Abstract
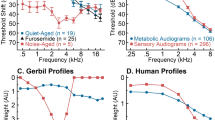
Presbyacusis, or age-related hearing loss, can be characterized in humans as metabolic and sensory phenotypes, based on patterns of audiometric thresholds that were established in animal models. The metabolic phenotype is thought to result from deterioration of the cochlear lateral wall and reduced endocochlear potential that decreases cochlear amplification and produces a mild, flat hearing loss at lower frequencies coupled with a gradually sloping hearing loss at higher frequencies. The sensory phenotype, resulting from environmental exposures such as excessive noise or ototoxic drugs, involves damage to sensory and non-sensory cells and loss of the cochlear amplifier, which produces a 50–70 dB threshold shift at higher frequencies. The mixed metabolic + sensory phenotype exhibits a mix of lower frequency, sloping hearing loss similar to the metabolic phenotype, and steep, higher frequency hearing loss similar to the sensory phenotype. The current study examined audiograms collected longitudinally from 343 adults 50–93 years old (n = 686 ears) to test the hypothesis that metabolic phenotypes increase with increasing age, in contrast with the sensory phenotype. A Quadratic Discriminant Analysis (QDA) was used to classify audiograms from each of these ears as (1) Older-Normal, (2) Metabolic, (3) Sensory, or (4) Metabolic + Sensory phenotypes. Although hearing loss increased systematically with increasing age, audiometric phenotypes remained stable for the majority of ears (61.5 %) over an average of 5.5 years. Most of the participants with stable phenotypes demonstrated matching phenotypes for the left and right ears. Audiograms were collected over an average period of 8.2 years for ears with changing audiometric phenotypes, and the majority of those ears transitioned to a Metabolic or Metabolic + Sensory phenotype. These results are consistent with the conclusion that the likelihood of metabolic presbyacusis increases with increasing age in middle to older adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presbyacusis (i.e., age-related hearing loss) is proposed to result from distinct cochlear pathologies that can produce unique threshold patterns in audiograms (Schmiedt 2010; Dubno et al. 2013). Accurate classification of pathologies based on audiometric data could improve our understanding of hearing loss mechanisms and their prevalence, as well as facilitate detailed diagnoses and enhance treatment strategies. Audiometric patterns from middle-aged to older adults can be challenging to interpret because they reflect contributions from noise history, injury, disease, medication, diet, heredity, and age. The search for robust audiometric patterns in presbyacusis is aided by algorithms that can detect or categorize audiograms within large databases (e.g., Allen and Eddins 2010; Dubno et al. 2013). These efforts have been enhanced with a theoretical framework based on studies of human temporal bones and animal models of hearing loss (Schmiedt 2010; Dubno et al. 2013), which relate distinct pathologies to audiometric patterns seen in audiograms from older adults.
Studies involving animal models have shown that degeneration of the stria vascularis and auditory nerve are primary contributors to presbyacusis (Mills et al. 1990; Tarnowski et al. 1991; Schmiedt 1996; Schmiedt et al. 2002; Mills et al. 2006). Most of these studies directly related cochlear pathology to changes in animal audiograms, which led to four proposed audiometric phenotypes of age-related hearing loss in humans: (1) Older-Normal, (2) Metabolic, (3) Sensory, and (4) Metabolic + Sensory (Dubno et al. 2013). Individuals classified in the Older-Normal phenotype have audiograms with thresholds in low-to-mid frequency <20 dB hearing level (HL) and slightly elevated thresholds for higher frequencies. The Metabolic phenotype is thought to result from the deterioration of the stria vascularis in the cochlear lateral wall, which normally produces endolymph to maintain the endocochlear potential (see Schmiedt 2010 for review). As a result of reduced endocochlear potential and decreased cochlear amplification, individuals classified in the Metabolic phenotype have audiograms that exhibit mild, flat hearing losses at lower frequencies and gradually sloping hearing loss at higher frequencies. The Sensory phenotype is thought to be related to damage to sensory cells in the inner ear and loss of the cochlear amplifier due to environmental exposures, including excessive noise or ototoxic drugs, resulting in steeply sloping 50–70 dB threshold shifts that predominantly affect higher frequencies. The Metabolic + Sensory phenotype is thought to reflect combined metabolic declines and sensory damage, which results in audiograms with mild, flat hearing loss at lower frequencies (the metabolic component) and steeply sloping hearing loss at higher frequencies (the sensory component). Audiometric patterns similar to the hypothesized phenotypes were observed in a data-driven principal component analysis of human audiograms (Allen and Eddins 2010), suggesting that these patterns commonly occur with increasing age.
Differences in the pathologies underlying the audiometric phenotypes led to distinct predictions regarding age-related changes in the likelihood of sensory or metabolic types. Histopathological studies of human temporal bones indicated that degeneration of the stria vascularis and auditory nerve contributes more to age-related hearing loss than sensory cell losses or conduction changes (Schuknecht and Gacek 1993). Similarly, gerbils raised in noise-limited environments to separate age-related declines from the effects of noise exposure consistently demonstrate strial and neural pathologies with only sparse sensory losses (Mills et al. 1990; Tarnowski et al. 1991; Schmiedt et al. 2002; Mills et al. 2006). Based on the gradual declines seen in the stria vascularis, the likelihood of a Metabolic phenotype was predicted to increase with age, along with concomitant age-related increases in pure-tone thresholds. Given the observations that sensory hearing losses result from damage rather than an aging process per se, threshold changes were predicted to be relatively restricted with increasing age for older adults with a Sensory phenotype.
Based on the theoretical framework for phenotypes of age-related hearing loss, Dubno et al. (2013) used algorithms to classify phenotypes for a cross-sectional sample of audiograms from 1728 ears (865 participants) to test the prediction that metabolic declines are a dominant factor in presbyacusis. After classifying each of the individual ears into a phenotype category using machine learning methods, their results showed that Metabolic and Metabolic + Sensory ears were on average the oldest, more likely to be female, and less likely to have a positive noise history than the other phenotypes. In contrast, the Sensory ears were younger on average, more likely to be male, and more likely to have a positive noise history compared to the Metabolic or Metabolic + Sensory ears. The positive noise history suggested that sex differences among the phenotypes were associated with different patterns of occupational and recreational noise exposure, which led to an increase in sensory loss for males. These age and noise history differences were consistent with predictions based on animal models, given that strial declines occur gradually with increasing age and result in Metabolic or Metabolic + Sensory phenotypes at a later age, compared to Sensory phenotypes.
