Abstract
Agonistic behavior involves the displays that arise when conspecifics compete for valuable resources such as territory. After conflict resolution, dominants obtain priority access to the resource while subordinates lose it. We aimed to evaluate how agonistic encounters mediate the acquisition of different sized territories in the weakly electric fish, Gymnotus omarorum, a species that displays a well-documented non-breeding agonistic behavior very unusual among teleosts. When tested in intrasexual and intersexual dyads in small arenas, a sex-independent dominant-subordinate status emerged after highly aggressive contests in which subordinates signaled submission by retreating and emitting submissive electric signals. We staged dyadic agonistic encounters in a large arena, in which the initial interindividual distance resembled the one observed in nature. We observed the emergence of a dominant-subordinate status after longer but milder contests with rare electric signaling of submission. We found the persistence of dominance over time with no outcome reversion. We observed how dominants exclude subordinates from their conquered resource during all the recording time. Although the territorial behavior of Gymnotus has been put forth since pioneer reports, this is the first study to show how agonistic behavior depends on the territory size in this genus. Agonistic encounters of G. omarorum in the small arena resemble the characteristics of violent-like behaviors. The ease of shifting from mild to high levels of aggression due to confinement, together with the use of electrical signaling of submission, makes this species an excellent model to explore new perspectives in territoriality assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal conflicts arise when conspecifics compete for different valuable resources (space, food, mates, shelters, breeding sites, etc.), whose control increases their individual fitness (Lorenz 1963; King 1973; Hungtinford and Turner 1987; Briffa and Hardy 2013). In dyadic agonistic interactions, conflicts are resolved when one individual obtains priority access to the resource (dominant) while the other contender loses it (subordinate) (Nelson 2006; Briffa and Sneddon 2010). Though the behavioral traits displayed during contests might be extremely diverse across species, agonistic encounters often follow three phases: evaluation (pre-contest), contest, and post-resolution, with overt aggression usually occurring during the contest phase (Summers and Winberg 2006).
When space is the resource animals compete for, territory is the area from which intruders are excluded by some combination of advertisement, threat, and/or attack (Brown 1975). As a form of social dominance, territoriality is often mediated by agonistic encounters between conspecifics (Wilson 1975; Kaufmann 1983). It is well-known in many vertebrates that reproductive males (and also male-female dyads) usually defend territories and prevent the intrusion of competitors during the breeding season (Brown 1964; Clarke 1970; Davies 1976; Armitage 1977; Bakker and Sevenster 1983; Pröhl 2005; Huang et al. 2011). Less frequently, when space itself is the resource animals fight for, territorial defense can also be observed in males and females all year round in several species independently of gonadal hormones (Caldwell et al. 1984; Wingfield and Hahn 1994; Chiver et al. 2014). In these cases, the defense of territories may ensure the access to foraging areas across seasons (Black-Cleworth 1970). In addition, in some species, increasing the distance from one’s nearest neighbors may give added protection from predators (Kaufmann 1983).
Population density is a well-known factor in determining the strength of intraspecific competition; as density increases (by increasing the number of individuals in a given space, or by space confinement), the rate at which animals interact with competitors obviously increases (King 1973; Kokko and Rankin 2006; Knell 2009). This general rule has been empirically confirmed in a wide variety of animals in which crowding generally increases aggressive behavior (Hazlet 1968; Alexander and Roth 1971; Turner et al. 2000; Buchwalder and Huber-Eicher 2004; Oldfield 2011). In particular, when the size of the territory was experimentally manipulated, an increase in intraspecific aggression was observed both in turkeys (Buchwalder and Huber-Eicher 2004) and Midas cichlids (Oldfield 2011).
South American freshwater weakly electric fish produce an electric organ discharge (EOD) commanded by a very well-known electromotor circuit (Stoddard 2002; Caputi et al. 2005), and shaped by their body into an asymmetric dipole-like electric field (Assad et al. 1999; Caputi and Budelli 2006; Pedraja et al. 2014). By means of this active electrosensory channel, electric fish can locate objects whose electrical properties differ from those of the surrounding water (electrolocation; Lissman 1958), and also communicate with conspecifics (electrocommunication; Hopkins 1972). In particular, fish can obtain important information of both the environment (territory quality) and the fighting ability of their contenders by information encoded in their EODs (Gómez-Sena et al. 2014; Pedraja et al. 2016). In addition, the EOD carries information about an individual’s species identity, sex, and physiological state, coded both by the rate and waveform of the EOD (Caputi et al. 2005). Thus, in any given motor behavior, electric fish display not only locomotor traits but also conspicuous social electric signals. Many studies have reported distinctive agonistic electric displays (either produced by dominants or subordinates) in several species of South American freshwater electric fish (Black-Cleworth 1970; Westby 1975a, b; Hagedorn and Zelick 1989; Hupé and Lewis 2008; Hupé et al. 2008; Triefenbach and Zakon 2008; Perrone et al. 2009; Batista et al. 2012; Perrone and Silva 2016, 2018).
