us with the laboratory space to perform these experiments. This work was partly funded by university of Tehran. Many thanks to M. Hedayati rad for her support during the study. We would also like to thank Dr. J. Mennigen for his useful comments and kind assistance. We thank the editor and 2 anonymous reviewers for their constructive and insightful comments.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forsatkar, M.N., Nematollahi, M.A., Amiri, B.M. et al. Fluoxetine inhibits aggressive behaviour during parental care in male fighting fish (Betta splendens, Regan). Ecotoxicology 23, 1794–1802 (2014). https://doi.org/10.1007/s10646-014-1345-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1345-0