Because the study by Dubno et al. (2013) was based on a cross-sectional sample of audiograms, their conclusions regarding age-related changes were based on participant age differences rather than age-related changes observed within subject. Although cross-sectional aging studies can be sensitive to changes that occur consistently with increasing age, individual age-related differences in hearing thresholds and phenotypes could also be influenced by other uncontrolled factors that add unexplained variance or co-vary with age differences. Nonetheless, the original cross-sectional design provided an initial validation for automatic phenotype classification and added support for the hypothesis that metabolic presbyacusis becomes more common with increasing age. Building on the framework and methods from Dubno et al. (2013), the current study tested age-related predictions for the hearing loss phenotypes with audiometric data collected longitudinally from a sample of older adults.
Longitudinal analyses were performed to test the hypothesis that metabolic phenotypes increase with age by comparing the average age for ears within each phenotype category. The number of ears that transitioned to a Metabolic or Metabolic + Sensory phenotype with increasing age were also compared. This study also built on the earlier findings (Dubno et al. 2013) by characterizing the stability of each phenotype. Longitudinally obtained audiograms could provide further validation for phenotype classification of audiograms by demonstrating that audiometric phenotypes were relatively stable across measurements and typically matched across left and right ears.
Another goal of the current study was to characterize longitudinal declines in hearing sensitivity for each audiometric phenotype, with the prediction that each audiometric phenotype should exhibit a unique pattern of threshold changes. Hearing sensitivity declines have been examined in longitudinal data in relation to increasing age (Cruickshanks et al. 2003; Lee et al. 2005; Echt et al. 2010). Maximum cochlear gain increases progressively from about 20 dB at the apex to 60 dB at the base, resulting in declines in the endocochlear potential that decrease sensitivity to high frequencies more than to low frequencies (Schmiedt 2010). Consequently, Metabolic and Metabolic + Sensory ears are predicted to exhibit gradual threshold changes that are larger for high frequencies than low frequencies. Sensory presbyacusis primarily involves inner and outer hair cell damage that can eliminate cochlear amplification, which typically results in substantial high-frequency hearing loss and relatively preserved endocochlear potential and lower frequency thresholds (Schmiedt 2010). To the extent that the Sensory phenotype reflects hearing damage, which is assumed to be restricted for older adults, only limited threshold changes were predicted for those ears (cf. Gates et al. 2000; Kujawa and Liberman 2006).
Sex-related differences in threshold changes have been observed longitudinally (Lee et al. 2005) and could partially reflect the unique distribution of females and males within each audiometric phenotype. Metabolic ears are more often female, and Sensory ears are more often male (Dubno et al. 2013). In studies of age-related hearing loss with human participants, audiograms from females appear more consistent with a Metabolic phenotype and audiograms from males are more consistent with a Sensory phenotype (Jerger et al. 1993; Demeester et al. 2009; Schmiedt 2010; Dubno et al. 2013; Kaya et al. 2015). These apparent sex differences in audiometric patterns have been attributed to social factors that could vary for males and females, such as recreational and occupational noise history (Jerger et al. 1993; Demeester et al. 2009; Dubno et al. 2013), rather than biologic factors. Consistent with these observations, screening for noise history can reduce sex differences in audiograms (Schmiedt 2010); an increased likelihood for positive noise history has been observed for mostly male, Sensory ears (Dubno et al. 2013). Thus, diminished sex differences in both audiometric configuration and threshold changes were expected for audiograms organized by phenotype.
In summary, the current study aimed to establish the longitudinal stability of phenotypes and declines in hearing sensitivity for older adults, as well as provide additional validation for the phenotype categories. Audiograms obtained longitudinally from large numbers of human participants were used to characterize age-related declines in hearing sensitivity for different audiometric phenotypes. An optimized algorithm was used to classify each ear as one of the four phenotypes; following that, descriptive statistics were calculated for ears classified in each phenotype. For example, comparisons of participant age were used to test the hypothesis that metabolic and sensory presbyacusis reflect distinct pathologies, with metabolic declines that predominantly contribute to age-related hearing loss in older humans. Annual threshold changes were estimated separately for ears with stable audiometric phenotypes and ears with changing phenotypes. The likelihood of phenotype transitions were used to test the prediction that the likelihood of Metabolic and Metabolic + Sensory phenotypes increases with increasing age.
Methods
Participants and Audiometric Measurements
Since 1987, the Hearing Research Program at the Medical University of South Carolina (MUSC) has collected audiometric and other data from more than 1500 participants enrolled in a longitudinal study of age-related hearing loss. None of the participants presented evidence of conductive hearing loss and otologic disease. Otologic examinations were performed for each participant, and audiometric measurements of pure-tone thresholds were collected at conventional frequencies (0.25, 0.5, 1, 2, 3, 4, 6, 8 kHz) and extended high frequencies (9, 10, 11, 12, 14, 16, 18 kHz; Matthews et al. 1997). Pure-tone thresholds were measured using a Madsen OB822 or OB922 clinical audiometer calibrated according to standards from the American National Standards Institute (ANSI 2010) with TDH-39 headphones in MX-41/AR cushions and the measurement protocol recommended by the American Speech-Language-Hearing Association (2005).
Noise history questionnaires were completed by all participants, which indicated that 47 % had a positive history for one or more of the following noise exposure categories: (1) noisy work environments, (2) guns, (3) loud music, (4) power tools, (5) farm machinery, and (6) sudden loud noises. Consistent with previous findings discussed above, the likelihood of a positive noise history differed significantly with the sex of the participant (Χ 2 = 105.42, P = 2.2 × 10−16), with a positive noise history for 23 % of the females and 79 % of the males. An elevated likelihood for positive noise history was predicted for Sensory ears, given that hearing damage is hypothesized to drive sensory declines. Additional details on the complete test battery are described in Dubno et al. (2013), including medical history questionnaires, hearing self-assessments, and other hearing-related measurements.