The weakly electric fish, Gymnotus omarorum, displays a well-documented non-breeding agonistic behavior very unusual among teleosts (Batista et al. 2012; Silva et al. 2013; Jalabert et al. 2015; Zubizarreta et al. 2015; Quintana et al. 2016). When gonads are regressed, and no reproductive motivation is expected to drive competition, males and females, tested in dyadic encounters in confined arenas, fiercely compete for space in intrasexual and intersexual encounters. Under these experimental conditions, subordinates G. omarorum signal submission by both retreating and emitting submissive electric signals. The cessation in the emission of electric signals (offs) has been interpreted as an initial submissive signal (Hopkins 1974; Westby 1975a; Hagedorn and Carr 1985; Zakon et al. 1991; Triefenbach and Zakon 2008; Fugère et al. 2011); chirps (brief, transient EOD modulations) have been described as late and more unambiguous signals of submission (Batista et al. 2012; Quintana et al. 2016) and an EOD rate rank between dominants and subordinates becomes evident immediately after contest resolution (Silva et al. 2013; Perrone and Silva 2018). The robustness and reliability of the agonistic behavior of G. omarorum in these laboratory conditions make this species an advantageous model system to contribute to the understanding of the neuroendocrine control of aggression (Zubizarreta et al. 2012; Silva et al. 2013; Perrone and Silva 2018). Recent field preliminary observations of G. omarorum spacing in the wild suggest territoriality (L. Zubizarreta, personal communication). In the non-breeding season, adult males and females G. omarorum rest more than 1 m apart from each other in the natural habitat. Although it is indisputable that in previously reported dyadic contests, individuals of G. omarorum compete for space, how agonistic encounters actually mediate territoriality remains unexplored in this species. Territoriality entails, by definition, the persistence of the dominant-subordinate status over time and the demonstration that the dominant proactively excludes the subordinate from the defended territory. Previous studies did not test neither the persistence of the hierarchy nor the exclusion of the subordinate except for a short time (10 min) after resolution. In addition, interindividual distance in the wild is approximately twice larger than the pre-contest interindividual distance of previous reports (L. Zubizarreta, personal communication). Therefore, it also remains unexplored if the size of the space animals compete for influences the characteristics of their agonistic behavior.
In this study, we aimed to demonstrate the territorial behavior of G. omarorum in laboratory settings by evaluating how spatial context impacts on the agonistic behavior of this species. We staged dyadic agonistic encounters using a large arena, in which the initial interindividual distance resembled the one observed in nature. We were thus able to demonstrate (a) the emergence of a clear dominant-subordinate status mediated by longer but milder contests in which electric submission signals are seldom observed, (b) the persistence of dominance over time with no outcome reversion, and (c) how dominants hold territory after contest resolution and exclude subordinates from their conquered resource.
Materials and methods
We used 42 non-breeding adult G. omarorum (Richer-de-Forges et al. 2009) that ranged from 15 to 27 cm in body length and 9 to 52 g in body weight. Sex in G. omarorum is not externally apparent (neither morphologically nor electrophysiologically) and was determined either after the behavioral experiments (experiment 1) or before (experiment 2, in which only males were used) by gonadal inspection (Jalabert et al. 2015).
Gymnotus omarorum were collected using a fish detector as described elsewhere (Silva et al. 2003) in Laguna del Sauce (34° 51′ S, 55° 07′ W, Department of Maldonado, Uruguay), and housed in individual compartments in 500-l outdoor tanks separated by an electrically transparent mesh for at least 15 days before the behavioral experiments. All environmental variables were kept within the normal range exhibited in the natural habitat in the non-breeding season. Water temperature ranged from 8 to 21 °C, and natural photoperiod ranged from LD10:14 to LD11:13. Water conductivity was adjusted and always maintained below 200 μS/cm by the addition of deionized water. Aquatic plants (Eichhornia crassipes, Pistia stratiotes, Salvinia sp.) covered the surface of the water and provided shelter for the fish. Fish were fed with Tubifex tubifex once a week.
Electric fish collection for experimental purposes was authorized by DINARA (National Direction of Aquatic Resources) and MGAP (Ministry of Agriculture and Fisheries), resolution No. 065/2004. All experimental procedures complied with ASAP/ABS Guidelines for the Use of Animals in Research and were approved by our institutional ethical committee (Comisión Bioética, Instituto Clemente Estable, MEC, 007/05/2012).
Laboratory settings
Fish were placed in an experimental tank that allowed simultaneous video and electric recordings as described elsewhere (Silva et al. 2007). Briefly, the electric signals of freely moving fish were detected by two pairs of orthogonal fixed electrodes attached to each tank wall, connected to two high-input impedance amplifiers (FLA-01, Cygnus Technologies Inc.). We used two types of experimental tanks: (a) the small arena, four 30-l glass aquaria (55 × 40 × 25 cm as described in (Batista et al. 2012); and (b) the large arena, one 120-l glass aquaria (110 × 80 × 25 cm, as described in (Pedraja et al. 2016). The day-night cycle and the physicochemical parameters (water temperature, conductivity, and pH) of indoor tanks matched those of the outdoor housing tanks. All the experiments were performed in total darkness illuminated by an array of infrared LEDs (L-53F3BT, Fablet & Bertoni Electronics) located above the tank. Weakly electric fish are not sensitive to infrared light (Ciali et al. 1997), and IR illumination has become the standard method to eliminate visual influences during behavioral testing (Maciver et al. 2001; Roth et al. 2011; Batista et al. 2012; Zubizarreta et al. 2015; Jun et al. 2016; Pedraja et al. 2018). An infrared-sensitive video camera (SONY CCD-Iris and RoHS CCD Digital Video Camera) was focused on the bottom of the tank. Images and electric signals were captured on a video card (EasyCap) and stored in the computer for analysis. The fish remained in the recording tank at constant temperature (16–20 °C) for 4–5 h before the experiments.
Behavioral experimental procedures
All behavioral experiments were performed during the non-breeding season (occurring during the Austral fall-winter time, May–July) of 2016 (experiment 1) and of 2017 (experiment 2) to avoid any other type of agonistic interactions related to reproduction, which normally occurs from November to February. We tested dyadic agonistic interactions of G. omarorum in experimental conditions in which space is the only resource that individuals fight for, providing symmetric resources and resource values for both contestants: equally-sized plain tanks, same residence time, and the same previous experience (Batista et al. 2012). In all cases, animals were kept in their individual housing compartments with no physical contact with conspecifics for at least 15 days before the behavioral experiment. We used dyads whose body weight difference ranged from 7 to 36% (n = 21), which allowed us to predict the contest outcome (Batista et al. 2012; Pedraja et al. 2016). Contest resolution was established when we observed the third consecutive retreat of one fish without attacking back (Batista et al. 2012; Pedraja et al. 2016).