Audiograms were obtained from each participant over a cluster of three to six visits that were needed to complete the entire test battery, with visits that typically occurred 1 month apart and clusters of visits repeating at 2–3-year intervals. Audiograms were averaged for each ear within a cluster of visits, which included three or more audiograms that were collected within a single year. Average audiograms were also used by Dubno et al. (2013) to improve threshold measurement accuracy by reducing error that results from within-subject variability and standard pure-tone threshold test increments of 5 dB HL. Left and right ears were analyzed separately to determine whether the two ears had the same or different audiometric phenotype. After excluding data from participants who were younger than 50 years old, participants with fewer than two clusters of visits, and audiograms with missing thresholds, the current study included data from 343 participants (n = 686 ears). A total of 7686 separately collected audiograms from those ears were averaged within clustered visits to produce the 1826 average audiograms that were used for all of the analyses.
Summary of Analyses
Because of the extensive number of analyses required, an overview of all analyses is presented before describing each in greater detail. First, tests were performed to optimize the accuracy of automatic classification of audiometric phenotypes based on threshold configurations in audiograms. Next, the longitudinally collected audiograms were classified into phenotype categories, and ears were divided into groups with longitudinally stable or changing phenotypes. Demographic characteristics were compared, and threshold changes were characterized separately for the ears with longitudinally stable or changing phenotypes. Finally, transitional probabilities were calculated for each phenotype to estimate the likelihood of longitudinal phenotype changes or no change.
Quadratic Discriminant Analysis
Audiometric phenotypes were classified using a machine learning algorithm, quadratic discriminant analysis (QDA; R-Project package MASS, 7.3-29). The QDA model was first trained with a dataset that included 897 first-year (baseline) audiograms that were classified by expert raters as one of the four audiometric phenotypes (Dubno et al. 2013). After training, the QDA model was used to estimate the posterior probability that quantifies the extent to which an audiogram matches the distribution of training examples for each of the phenotypes. An audiogram is classified as the phenotype category with the highest posterior probability, and the probability for the classified phenotype is referred to as its classification probability.
Shape-related information (e.g., intercept, slope, quadratic) guided the expert ratings of audiograms and has been used in the literature to characterize audiometric differences (e.g., Demeester et al. 2009; Allen and Eddins 2010). To provide similar details to the classification algorithm, orthogonal polynomial curves were fit to pure-tone thresholds in each audiogram prior to classification (R-Project package nlme version 3.1-113). Orthogonal polynomials consist of independent factors that are orthogonalized, so additional higher order factors do not alter the values of lower order factors. For example, the fitted intercept term is the average pure-tone threshold, whether or not a slope term is added to the model and fitted to the data. This facilitates model optimization because each term that is added to the model accounts only for variance that the other terms do not. The shape parameters from each pooled audiogram in the longitudinal dataset were submitted as multivariate predictors to the QDA, which classified phenotypes after training with the shape parameters derived from the training dataset (Fig. 1). Importantly, the QDA was naïve regarding the identity, sex, or age of individual ears, so classifications were determined solely on the basis of the shape of a pooled audiogram in relation to the prior distributions of the training dataset.
QDA classification probability (CP) quantifies the similarity of an audiogram (points) to the distribution of expert-labeled training data (shaded regions: Older-Normal (gray), Metabolic (green), Sensory (red), Metabolic + Sensory (blue)). Pure-tone thresholds and fitted curves from an example audiogram for each category are shown in relation to the distribution. Higher CPs are typically obtained for correct classifications than for incorrect ones.
Optimizing Classification Accuracy
A feature selection process was used to train and test the QDA model with an increasing number of shape parameters from the training dataset. This approach maximized classification accuracy in matching expert labels, while increasing the likelihood of successful generalization of the classifier to new data. After determining the optimal number of shape parameters based on the expert-labeled training dataset, the QDA model was used to classify audiograms in the longitudinal dataset. Note that shape parameters were only used for classifying phenotypes. Pure-tone thresholds were used for more detailed statistical analyses of threshold changes and audiograms.
Machine learning methods often demonstrate improved classification accuracy after training with more examples or data points (Figueroa et al. 2012). For this reason, QDA was tested with a larger training dataset that contained diverse examples of each phenotype and a smaller subset of training data that contained more representative examples of each phenotype. As part of the study by Dubno et al. (2013), a collection of audiograms was labeled by two expert raters and 338 exemplars were selected that were judged to be good examples of each phenotype. There was also an expert labeled set of 559 non-exemplar audiograms. The classification accuracy of the QDA model was tested after training with the smaller set of exemplar audiograms, and the results were compared to the QDA model trained with the combined set of exemplar and non-exemplar audiograms (N = 897). This approach allowed us to (1) demonstrate similar classification accuracy with the shape parameters compared to pure-tone thresholds that were used for QDA classification in Dubno et al. (2013) and then (2) extend the approach to a larger dataset to increase the accuracy and validity of the classifier.
A leave-one-out cross-validation (LOOCV) was used to establish classification accuracy. This procedure preserved the independence of audiograms that were used to train the QDA and tests its accuracy in matching expert-based labels. The LOOCV procedure iteratively trains the QDA model with all of the audiograms in the training dataset, except for one. Then, the model is tested by classifying the remaining audiogram to determine whether the QDA-based phenotype matched the expert label. Because this cross-validation method is unlikely to over-estimate classification accuracy for new audiograms that were not part of the training dataset (i.e., generalization), this method was used to optimize the accuracy of QDA-based phenotype classification.
Classification of Ears with Longitudinally Stable or Changing Phenotypes
The QDA model was used to classify the phenotype for each audiogram in the longitudinal dataset. Then, individual ears were identified with phenotypes that appeared to change or not change with increasing age. Comparisons of phenotypes for left and right ears were made with the prediction that phenotypes were likely to match within subject. Ears with stable phenotypes or changing phenotypes were also tallied to determine the probability of transitions from one phenotype to another.
Phenotype Differences in Age, Sex, and Noise History
Summary statistics were calculated from the demographic data to assess the validity of classifications, separately for ears with stable or changing phenotypes. Based on the hypothesis that the likelihood of metabolic type hearing loss increases with age, predictions were tested that Metabolic and Metabolic + Sensory ears were more likely to include older participants and females and were less likely to have a positive noise history, compared to Sensory cases.
Estimating Longitudinal Threshold Changes by Phenotype
Longitudinal changes in audiometric data were examined separately for ears with stable or changing phenotypes to characterize their progression as participants became older. We predicted that pure-tone thresholds increase with age over a relatively wide frequency range in ears with metabolic declines (i.e., Metabolic or Metabolic + Sensory phenotypes). The Older-Normal phenotype and Sensory phenotype were predicted to demonstrate relatively fewer cases with significant threshold changes.
Generalized linear mixed model (GLMM)-based regression analyses were performed separately for each frequency and phenotype to characterize age-related hearing loss for each phenotype. The GLMMs were used to estimate threshold changes per year, because this type of regression model can estimate fixed effects across participants (e.g., threshold change per year of age) and between-subject random effects (e.g., differences such as overall hearing loss or age at the first visit). The GLMM also can be used to estimate effects for unbalanced datasets that include different numbers of observations across participants and with varying time intervals between clusters of visits. The GLMM equation was formulated to predict thresholds (PT) for each subject (s) at time (t) as a function of age and random subject effects (1|s), which can be expressed as PT s,t = age s,t + (1|s) + error. After fitting the GLMMs, estimated threshold changes per year were used to produce simulated hearing loss for specified ages within each phenotype category. The GLMM was also modified to test for interactions between sex and age, in relation to thresholds: PT s,t = age s,t + sex s + age s,t × sex s + (1|s) + error. Bonferroni correction was used to adjust the alpha (α = 0.05 / 24 = 0.0021) for 24 GLMM tests performed to estimate age and gender effects on thresholds within each phenotype, based on eight frequencies × three groups [females, males, combined]. A control analysis was performed to demonstrate that sex differences in age-related threshold changes occurred when the audiograms were not separately modeled within phenotype categories. In summary, the GLMM regression analyses were used both to estimate average threshold changes (dB/year) and to test their statistical significance.
Results
Optimal QDA Classification Accuracy
The optimized QDA model correctly matched expert labels for the 897-audiogram training dataset with 80 % accuracy, based on the LOOCV tests. The classification accuracy for the optimized QDA model within each phenotype category was 91 % Older-Normal, 76 % Metabolic, 84 % Sensory, and 72 % Metabolic + Sensory. The QDA model with five shape parameters demonstrated optimal classification accuracy after testing the entire range of two to seven shape parameters.
Classification probabilities for the predicted phenotype indicate the similarity of each audiogram to the training data and were significantly higher for correct than incorrect classifications, t(895) = 14.52, two-tailed P = 2.2 × 10−16. This result indicated that QDA and expert labels were less likely to match when an audiogram was not similar to the phenotype examples in the training dataset.
Phenotype classifications were more accurate when the QDA was trained with the larger dataset (exemplars + non-exemplars) compared to the QDA trained with exemplars only. When classification accuracy was calculated across all of the labeled audiograms (N = 897), exemplar-only training resulted in 72 % classification accuracy, whereas the expanded training dataset resulted in 80 % accuracy. These results showed that a larger training dataset increases classification accuracy, which likely reflects greater multivariate statistic power (Figueroa et al. 2012).
Based on the optimization test results, five shape parameters and the large training dataset (N = 897 audiograms) were selected for the subsequent QDA analyses.
Longitudinally Stable Phenotypes
Audiometric phenotypes were stable across all time points for 422 out of 686 ears (61.5 %), although thresholds increased with age (Lee et al. 2005; Echt et al. 2010). Audiograms were collected over an average of 5.5 ± 3.4 years from ears with stable audiometric phenotypes, and classification probabilities were observed to vary across serial measurements (Fig. 2). As expected, a majority of participants with stable phenotypes for both ears across all time points (90 %; N = 138/154) were classified with the same phenotype for both ears. Stable phenotype classifications were observed for one ear only in an additional 114 participants.
Most individual ears (lines) were classified as the same phenotype with increasing age (four shaded regions; classification probabilities on the ordinate), although these classification probabilities varied with age (abscissa, points). While the phenotype categories were stable longitudinally for these ears, classification probabilities were observed to vary with increasing age.
For the ears with longitudinally stable phenotypes, the oldest ears were classified as Metabolic and Metabolic + Sensory phenotypes, consistent with age-related metabolic declines observed in animal models. The results of a one-way ANOVA showed that average participant ages differed significantly between phenotypes, F(3, 418) = 30.11, P = 2.2 × 10−16, with significantly younger Older-Normal ears compared to the other phenotypes (Tukey P < 0.001) and significantly older Metabolic + Sensory ears compared to the Sensory ears (Tukey P < 0.01).
Sex differences were also observed among the phenotypes (Χ 2 = 43.50, P = 1.9 × 10−9). As in Dubno et al. (2013), males were significantly more likely than females to be classified as Sensory and less likely to be classified as Metabolic (Χ 2 = 7.05, P = 0.0079), and Metabolic + Sensory ears were evenly divided among males and females. Demographic information for the ears with longitudinally stable phenotypes is summarized in Table 1.
Consistent with the view that rates of noise exposure would systematically differ among the four audiometric phenotypes, positive noise history varied significantly across phenotypes (Χ 2 = 12.17, P = 0.0068) with a majority of positive ears in both the Sensory and Metabolic + Sensory phenotypes. As expected, positive noise history was significantly more likely for Sensory ears (Χ 2 = 7.74, P = 0.0054) than Older-Normal ears. Metabolic + Sensory ears were also significantly more likely to have a positive noise history than Older-Normal ears (Χ 2 = 6.69, P = 0.0097), but there was not a significant difference between Metabolic and Older-Normal ears (Χ 2 = 0.04, P = 0.83). The differences in positive noise history among the phenotypes were consistent with the sex differences for both positive noise history and phenotypes.