Experiment 1
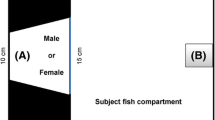
To test the effect of territory size on the establishment of the dominant-subordinate status, we recorded the agonistic behavior of G. omarorum in similar conditions in both the small and the large arenas (Fig. 1A). As originally described in both contexts (Batista et al. 2012; Pedraja et al. 2016), we used indistinctively intrasexual and intersexual adult dyads (small arena n = 6; large arena n = 8). In all cases, a removable glass gate was raised 5–10 min after artificial sunset, and fish were separated 10 min following conflict resolution. While in the small arena fish were freely moving in each compartment prior to the contest, 3 plastic partitions ensured that fish were separated by more than 100 cm in the large arena before the agonistic encounter (Fig. 1A).
Experimental design. A Experiment 1. Each fish is placed in one separate compartment (a). In the large arena, 3 plastic partitions are used to separate fish. Five min after the light is turned off, the gate is removed (b) and the agonistic encounter begins. Post-resolution phase starts after conflict resolution (gray circle) and has an arbitrary duration of 10 min. B Experiment 2. (a) and (b) as in A in the large arena. (c) the post-resolution phase is recorded for 36 h after contest resolution. A central shelter was added to enrich territory value
Experiment 2
To test the maintenance of the dominant-subordinate status and dominants’ territorial defense over time, we performed a different set of dyadic encounters in the large arena prolonging the recording of the agonistic behavior of G. omarorum up to 36 h after contest resolution, and enriched the resource value of the territory by adding one shelter in the middle of the arena (n = 7 male-male dyads; Fig. 1B). In order to identify unambiguously both contenders in the video recordings, potential subordinates were marked with a slight cut in the anal fin (1–2 mm long), previous to the agonistic encounter. We have used this slight cut as a routine marking procedure for years after confirming it is harmless for the fish and does not alter its behavior. Similarly, as described above, fish were isolated before the contest in opposite corners of the arena by plastic partitions, which were removed (together with the medial glass gate) 5–10 min after artificial sunset to allow the physical interaction between individuals. The locomotor and electric displays of the agonistic behavior of G. omarorum were continuously recorded for 30 min after gate removal, and then recorded in samples of 2 min each 30 min during the following 35 h.
Behavioral data processing
Locomotor displays
In both experiments, we analyzed the locomotor displays of the tested individuals to identify the 3 phases of the agonistic encounter following Batista et al. (2012): (a) evaluation phase (pre-contest): from time 0 (gate removal) to the occurrence of the first attack; (b) contest phase: from the first attack to conflict resolution (resolution time); and (c) post-resolution phase (post-contest), which was recorded for 10 min after conflict resolution in experiment 1, and for 30 min in experiment 2 (early post-resolution, EPR). We measured the following locomotor parameters in all the experiments (experiment 1 in both the small and large arenas, and experiment 2): latency to the first attack, contest duration, and contest attack rate (number of attacks/contest duration in seconds) of dominants and subordinates. In experiment 1, we measured post-resolution attack rate of dominants as the number of attacks/600 s, and post-resolution retreat rate of dominants and subordinates as the number of retreats/600 s. In experiment 2, we measured post-resolution attack rate and retreat rate of dominants and subordinates as the number of attacks or retreats per min performed in the EPR (30 min after resolution). We also measured the number of attacks per min, the number of retreats per min, the position of contenders, and the shelter occupancy of both the dominant and the subordinate in 2-min samples each 30 min during approximately 35 h (late post-resolution, LPR). We calculated an index of shelter occupancy as the number of samples in which either the dominant or the subordinate were found inside the shelter divided by the total number of samples. We calculated a territory access index using ordinal scores depending on the position of each individual with respect to the shelter in all the samples as follows: score 5 (inside the shelter), score 3 (inside a circle whose diameter was twice the shelter length and was centered in the middle of the shelter), and score 1 (beyond this circle). The maximum score for each 2-min sample was used as the representative score sample value, and the mean value of all these scores was used as the territory access index for each individual.
Electric signals
EOD rate was calculated as the mean instantaneous frequency in 5–10-s samples obtained from the evaluation and post-resolution phases. In all the experiments, the EOD rate change index was calculated as ((EOD rate in the post-resolution phase)-(EOD rate in the evaluation phase))/(EOD rate in the evaluation phase) in percentage. Positive values of the index represent an increase in the EOD rate, and negative values of the index a decrease in the EOD rate in the post-resolution phase.
We measured the occurrence and timing of offs (interruptions of EOD emission) and chirps (transient increases in EOD rate with waveform distortion). We calculated first off and first chirp latency as the time to first off/chirp minus the time of occurrence of the first attack. As EOD cessations are observed in both the contest and post-resolution phase (Batista et al. 2012; Quintana et al. 2016), we calculated off rate as follows: (number of offs during contest + post-resolution phase) divided (contest duration + 600 s, the arbitrary recorded duration of the post-resolution phase). As chirps are late submissive electric displays mostly observed after contest resolution (Batista et al. 2012; Quintana et al. 2016), we calculated chirp rate by dividing the number of post-resolution chirps by 600 s. As no submissive electric signals were ever observed after the initial establishment of the dominant-subordinate status, these signals were not evaluated after the first 600 s post-resolution in experiment 2.
Statistics
All data were analyzed by non-parametric tests using the software GraphPad Prism (v 7-2016): Mann-Whitney U test (independent variables using sets of data from different fish) for comparing dominants versus subordinates, and small versus large arenas. We used chi-square tests 2 × 2 (χ2) to verify if the big fish wins in the same proportion in both conditions (large arena versus small arena).