Distinct patterns of age-related hearing loss were observed based on the audiograms from ears with longitudinally stable phenotypes (Fig. 3), and the average threshold changes (dB/year) also appeared consistent with the characteristic audiometric phenotype profiles (Fig. 4). The Older-Normal ears did not exhibit significant age-related increases of pure-tone thresholds for frequencies less than 8 kHz (Fig. 4, upper left panel), over an average of 4.02 ± 1.97 years. In contrast, significant age-related threshold increases were observed across the tested frequency range for Metabolic and Metabolic + Sensory ears (Fig. 4). Similarly, Sensory ears demonstrated significant age-related threshold increases, but only for frequencies ≥0.5 kHz (Fig. 4). Few significant interactions between sex and age were observed: Males exhibited smaller threshold changes than females at 4 and 8 kHz (Metabolic + Sensory). When threshold changes were tested without separating phenotypes, there were significant sex interactions with age (Bonferroni-corrected P value <0.05) such that males exhibited significantly larger threshold changes at 2 kHz and females exhibited larger changes at 4, 6, and 8 kHz. Together with the sex distributions reported in Table 1, these results are consistent with the prediction that distinct phenotypes (e.g., more Sensory males, more Older-Normal or Metabolic females) can account for sex differences in age-related hearing loss. Detailed results from the GLMM regression models are provided in Supplementary Tables 1 and 2.
Audiometric patterns consistent with each phenotype were preserved despite increasing thresholds. The simulated pure-tone threshold values were produced with a GLMM linear regression model that estimated age-related changes separately for each frequency and phenotype for females (left column), males (open circles, middle column), and their combined data (right column).
Mean threshold changes as a function of frequency (dB/year, SEM bars), estimated with separate GLMM regressions for frequency and phenotype. Asterisks denote significant hearing sensitivity declines with increasing age: *P < 0.05 and **P < 0.001 (Bonferroni corrected). Changes were estimated and plotted separately where sex significantly interacted with age effects, which only occurred for the Metabolic + Sensory ears at 4 and 8 kHz (Bonferroni corrected P < 0.05; open circles depict mean threshold change for males).
Phenotypes that Changed with Increasing Age
Evidence for longitudinal changes in phenotype was observed for 264 out of 686 ears (38.5 %) in addition to increased hearing loss. Audiograms were collected for an average period of 8.2 ± 4.8 years for the ears with changing audiometric phenotypes. Audiometric phenotypes appeared to change at an average transition age of 74.2 ± 6.4 years. Transition age significantly differed in relation to the phenotype to which each ear changed (i.e., final phenotype; F(3, 260) = 20.35, P = 7.04 × 10−12); all post hoc pairwise comparisons were significant (Tukey P < 0.05). For example, ears that were classified as Older-Normal phenotype transitioned to Sensory or Metabolic phenotypes when participants were in their early 70s, whereas ears changed from Metabolic or Sensory phenotypes to Metabolic + Sensory when participants were in their mid-70s. The final phenotype observed for each ear significantly differed depending on the initial phenotype for each ear (Χ 2 = 99.32, P = 0.0005). Consistent with age-related strial declines, the majority of ears with changing phenotypes transitioned to metabolic types: 54.2 % became Metabolic + Sensory (130 ears), and 21.7 % became Metabolic (52 ears). Demographic characteristics of ears with changing phenotypes are summarized by initial phenotype in Tables 2 and 3.
Figure 5 shows examples of Older-Normal ears that exhibited changes in audiometric shape over the course of 8 to 15 years, such that their classification also transitioned to Metabolic, Sensory, or Metabolic + Sensory phenotypes. Figure 5 also shows examples of Metabolic or Sensory ears that exhibited characteristic low-frequency threshold increases that are proposed to reflect metabolic declines, resulting in their transition to the Metabolic + Sensory phenotype over 13–19 years. These examples support the implicit assumption that, in certain cases, ears can change from one phenotype category to another with increasing age, over the course of years or even decades.
Example audiogram data from individual ears that changed audiometric phenotype with increasing age. Ears that were initially classified as Older-Normal phenotype and later transitioned to Metabolic, Sensory, or Metabolic + Sensory phenotypes (top row). Ears initially classified as Metabolic or Sensory phenotype and later transitioned to Metabolic + Sensory phenotype (bottom row).
The QDA classification probabilities plotted as a function of age within each phenotype transition type (Fig. 6) revealed substantial variability in the trajectory across individual ears. Because the classification probability reflects the similarity of an averaged audiogram to the audiograms in the training dataset, changes in classification probability with increasing age indicate that audiograms became less similar to one phenotype and more similar to another. The example audiograms (Fig. 5) and estimated threshold changes (Fig. 7) both show that phenotype changes occurred relatively gradually.
Most of the individual ears that changed phenotypes with increasing age (four shaded regions; classification probabilities on the ordinate) transitioned to a Metabolic or Metabolic + Sensory phenotype (O-N Older-Normal, MET Metabolic, SENS Sensory, MET + SENS Metabolic + Sensory). Classification probabilities (abscissa, points) varied with age. Each subplot displays a particular phenotype transition (e.g., Older-Normal to Metabolic (top row)). The Older-Normal ears that appeared to transition directly to Metabolic + Sensory changed phenotypes during a relatively long interval between clustered visits (8.74 ± 1.17 years), compared to those that changed to Metabolic phenotypes (5.98 ± 0.88 years) or Sensory phenotypes (5.03 ± 0.51 years).
Mean threshold changes as a function of frequency (dB/year, SEM bars) for ears with changing phenotypes, estimated with separate GLMM regressions for frequency and phenotype (same procedure as shown in Fig. 4). Asterisks denote significant hearing sensitivity declines with increasing age: *P < 0.05 and **P < 0.001 (Bonferroni corrected). There were no significant interactions between participant sex and age-related threshold changes.
Participant age during the first cluster of visits was significantly different among initial phenotypes, F(3, 260) = 12.04, P = 2.09 × 10−07, with Older-Normal ears younger than the others (Tukey P < 0.001). Phenotype transitions occurred at different ages for the phenotypes, F(3, 260) = 4.96, P = 0.0023, and pairwise comparisons showed that Older-Normal ears changed at younger ages than Sensory ears (Tukey P < 0.001).
Sex differences were observed for the classified ears (Χ 2 = 17.33, P = 0.0006), as a smaller proportion of ears that were initially Sensory were females (54 %) compared to the other phenotypes (Χ 2 = 10.47, P = 0.0012), which were predominantly female (74 %). Positive noise history differed across initial phenotype categories for ears with changing phenotypes (Χ 2 = 13.26, P = 0.004). Post hoc comparisons showed that Sensory and Metabolic + Sensory ears both were again more likely to have a positive noise history compared to Older-Normal ears (Χ 2 = 4.43, P = 0.036; Χ 2 = 5.98, P = 0.017, respectively). In contrast, Metabolic ears were no more likely to have a positive noise history compared to Older-Normal ears (Χ 2 = 2.20, P = 0.19). The demographic differences among phenotypes are summarized in Tables 2 and 3.
GLMMs were used to estimate age-related threshold changes separately within each pure-tone frequency, for each observed phenotype transition type (Fig. 7). Older-Normal ears that transitioned to Metabolic, Sensory, or Metabolic + Sensory phenotypes exhibited significant threshold changes for frequencies above 0.25 kHz, unlike the stable Older-Normal ears with threshold changes <0.5 dB per year for all but one frequency (Fig. 4). Metabolic ears and Sensory ears that transitioned to the Metabolic + Sensory phenotype showed significant threshold increases across the measured frequencies, with larger estimated changes at lower frequencies than their stable phenotype counterparts. Age-sex interactions were tested, but none was statistically significant, suggesting that threshold changes were no different for female and male ears that transitioned from one phenotype category to another. However, sensitivity to sex-related differences in threshold changes was potentially limited by the sample size for some phenotype transitions (e.g., 49 females and 5 males classified as Older-Normal phenotype).
The Likelihood of Changing Phenotypes
Phenotype category changes differed among the phenotypes. The conditional likelihood of each observed phenotype change was calculated based on ears with the same audiometric phenotype during their initial visit (Fig. 8). Older-Normal ears demonstrated the highest likelihood of a phenotype transition (55 % changed), whereas the least likely change was observed for Metabolic + Sensory ears (11 %). This trajectory was expected, given the capacity for threshold changes, but this observation provided additional validation for QDA classifications showing phenotypes that change with increasing age.
The likelihood of phenotype change or no change (left). Filled circles depict the phenotype classification at the initial visit (Older-Normal (gray), Metabolic (green), Sensory (red), Metabolic + Sensory (blue)), and same-colored lines depict phenotype changes by the final visit (e.g., 30 % of Sensory ears became Metabolic + Sensory). Closed loops show ears that were classified with the same phenotype at the initial and final visit. The transitional probabilities sum to 100 % for each phenotype, although the total for the rounded percentages was 101 % for the Older-Normal phenotype. A frequency matrix shows the total number of ears that were classified with each phenotype at their initial visit (rows) and final visit (columns) (right). Ears with the same phenotype at the initial and final visit (diagonal) included 422 ears with stable phenotypes as well as 24 ears with a different intermediate classification, which could reflect an ambiguous audiometric configuration or classification error.
Transitional counts showed that transitions to Metabolic and Metabolic + Sensory categories were quite common for older adults with increasing age. Although the largest portion of changing Older-Normal ears became Sensory, ears in the Metabolic and Sensory phenotypes predominantly became Metabolic + Sensory. With 52 ears added to the Metabolic phenotype and 130 added to the Metabolic + Sensory phenotype by their final visit, 76 % of the ears with changing phenotypes and 27 % of all the ears in the sample transitioned to a metabolic type of hearing loss. These results support the conclusion that the likelihood of Metabolic or Metabolic + Sensory phenotypes increases with increasing age in middle to older adulthood.
Positive noise history differed based on the initial phenotype across all ears (Χ 2 = 24.00, P = 2.50 × 10−5; percentages reporting noise history: Older-Normal = 31 %, Metabolic = 35 %, Sensory = 53 %, Metabolic + Sensory = 53 %). The results of a logistic regression demonstrated that the likelihood of a positive noise history was significantly higher for ears initially classified as Sensory (Z = 2.54, two-tailed P = 0.011) or Metabolic + Sensory (Z = 2.54, P = 0.011) but was not significantly different for the final phenotypes when the initial and final classes were included in the same regression model (Sensory Z = 1.20, P = 0.23; Metabolic + Sensory Z = 0.76, P = 0.45). Self-reported noise history was uniquely associated with the initial audiometric phenotype and not the final phenotype, which indicates that noise history did not affect longitudinal phenotype changes or stability.
Discussion
The results from this longitudinal study demonstrate that Metabolic and Metabolic + Sensory phenotypes are more likely with increasing age. Most ears exhibited a stable audiometric phenotype for several years, and those classified as Metabolic or Metabolic + Sensory were older on average than ears from the other phenotypes. Among the ears with audiometric phenotypes that transitioned to a different phenotype with increasing age, the majority transitioned to a Metabolic or Metabolic + Sensory phenotype at an older average age than the others. Age-related differences in phenotype and increased likelihood of metabolic-type age-related hearing loss in the longitudinal dataset reinforce the conclusion that gradual age-related metabolic declines are a primary contributor to age-related hearing loss (Dubno et al. 2013). The Sensory and Metabolic + Sensory ears were more likely to have a positive noise history than the Older-Normal ears, while Metabolic ears were not, consistent with the premise that noise-related damage is more likely to be involved for ears with sensory declines.
Ears with stable phenotypes nonetheless demonstrated significant threshold changes that conformed to the characteristic audiometric patterns for each phenotype. For example, Older-Normal ears only exhibited a significant threshold change at the highest frequency, consistent with relatively flat, mild hearing loss seen in their audiograms. The Metabolic and Metabolic + Sensory ears exhibited larger threshold changes for higher frequencies than lower frequencies, which would form the sloping pattern of hearing loss common for those phenotype categories. The Sensory ears also exhibited threshold changes for frequencies ≥2 kHz and above, which leads to the steeply sloping hearing loss in their audiograms. Threshold changes of this magnitude were not predicted for ears with sensory hearing loss, which is hypothesized to result from sensory cell damage related to environmental exposures.