Results
All dyads of non-breeding G. omarorum tested (small and large arenas) displayed agonistic behavior shortly after the gate was removed, which ended with the establishment of a clear dominance-subordination status within few minutes in all cases (Fig. 2; less than 5 min in the small arena, n = 6; around 12 min in the large arena, n = 15). All the agonistic encounters also followed the typical 3 phases (pre-contest, contest, post-resolution); and in most cases (6 out of 6 and 13 out of 15 in the small and the large arena, respectively), the larger fish resulted the dominant (Table 1).
Time structure of agonistic encounter in experiment 1. Agonistic behavior has three different stages: evaluation phase, from time 0 (gate removal, b) to the occurrence of the first attack; contest phase from the occurrence of the first attack to conflict resolution; and post-resolution phase. During the contest and post-resolution phase, conspicuous electric signals are observed. The duration of each phase and the latencies of motor and electrical displays are represented by the mean values (small arena n = 6; large arena n = 8). A Small arena. B Large arena
Experiment 1
Although G. omarorum engaged in dyadic agonistic interactions that reached the establishment of the dominance-subordination status in both arenas, we observed important differences between them in the time structure of the agonistic behavior and in its levels of both aggression and subordination (Fig. 2; Table 1). In line with previous reports (Batista et al. 2012; Quintana et al. 2016; Perrone and Silva 2018), the agonistic behavior of the small arena (Fig. 2A) was characterized by (a) a short pre-contest of around 15 s; (b) the contest, with highly aggressive displays by both contenders; and (c) the 10-min post-resolution phase, in which dominants persisted in attacking, while subordinates attempted to flee and emitted submissive electric signals. On the other hand, the agonistic behavior in the large arena (Fig. 2B) was characterized by (a) a longer pre-contest of around 1 min; (b) a longer contest of more than 10 min, with milder aggressive displays by both contenders; and (c) the 10-min post-resolution phase, in which dominants patrolled the conquered territory and excluded subordinates less aggressively inducing subordinates to flee without emitting submissive electric signals. As shown in Table 1, not only the temporal parameters (first attack latency, contest duration) were significantly different between the small and large arenas, but also the intensity of aggression of dominants, subordinates’ retreats, and the displays of electric submission. In the small arena, subordinates decreased their EOD rate after contest resolution, and thus showed a negative EOD rate change index – 7.9 (± 1.54); Table 1), while dominants did not change their EOD rate during the contest and showed a nearly null EOD rate change index of 0.11 (± 0.07); Table 1). Interestingly, the EOD rate decrease observed in subordinates after contest resolution in the small arena was not observed in the large arena, resulting in no significant differences in the EOD rate rank index between dominants and subordinates in the post-resolution phase of the large arena (Table 1). In line with this result, chirps were profusely emitted by subordinates in the small arena during the post-resolution phase, but were almost absent in the agonistic encounters held in the large arena (Table 1). In contrast, off rate was not significantly different between both arenas (Table 1).
Experiment 2
Experiment 2 was carried out in the large arena; thus, the timing and general features of the agonistic behavior displayed by the male-male dyads involved in this experiment (n = 7) were similar to those observed in the large arena of experiment 1 (Fig. 2B and Table 1). In experiment 2, 6 out of the 7 larger males won the fight; first attack latency was of 65 (± 49) s; contest lasted 259 (± 151) s; and contest attack rate of dominants and subordinates was of 0.04 (± 0.01)/s and 0.01 (± 0.008)/s, respectively. None of these characteristics were significantly different from the ones recorded in the large arena of experiment 1 (Mann-Whitney U test; large arena experiment 1 versus experiment 2; first attack latency p = 0.95; contest duration p = 0.18; dominants’ contest attack rate p = 0.13; subordinates’ contest attack rate p = 0.23).
During the EPR, 30 min immediately after the dominant-subordinate status was established (Fig. 3), we observed a clear asymmetry in the locomotor displays of dominants and subordinates. Dominants attacked (0.03 (± 0.03)) and never retreated (0 (± 0)) while subordinates retreated (0.36 (± 0.21)) and never attacked (0 (± 0)). Further, dominants’ attacks and subordinates’ retreats were positively correlated (Fig. 3C, r2 = 0.80, p = 0.007). Interestingly, during the LPR (next 35 h of the post-resolution phase), the dominant-subordinate status consolidated with no reversion (Fig. 3). The same asymmetric behavior between dominants and subordinates was also observed in the LPR. Dominants attacked (0.02 (± 0.02)) and never retreated (0 (± 0)) while subordinates retreated (0.19 (± 0.12)) and never attacked (0 (± 0)). In addition, the correlation between dominants’ attacks and subordinates’ retreats persisted in long-term recordings (Fig. 3C, r2 = 0.80, p = 0.007). Furthermore, Fig. 4A shows the temporal association of dominant attacks usually preceding subordinate retreats.
Locomotor agonistic displays in the post-resolution phase. EPR early post-resolution, 30 min post-resolution. LPR late post-resolution, 35 h post-resolution. A Attack rate. EPR: Mann-Whitney U test, p = 0.07; LPR: Mann-Whitney U test, p = 0.07. B Retreat rate. EPR: Mann-Whitney U test, p = 0.0006; LPR: Mann-Whitney U test, p = 0.02. C Correlation between subordinates’ retreat rate and dominants’ attack rate. Results in A and B are depicted by boxplots with a dark line representing the median and the whiskers minimum to maximum values
Persistence of the dominant-subordinate status and territorial behavior during LPR. A Locomotor agonistic displays recorded in one representative dyad over 35 h. Total time was subdivided in 1 h bins. The white-black bar represents daytime and nighttime, respectively. B Shelter occupancy. Note that subordinates are never found inside the shelter. Mann-Whitney U test, p = 0.0006, n = 7. C Territory access index. Note that dominants have priority access to the central part of the arena. Mann-Whitney U test, p = 0.0006, n = 7. Results in B and C are depicted by boxplots with a dark line representing the median and the whiskers minimum to maximum values
In experiment 2, the large arena was enriched by the presence of a central shelter, whose occupancy and defense allowed us to make evident both dominant status and territorial behavior. As shown in Fig. 4A with one representative dyad, only the dominant fish occupied the shelter; it rested inside the shelter during all daytime, and sheltered briefly several times during both active nights. Our video recordings clearly showed how dominants proactively excluded the access of subordinates to the shelter, chasing them when they attempted to approach it. Because of this agonistic interaction, we never found subordinates inside the shelter (Fig. 4B; Mann-Whitney U test; shelter occupancy dominants versus subordinates; p = 0.0006). The overall position of dominants and subordinates with respect to the shelter was evinced by calculating the territory access index. As shown in Fig. 4C, dominants exhibited a significantly higher territory access index than subordinates, indicating that dominants not only occupied the shelter but also patrolled the surrounding area more than subordinates (Mann-Whitney U test, territory access index dominants versus subordinates; p = 0.0006).