A few potential explanations were considered for the unexpected result that indicated a continued increase in higher frequency thresholds for the stable Sensory ears. The estimated threshold changes were faster for frequencies ≥2 kHz and slower for frequencies <2 kHz among Sensory ears, which would produce a pattern of hearing loss that is consistent with the typical Sensory audiometric shape. Because the threshold changes would produce a characteristic Sensory profile, these results appeared unlikely to reflect misclassified audiograms. Certain mutant mouse strains (e.g., CBA/J mice) exhibit age-related sensory cell losses that are most concentrated at the apex (Sha et al. 2008), although characterizing genetic predispositions is beyond the scope of the current study. Another possibility is that the Sensory ears had ongoing noise exposure that continued to increase hearing thresholds over time. This is difficult to assess because a positive noise history would simply remain positive longitudinally, which is why the likelihood of a positive noise history was significantly higher for some initial phenotypes when controlling for final phenotypes, but not for the final phenotypes. Nonetheless, self-reported noise history in this dataset confirmed that the likelihood of a positive noise history differed based on phenotype, with the highest rates observed for the Sensory and Metabolic + Sensory phenotypes. Finally, it has been proposed that hearing damage could trigger cochlear pathologies that endure for many years, which could also account for the continued threshold increases for the Sensory ears (Gates et al. 2000; Kujawa and Liberman 2006). However, those studies reported slower age-related threshold changes for frequencies with the most hearing damage and faster losses in adjacent regions of the audiogram, which contrast with our results that faster threshold changes occurred for the frequencies with the most hearing loss. Consistent with Lee et al. (2005), we did not observe differences in the rate of threshold change for Sensory ears based on self-reported noise history, which would be expected if progressive age-related sensory losses occurred independently of noise exposure or if noise exposure resulted in faster declines over many years. It is unclear why significant high-frequency threshold changes were seen for the stable Sensory ears.
Few significant differences were observed between males and females when threshold changes were estimated within each phenotype category. As noted in the introduction, sex differences in hearing loss are commonly observed in studies of age-related hearing loss and are hypothesized to reflect differences in noise exposure (Jerger et al. 1993; Demeester et al. 2009; Dubno et al. 2013). Consistent with the view that noise-induced hearing damage affects more males than females, Sensory ears were more likely to be male, while Metabolic and Older-Normal ears included more females in the current longitudinal results as well as the previous cross-sectional results (Dubno et al. 2013). These findings suggest that phenotype differences with distinct cochlear pathologies could be misattributed to sex differences. For example, relatively slower threshold changes were observed for an all-male sample of older adults studied by Echt et al. (2010) and relatively faster threshold changes were observed for an older adult sample with males and females (Lee et al. 2005). Because males are relatively more likely to exhibit sensory hearing loss and females are more likely to exhibit metabolic hearing loss, these threshold changes potentially differ in rate and pattern as a result of phenotype differences. Age-related hearing loss may be characterized more accurately by examining longitudinal changes in relation to distinct audiometric phenotypes in addition to sex differences.
The current results appear to demonstrate changes in audiometric phenotype that occurred within individual participants, which validated an implicit assumption that transitions between phenotypes occur for older adults. Ears with stable phenotypes and changing phenotypes both exhibited classification probabilities that varied with increasing age, and a phenotype transition occurred whenever the probability for an ear’s initial phenotype was exceeded by the probability of a different phenotype. Because classification probability reflects the extent to which an audiogram’s shape matched those in the training dataset, this progression indicates that audiograms with changing phenotypes gradually shifted from one audiometric configuration to another with increasing age. Example audiograms for individuals that transitioned from Older-Normal to Metabolic, Sensory, or Metabolic + Sensory phenotypes with increasing age are shown in Figure 5. Because their threshold changes were larger and occurred more quickly than for the stable ears, longer periods of time are likely needed to observe these changes for most ears.
When different phenotype transitions were examined, it was apparent that not all transitions were equally likely. Most of the ears that changed were observed to transition to a Metabolic or Metabolic + Sensory phenotype, confirming the prediction that those phenotypes are increasingly likely for older adults with increasing age. The likelihood of a phenotype change or no change also depended on the initial phenotype. First, there were differences in the capacity for threshold changes that restrict potential changes in phenotype. For example, Older-Normal ears were the most likely to transition to any other phenotype with increasing age, whereas the Metabolic + Sensory ears were the least likely to transition to anything else. Second, distinct audiometric shapes and potentially distinct underlying pathologies limited exchange between the Metabolic and Sensory phenotypes, aside from classification errors. There appeared to be two distinct routes from the Older-Normal phenotype to the Metabolic + Sensory phenotype, which typically involved an intermediate stage of Metabolic or Sensory phenotype. The Older-Normal ears that appeared to directly transition to Metabolic + Sensory phenotype changed during relatively long intervals between clustered visits, so an intermediary stage of Metabolic or Sensory phenotype cannot be ruled out.
As previously noted (Schmiedt 2010; Dubno et al. 2013), animal models that were used to establish audiometric patterns from distinct cochlear pathologies were obtained in acoustically controlled environments. In contrast, age-related hearing loss for older adults is caused by a complex mixture of genetic, health, and environmental risk factors. An accumulation of risk factors, exposures, and damage across the lifespan produces age-related hearing loss with metabolic and sensory characteristics for many older adults (Dubno et al. 1984; Allen and Eddins 2010). Age-related hearing loss could potentially be modeled effectively with distinct metabolic and sensory mechanisms that differentially combine to produce a variety of metabolic, sensory, and metabolic + sensory pathologies.
Limitations
Audiograms were categorized within audiometric phenotypes based on their similarity to a distribution of expert-labeled audiograms. One important limitation of the current approach is that audiometric configurations for individuals are not always well-represented by the four phenotype categories. Careful screening of participants and audiograms can limit misclassification of presbyacusis phenotypes for children, younger adults, or people with a congenital hearing loss (i.e., “corner audiograms”) instead of age-related hearing loss, per se. Because audiograms from older adults reflect an accumulation of combined genetic, health, and environmental risk factors that impact hearing sensitivity across the lifespan, even carefully selected and correctly classified audiograms will vary considerably within phenotype categories. Hearing loss reflects a mixture of distinct influences that could be useful to disentangle in future studies, for example, by estimating the relative contribution of the sensory or metabolic declines.
Another consideration regarding the current findings is the sample composition. Participants with longitudinal data included a relatively large proportion of Metabolic + Sensory ears, more females than males, and 2–5-year intervals of longitudinal data. While the current longitudinal dataset established relative differences in phenotype transitions and patterns of threshold change, a larger dataset could produce more detailed information about the distribution of phenotypes for the larger population. A clinical database could potentially be illustrative, although careful screening would be necessary to avoid misclassification of hearing loss that is not related to aging per se.