Discussion
Territoriality embraces both behavioral and ecological perspectives (Maher and Lott 1995). The exclusive use of an area claimed by ecological definitions refers to the allocation of resources among individuals, while the behavioral approach intends to assess how that allocation was produced. Although the all year round territorial behavior of Gymnotus has been put forth since pioneer reports (Black-Cleworth 1970), this is the first study to show explicitly how agonistic encounters mediate territoriality in this genus. In dyadic interactions (experiment 2), males G. omarorum engage in aggressive agonistic encounters, after which a clear dominant-subordinate status emerges with no outcome reversion over time. More importantly, dominants show exclusive access to the most valuable territory (shelter), priority access to its surroundings, and proactively exclude subordinates from this conquered space. The unusual non-breeding territory defense has been associated with feeding habits in different classes of vertebrates (Crook 1965; Lorenz 1963). Although this assumption needs to be tested in the field, feeding demands is the most likely drive for territorial defense in G. omarorum as the dispersion of conspecifics allows an even exploitation of the habitat. Interestingly, as in other sexually monomorphic species that display territorial defense across seasons, territories are defended equally by both sexes (Randall 1984, Hau et al. 2004, Sogge et al. 2007).
Territory is defined as a fixed area defended by an animal, from which it excludes rival intruders (Brown 1975). To do so, animals use diverse types of threats as well as actual attacks, usually termed territorial aggression (Wilson 1975b; Hau et al. 2000). Territory ownership is a major determinant of fitness and the way animals defend territories has important implications for population structure and dynamics (Balthazart et al. 1999; Adams 2001; Morrell and Kokko 2005). There are three criteria for the operational definition of territoriality: (1) defended area, (2) exclusive use, and (3) site-specific dominance (Kaufmann 1983; Maher and Lott 1995). The diagnosis of territoriality for any given species meets at least one of these requirements. For example, the black-capped chickadee (Parus atricapillus), was characterized as territorial because they show site-specific dominance, although they do not fulfill the criteria of defended area nor exclusive use (Desrochers and Hannon 1989). As shown in Fig. 4, we were able to demonstrate that G. omarorum meets all the three criteria of territoriality: (1) dominants defend the central territory and chase subordinates from it, (2) the shelter is exclusively used by dominants that remain inside it during all the diurnal resting phase, and (3) dominants have priority access to a fixed area with no reduction of its boundaries over time. Indirect evidence of the persistence of territory ownership by dominants is also shown in Fig. 3, in which attacks and retreats are not only asymmetric between dominants and subordinates but also dominant attacks correlate with subordinate retreats in a similar way for 36 h after resolution.
When space is the resource animals compete for, agonistic encounters mediate the establishment of territories and thus the space distribution of a given population. The large arena, presented in this study (experiments 1 and 2) and initially reported by (Pedraja et al. 2016), contributes a naturalistic scenario to test the agonistic behavior in G. omarorum. Pre-contest individual distance mimics the one observed in nature (L. Zubizarreta, personal communication). Agonistic contests in the large arena follow the three expected phases (evaluation, contest, and post-resolution), with a stable status establishment in which dominants hold the central territory while subordinates are excluded to the periphery. It is interesting to note that though the volume of water of the large arena is enough to allocate two or more fish, the aggressive contest phase seems unavoidable to solve dyadic agonistic encounters in G. omarorum. This is somehow unexpected as individuals of G. omarorum can infer the size of their contenders by electric cues at intermediate distances (Pedraja et al. 2016). However, instead of taking advantage of the electric channel of communication to avoid energy demanding and injure costly contests, they disregard this information and always engage in actual fights to settle the use of space.
The time structure, aggression levels, and submissive displays of G. omarorum dyadic agonistic encounters show dramatic differences between the small and the large arenas (Fig. 2; Table 1). Dyads display a more robust and exaggerated agonistic behavior in the small arena (extensively described in previous reports (Batista et al. 2012; Quintana et al. 2016; Perrone and Silva 2018) than in the large arena. Contest dynamics are extremely short in the small arena, with an evaluation phase of only 15 s and a contest duration < 3 min. During contest, dominants’ aggression levels, but not subordinates’, are higher in the small arena with respect to the large one. Even more obvious changes between arenas are observed during the post-resolution phase, which is characterized exclusively in the small arena by the persistence of dominants’ aggression and the profuse emission of subordinates’ electric signaling of surrender. For example, the status-dependent EOD rate rank attained by the significant decrease in the EOD rate of the defeated fish after contest in the small arena (Perrone and Silva 2018) is not observed in the large arena. In addition, the emission of chirps, the latest signal of submission interpreted as the most explicit and unambiguous one (Batista et al. 2012; Quintana et al. 2016), is only observed in the small arena. This comparative analysis reinforces the idea that the experimental conditions of the large arena resembles the natural agonistic behavior of G. omarorum as in these conditions the communication codes exchanged by the contenders during contest lead to a peaceful agreement in how to distribute space. In contrast, when confined in the small arena, a hyper-aggressive agonistic behavior arises. The fact that subordinates cannot flee in the small arena may mislead dominants’ interpretation of subordinates’ surrender despite subordinates broadcast their defeat by a sequence of progressively unambiguous signals. The comparison of the agonistic behavior of G. omarorum between the small and large arenas also contributes a very clear example of how subordinates’ signaling is adjusted in response to dominants’ behavior. It has already been reported in the small arena that the intensity of aggression is evaluated directly between contenders, and that subordinates assess how hard they are attacked to escalate during contest or to decide when to retreat and to emit submissive electric signals (Zubizarreta et al. 2015; Quintana et al. 2016). In line with these results, we observed in this study that the milder contests of the large arena did not force subordinates to increase their signaling of submission.
Contest outcome in G. omarorum depends on body size asymmetry regardless the size of the arena in which the agonistic behavior has been tested (Table 1). Body size is the most common predictor of fighting ability and thus of contest outcome across taxa (Jennions and Backwell 1996; Umbers et al. 2012). In theory, if resource value is symmetric among contestants, contest outcome is expected to depend only on fighting ability asymmetries (Maynard Smith and Parker 1976; Parker and Rubenstein 1981). This is indeed the case of the non-breeding agonistic behavior of G. omarorum, in which no resource value asymmetry is observed between contenders, and hence, their body mass difference is the only predictor of contest outcome (Batista et al. 2012). Assuming that G. omarorum natural territorial behavior is also mediated by agonistic encounters, two predictions arise from this study to be tested in the wild during the non-breeding season: (1) we expect body size to be the only proxy of territory size and (2) we expect no sex differences in territory size.
The features displayed in the agonistic behavior of G. omarorum in the small arena resemble the characteristics of violent-like behaviors (de Boer et al. 2009, 2016). From this perspective, violence is defined as an exaggerated form of escalated aggressive behavior that has lost its adaptive function in social communication. Violence is expressed out of context, out of inhibitory control; and it is thus characterized by highly aggressive short-latency agonistic encounters in which dominants persist attacking even after subordinates’ surrender (de Boer et al. 2009). Accordingly, the agonistic encounter of G. omarorum in the small arena shows an extremely short latency (around 15 s), after which dominants display an escalated and persistent aggression regardless subordinates’ defeat and their profuse electric signaling of submission. Traditional models for the study of violence have been developed in laboratory-bred feral rats and mice (Miczek et al. 2007). These studies claim for novel models to test predictions of probably conserved mechanisms governing exaggerated aggression across evolution. The inclusion of G. omarorum as a novel model of violent-like behavior is thus timely and promising. It contributes a teleost model whose territorial behavior is crucial for population structure in nature, can be mimicked in laboratory settings, it only requires confinement to shift from normal adaptive aggression into violent behavior, and it offers an interesting additional dimension to the assessment of territoriality by means of its electric signaling.
References
Adams ES (2001) Approaches to the study of territory size and shape. Annu Rev Ecol Syst 32:277–303
Alexander BK, Roth EM (1971) The effects of acute crowding on aggressive behavior of Japanese monkeys. Behaviour 39:73–90
Armitage KB (1977) Social variety in the yellow-bellied marmot: a population-behavioural system. Anim Behav 25:585–593
Assad C, Rasnow B, Stoddard PK (1999) Electric organ discharges and electric images during electrolocation. J Exp Biol 202:1185–1193
Bakker TCM, Sevenster P (1983) Determinants of dominance in male sticklebacks (Gasterosteus aculeatus L.). Behaviour 86:55–71
Balthazart J, Foidart A, Baillien M, Silverin B (1999) Brain aromatase in laboratory and free-living songbirds: relationships with reproductive behaviour. NJ Adams RH Slotow, pp 1257–1289
Batista G, Zubizarreta L, Perrone R, Silva A (2012) Non-sex-biased dominance in a sexually monomorphic electric fish: fight structure and submissive electric signalling. Ethology 118:398–410
Black-Cleworth P (1970) The role of electrical discharges in the non-reproductive social behaviour of Gymnotus carapo. Anim Behav Monogr 3:1–77
Briffa M, Hardy ICW (2013) Introduction to animal contest. In: ICW H, Briffa M (eds) Animal contest. Cambridge University Press, New York, pp 1–4
Briffa M, Sneddon LU (2010) Contest behavior. In: Westneat DF, Fox CW (eds) Evolutionary behavioral ecology. Oxford University Press, New York, pp 246–265
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull, pp 160–169
Brown JL (1975) The evolution of behavior. WW Norton, New York
Buchwalder T, Huber-Eicher B (2004) Effect of increased floor space on aggressive behaviour in male turkeys (Meleagris gallopavo). Appl Anim Behav Sci 89:207–214
Caldwell GS, Glickman SE, Smith ER (1984) Seasonal aggression independent of seasonal testosterone in wood rats. Proc Natl Acad Sci 81:5255–5257
Caputi AA, Budelli R (2006) Peripheral electrosensory imaging by weakly electric fish. J Comp Physiol A 192:587–600
Caputi A, Carlson B, Macadar O (2005) Electric organs and their control. In: Bullock TH, Hopkins CD, Popper AN, Fay RR (eds) Electroreception. Springer, New York, pp 410–451
Chiver I, Stutchbury BJM, Morton ES (2014) Seasonal variation in male testosterone levels in a tropical bird with year-round territoriality. J F Ornithol 85:1–9
Ciali S, Gordon J, Moller P (1997) Spectral sensitivity of the weakly discharging electric fish Gnathonemus petersii using its electric organ discharges as the response measure. J Fish Biol 50:1074–1087
Clarke TA (1970) Territorial behavior and population dynamics of a pomacentrid fish, the garibaldi, Hypsypops rubicunda. Ecol Monogr 40:189–212