Although this was not a limitation of the current study, we note that several measures were taken in the current analyses to prevent circularity in the analyses and results. First, the optimal number of shape parameters and the best training dataset were determined on the basis of LOOCV procedures to preserve independence between the audiograms used to train and test the QDA model, described earlier. Second, the longitudinal data were largely distinct from the cross-sectional audiogram data used to train the QDA model. Furthermore, the QDA classifications for audiograms were naïve with respect to the specific identity of each ear, sex, noise history, and age, so differences in those characteristics were not influenced by the categorization process. Audiometric differences were not directly tested between the phenotypes, because those differences would depend on the same data that generated the phenotype classifications.
The current study provided additional evidence for distinct audiometric phenotypes that can change with increasing age in middle to older adulthood, as well as further validation for phenotype classification. The classification accuracy was observed to improve within a larger training dataset, which suggests that the development of more extensive training datasets could further enhance this method. Future studies could also use a similar automatic classification approach to characterize the relative contributions of distinct mechanisms in presbyacusis and determine the prevalence of audiometric phenotypes in a clinical audiometric database.
Conclusions
Building on previous observations with a cross-sectional audiogram dataset (Dubno et al. 2013), the current study used pure-tone thresholds measured longitudinally to classify phenotypes that are hypothesized to reflect distinct etiologies. Consistent with the view that age-related hearing loss is a metabolic rather than a sensory disorder, individuals categorized within the Metabolic and Metabolic + Sensory phenotypes were the oldest. Furthermore, most individuals that exhibited a change in phenotype category transitioned to Metabolic phenotypes at older ages compared to other phenotype transitions. The longitudinal audiometric data revealed threshold changes that produce characteristic audiogram configurations for each phenotype, including age-related threshold changes at higher frequencies for the Sensory phenotype that were larger than expected. Furthermore, well-established sex differences in audiometric change were minimized when age-related threshold changes were estimated separately for each phenotype. Phenotype classification methods have the potential to facilitate our ability to accurately categorize hearing loss mechanisms and predict declines based on audiometric data. The current results from classifying longitudinal audiograms provided additional evidence that age-related hearing loss commonly includes metabolic-type declines for middle-aged to older adults.
References
Allen PD, Eddins DA (2010) Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hear Res 264:10–20
American National Standards Institute (2010) Specification for audiometers. ANSI S3.6–2010. American National Standards Institute, New York
American Speech-Language-Hearing Association (2005) Guidelines for manual pure-tone threshold audiometry. American Speech–Language–Hearing Association, Rockville, MD
Cruickshanks KJ, Tweed TS, Wiley TL et al (2003) The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg 129:1041–1046
Demeester K, Van Wieringen A, Hendrick J et al (2009) Audiometric shape and presbycusis. Int J Audiol 48:222–232
Dubno JR, Dirks DD, Morgan DE (1984) Effects of age and mild hearing loss on speech recognition in noise. J Acoust Soc Am 76:87–96
Dubno JR, Eckert MA, Lee FS et al (2013) Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol 14:687–701
Echt KV, Smith SL, Burridge AB, Spiro A (2010) Longitudinal changes in hearing sensitivity among men: the veterans affairs normative aging study. J Acoust Soc Am 128:1992–2002
Figueroa RL, Zeng-Treitler Q, Kandula S, Ngo LH (2012) Predicting sample size required for classification performance. BMC Med Inform Decis Mak 12:8
Gates GA, Schmid P, Kujawa SG et al (2000) Longitudinal threshold changes in older men with audiometric notches. Hear Res 141:220–228
Jerger J, Chmiel R, Stach B, Spretnjak M (1993) Gender affects audiometric shape in presbyacusis. J Am Acad Audiol 4:42–49
Kaya KH, Koç AK, Sayın İ et al (2015) Etiological classification of presbycusis in Turkish population according to audiogram configuration. The Turkish Journal of Ear Nose and Throat 25:1–8
Kujawa SG, Liberman MC (2006) Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26:2115–2123
Lee FS, Matthews LJ, Dubno JR, Mills JH (2005) Longitudinal study of pure-tone thresholds in older persons. Ear Hear 26:1–11
Matthews LJ, Lee FS, Mills JH, Dubno JR (1997) Extended high-frequency thresholds in older adults. J Speech Lang Hear Res 40:208–214
Mills JH, Schmiedt RA, Kulish LF (1990) Age-related changes in auditory potentials of Mongolian gerbil. Hear Res 46:201–210
Mills JH, Schmiedt RA, Schulte BA, Dubno JR (2006) Age-related hearing loss: a loss of voltage, not hair cells. Semin Hear 27:228–236
Schmiedt RA (2010) The physiology of cochlear presbycusis. In: Gordon-Salant S, Frisina RD, Popper AN, Fay R (eds) The aging auditory system. Springer, New York, pp. 9–38
Schmiedt RA (1996) Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hear Res 102:125–132
Schmiedt RA, Lang H, Okamura H-O, Schulte BA (2002) Effects of furosemide applied chronically to the round window: a model of metabolic presbyacusis. J Neurosci 22:9643–9650
Schuknecht HF, Gacek MR (1993) Cochlear pathology in presbyacusis. Ann Otol Rhinol Laryngol 102:1–16
Sha SH, Kanicki A, Dootz G et al (2008) Age-related auditory pathology in the CBA/J mouse. Hear Res 243:87–94
Tarnowski BI, Schmiedt RA, Hellstrom LI et al (1991) Age-related changes in cochleas of mongolian gerbils. Hear Res 54:123–134
Acknowledgments
This work was supported by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (P50 DC 000422) and the South Carolina Clinical and Translational Research (SCTR) Institute, NIH/NCRR grant number UL1 RR029882. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program, NIH/NCRR grant number C06 RR14516. We also thank the study participants.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors state that they have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Vaden, K.I., Matthews, L.J., Eckert, M.A. et al. Longitudinal Changes in Audiometric Phenotypes of Age-Related Hearing Loss. JARO 18, 371–385 (2017). https://doi.org/10.1007/s10162-016-0596-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-016-0596-2