Crook JH (1965) The adaptive significance of avian social organization. Symp Zool Soc Land 14:181–218
Davies NB (1976) Food, flocking and territorial behaviour of the pied wagtail (Motacilla alba yarrellii Gould) in winter. J Anim Ecol 45:235–253
de Boer SF, Caramaschi D, Natarajan D, Koolhaas JM (2009) The vicious cycle towards violence: focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front Behav Neurosci 3:52. https://doi.org/10.3389/neuro.08.052.2009
de Boer SF, Buwalda B, Koolhaas JM (2016) Aggressive behavior and social stress. In: Stress: concepts, cognition, emotion, and behavior. Elsevier, pp 293–303
Desrochers A, Hannon SJ (1989) Site-related dominance and spacing among winter flocks of Black-capped Chickadees. Condor 91:317–323
Fugère V, Ortega H, Krahe R (2011) Electrical signalling of dominance in a wild population of electric fish. Biol Lett 7:197–200. https://doi.org/10.1098/rsbl.2010.0804
Gómez-Sena L, Pedraja F, Sanguinetti-Scheck JI, Budelli R (2014) Computational modeling of electric imaging in weakly electric fish: insights for physiology, behavior and evolution. J Physiol Paris 108:112–128
Hagedorn M, Carr C (1985) Single electrocytes produce a sexually dimorphic signal in South American electric fish, Hypopomus occidentalis (Gymnotiformes, Hypopomidae). J Comp Physiol A 156:511–523. https://doi.org/10.1007/bf00613975
Hagedorn M, Zelick R (1989) Relative dominance among males is expressed in the electric organ discharge characteristics of a weakly electric fish. Anim Behav 38:520–525
Hau M, Wikelski M, Soma KK, Wingfield JC (2000) Testosterone and year-round territorial aggression in a tropical bird. Gen Comp Endocrinol 117:20–33
Hau M, Stoddard ST, Soma K (2004) Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm Behav 45:40–44
Hazlet BA (1968) Effects of crowding on the agonistic behavior of the hermit crab (Pagurus bernhardus). Ecology 49:573–575
Hopkins CD (1972) Sex differences in electric signaling in an electric fish. Science 176:1035–1037
Hopkins CD (1974) Electric communication: functions in the social behavior of Eigenmannia virescens. Behaviour 50:270–304
Huang W-S, Greene HW, Chang T-J, Shine R (2011) Territorial behavior in Taiwanese kukrisnakes (Oligodon formosanus). Proc Natl Acad Sci 108:7455–7459
Hungtinford F, Turner A (1987) Animal conflict. Chapman and Hall Ltd, London
Hupé GJ, Lewis JE (2008) Electrocommunication signals in free swimming brown ghost knifefish, Apteronotus leptorhynchus. J Exp Biol 211:1657–1667
Hupé GJ, Lewis JE, Benda J (2008) The effect of difference frequency on electrocommunication: chirp production and encoding in a species of weakly electric fish, Apteronotus leptorhynchus. J Physiol Paris 102:164–172
Jalabert C, Quintana L, Pessina P, Silva A (2015) Extra-gonadal steroids modulate non-breeding territorial aggression in weakly electric fish. Horm Behav 72:60–67. https://doi.org/10.1016/j.yhbeh.2015.05.003
Jennions MD, Backwell PRY (1996) Residency and size affect fight duration and outcome in the fiddler crab Uca annulipes. Biol J Linn Soc 57:293–306. https://doi.org/10.1111/j.1095-8312.1996.tb01851.x
Jun JJ, Longtin A, Maler L (2016) Active sensing associated with spatial learning reveals memory-based attention in an electric fish. J Neurophysiol 115:2577–2592. https://doi.org/10.1152/jn.00979.2015
Kaufmann JH (1983) On the definitions and functions of dominance and territoriality. Biol Rev 58:1–20
King JA (1973) The ecology of aggressive behavior. Annu Rev Ecol Syst 4:117–138
Knell RJ (2009) Population density and the evolution of male aggression. J Zool 278:83–90
Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Phil Trans R Soc B 361:319–334
Lissman H (1958) On the function and evolution of electric organs in fish. J Exp Biol 35:156–191
Lorenz K (1963) On aggression. Harcourt, Brace and World, New York
Maciver MA, Sharabash NM, Nelson ME (2001) Prey-capture behavior in gymnotid electric fish: motion analysis and effects of water conductivity. J Exp Biol 204:543–557
Maher CR, Lott DF (1995) Definitions of territoriality used in the study of variation in vertebrate spacing systems. Anim Behav 49:1581–1597
Maynard Smith J, Parker GA (1976) The logic of asymmetric contests. Anim Behav 24:159–175
Miczek KA, de Almeida RMM, Kravitz EA, Rissman EF, de Boer SF, Raine A (2007) Neurobiology of escalated aggression and violence. J Neurosci 27:11803–11806
Morrell LJ, Kokko H (2005) Bridging the gap between mechanistic and adaptive explanations of territory formation. Behav Ecol Sociobiol 57:381–390
Nelson RJ (2006) Biology of aggression. Oxford University Press, New York
Oldfield R (2011) Aggression and welfare in a common aquarium fish, the Midas cichlid. J Appl Anim Welf Sci 14:340–360
Parker GA, Rubenstein DI (1981) Role assessment, reserve strategy, and acquisition of information in asymmetric animal conflicts. Anim Behav 29:221–240
Pedraja F, Aguilera P, Caputi AA, Budelli R (2014) Electric imaging through evolution, a modeling study of commonalities and differences. PLoS Comput Biol 10:e1003722. https://doi.org/10.1371/journal.pcbi.1003722
Pedraja F, Perrone R, Silva A, Budelli R (2016) Passive and active electroreception during agonistic encounters in the weakly electric fish Gymnotus omarorum. Bioinspir Biomim 11(6):065002. https://doi.org/10.1088/1748-3190/11/6/065002
Pedraja F, Hofmann V, Lucas KM, Young C, Engelmann J, Lewis JE (2018) Motion parallax in electric sensing. Proc Natl Acad Sci 115:573–577
Perrone R, Silva A (2016) Vasotocin increases dominance in the weakly electric fish Brachyhypopomus gauderio. J Physiol Paris 110:119–126
Perrone R, Silva AC (2018) Status-dependent vasotocin modulation of dominance and subordination in the weakly electric fish Gymnotus omarorum. Front Behav Neurosci 12:1
Perrone R, Macadar O, Silva A (2009) Social electric signals in freely moving dyads of Brachyhypopomus pinnicaudatus. J Comp Physiol A 195:501–514
Pröhl H (2005) Territorial behavior in dendrobatid frogs. J Herpetol 39:354–365
Quintana L, Zubizarreta L, Jalabert C, Batista G, Perrone R, Silva A (2016) Building the case for a novel teleost model of non-breeding aggression and its neuroendocrine control. J Physiol Paris 110:224–232
Randall JA (1984) Territorial defense and advertisement by Footdrumming in Bannertail Kangaroo Rats (Dipodomys spectabilis) at high and low population densities. Behav Ecol Sociobiol 16:11–20
Richer-de-Forges MM, Crampton WGR, Albert JS (2009) A new species of Gymnotus (Gymnotiformes, Gymnotidae) from Uruguay: description of a model species in neurophysiological research. Copeia 2009:538–544
Roth E, Zhuang K, Stamper SA, Fortune ES, Cowan NJ (2011) Stimulus predictability mediates a switch in locomotor smooth pursuit performance for Eigenmannia virescens. J Exp Biol 214:1170–1180
Silva A, Quintana L, Galeano M, Errandonea P (2003) Biogeography and breeding in Gymnotiformes from Uruguay. Environ Biol Fish 66:329–338
Silva A, Perrone R, Macadar O (2007) Environmental, seasonal, and social modulations of basal activity in a weakly electric fish. Physiol Behav 90:525–536
Silva AC, Perrone R, Zubizarreta L, Batista G, Stoddard PK (2013) Neuromodulation of the agonistic behavior in two species of weakly electric fish that display different types of aggression. J Exp Biol 216:2412–2420
Sogge MK, Koronkiewicz TJ, van Riper C III, Durst S (2007) Willow flycatcher nonbreeding territory defense behavior in Costa Rica. Condor 109:475–480
Stoddard P (2002) The evolutionary origins of electric signal complexity. J Physiol 96:485–491
Summers CH, Winberg S (2006) Interactions between the neural regulation of stress and aggression. J Exp Biol 209:4581–4589
Triefenbach F, Zakon H (2008) Changes in signalling during agonistic interactions between male weakly electric knifefish, Apteronotus leptorhynchus. Anim Behav 75:1263–1272
Turner SP, Ewen M, Rooke JA, Edwards SA (2000) The effect of space allowance on performance, aggression and immune competence of growing pigs housed on straw deep-litter at different group sizes. Livest Sci 66:47–55
Umbers KD, Osborne L, Keogh JS (2012) The effects of residency and body size on contest initiation and outcome in the territorial dragon, Ctenophorus decresii. PLoS One 7:e47143
Westby G (1975a) Further analysis of the individual discharge characteristics predicting social dominance in the electric fish. Anim Behav 23:249–260
Westby GWM (1975b) Comparative studies of the aggressive behaviour of two gymnotid electric fish (Gymnotus carapo and Hypopomus artedi). Anim Behav 23:192–213
Wilson EO (1975) Sociobiology: The New Synthesis. University Press, Harvard
Wingfield JC, Hahn TP (1994) Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim Behav 47:77–89. https://doi.org/10.1006/anbe.1994.1009
Zakon HH, Thomas P, Yan H-Y (1991) Electric organ discharge frequency and plasma sex steroid levels during gonadal recrudescence in a natural population of the weakly electric fish Sternopygus macrurus. J Comp Physiol A 169:493–499. https://doi.org/10.1007/bf00197661
Zubizarreta L, Perrone R, Stoddard PK, Costa G, Silva AC (2012) Differential serotonergic modulation of two types of aggression in weakly electric fish. Front Behav Neurosci 6(77). https://doi.org/10.3389/fnbeh.2012.0007
Zubizarreta L, Stoddard PK, Silva A (2015) Aggression levels affect social interaction in the non-breeding territorial aggression of the weakly electric fish, Gymnotus omarorum. Ethology 121:8–16
Acknowledgments
We specially thank Laura Quintana and Lucía Zubizarreta for their generous comments and suggestions to our manuscript. We are very grateful to Adriana Migliaro, Carlos Passos, Laura Quintana, Federico Reyes, and Lucía Zubizarreta for their useful discussions during the BERTA Workshop, Cerro del Toro, Piriápolis, Uruguay.
Funding
This work was supported by National Agency for Research and 50 Innovation (ANII), projects FCE 569 and FCE 4272.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Electric fish collection for experimental purposes was authorized by DINARA (National Direction of Aquatic Resources) and MGAP (Ministry of Agriculture and Fisheries), resolution No. 065/2004. All experimental procedures complied with ASAP/ABS Guidelines for the Use of Animals in Research and were approved by our institutional ethical committee (Comisión Bioética, Instituto Clemente Estable, MEC, 007/05/2012).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perrone, R., Pedraja, F., Valiño, G. et al. Non-breeding territoriality and the effect of territory size on aggression in the weakly electric fish, Gymnotus omarorum. acta ethol 22, 79–89 (2019). https://doi.org/10.1007/s10211-019-00309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10211-019-00309-7