Abstract
Background
Experimental studies suggest a detrimental role for cyclic adenosine monophosphate (cAMP) and vasopressin in the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD). It is unknown, however, whether urinary cAMP and copeptin concentration are associated with disease severity in patients with ADPKD.
Methods
Urinary cAMP (u-cAMP) and copeptin concentration (u-copeptin) were measured by immunoassay in ADPKD patients with CKD stage ≤4. We compared our measurements with clinical parameters including estimated glomerular filtration rate (eGFR), total kidney volume (TKV), and height-adjusted TKV (htTKV). Logarithmic transformation of all variables was performed to fulfill the requirement of equal distribution of the residuals.
Results
We included 50 patients in this study (24 females and 26 males; mean age: 49.3 years). The median eGFR and TKV were 53.2 ml/min/1.73 m2 (interquartile range: IQR; 29.4–68.45) and 1138.1 ml (IQR; 814.7–2065.0), respectively. The median u-copeptin level was 12.19 (IQR; 6.91–22.32) ng/ml. Although u-cAMP/u-Cr was not significantly correlated with TKV (R = −0.006, p = 0.967) and eGFR (R = 0.077, p = 0.602), urinary copeptin/u-Cr was statistically associated with the various markers of disease severity in ADPKD [positively with TKV (R = 0.351, p = 0.014), htTKV (R = 0.383, p = 0.008) and negatively with eGFR (R = −0.304, p = 0.036)].
Conclusions
In ADPKD subjects, a higher u-copeptin is associated with disease progression, suggesting that u-copeptin may be a new surrogate marker to predict renal prognosis in ADPKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common progressive hereditary kidney disease. Since the intact kidneys can compensate for the loss of glomerular filtration in ADPKD patients, renal insufficiency usually remains undetected until close to almost the fourth decade of life, progressing rapidly once a critical expansion of renal cysts is reached [1]. Hence, reliable diagnostic and prognostic biomarkers to identify ADPKD progression are urgently needed.
When arginine vasopressin (AVP) is bound to the V2 receptor (V2R), cyclic adenosine monophosphate (cAMP) production is stimulated. In turn, cAMP leads to the proliferation of epithelial cells and stimulates cyst formation [2]. AVP measurement is often cumbersome because it is unstable with a short half-life [3] and is difficult to measure [4]. Copeptin consists of the C-terminal portion of pro-AVP and is produced in equimolar amounts as AVP during precursor processing [5]. Copeptin has been shown to be a relatively easily measured [6], reliable and stable substitute for circulating AVP concentration [7–9].
Recently, several studies reported that plasma copeptin concentration was associated with ADPKD progression [10–13]. As far as we know, however, there are no previous reports concerning either the efficacy of urine copeptin as a surrogate marker of ADPKD progression or the association between urine cAMP levels and disease severity in ADPKD patients.
The novel treatment of V2R antagonist for ADPKD began in Japan under national health insurance in 2014. As we urgently need precise and handy biomarkers of disease progression associated with ADPKD advancement, we investigated the association between urine copeptin and urine cAMP concentration and ADPKD disease progression in this study, measured as estimated glomerular filtration rate (eGFR) and total kidney volume (TKV) in ADPKD patients.
Materials and methods
Study population and design
The present study enrolled individuals from January to October, 2014 at our outpatient clinic who had been diagnosed as having ADPKD according to Ravine’s criteria [14]. We included ADPKD patients with CKD stage ≤4. Exclusion criteria were as follows: use of medication influencing renal concentration capacity, such as diuretics and postmenopausal hormone therapy; history of diseases influencing renal concentration capacity, such as diabetes mellitus, diabetes insipidus, hypothalamic–pituitary–adrenal deficiency, or kidney diseases other than ADPKD; other factors that can influence renal concentration capacity such as menstruation, urinary tract infection, and pregnancy; and contraindication of magnetic resonance imaging (MRI). However, patients on antihypertensive agents were eligible for inclusion in this study.
This study was approved by our institutional review board (approval number: 070475) and was performed in adherence to the Declaration of Helsinki. All participants gave written informed consent. We excluded the patients without informed consent in this study.
Weight, height, body mass index (BMI) calculated as weight (kg) divided by height (m2), and waist circumference were measured upon admission. Blood pressure was measured in the morning before antihypertensive medication intake. Systolic and diastolic BP values were used to calculate mean arterial pressure (MAP) using the standard formula 2/3 diastolic BP + 1/3 systolic BP.
Blood and spot urine samples were collected in the morning prior to taking medications or food. Plasma osmolality (s-OSM), sodium (s-Na), creatinine (s-Cr), and cystatin C were measured in these blood samples using standard laboratory techniques. Urinary sodium (u-Na), N-acetyl-β-D-glucosaminidase (NAG), creatinine (u-Cr), and osmolality (u-OSM) were measured in these spot urine samples by freezing point depression. Fractional excretion of sodium (FENa) was calculated as [(urinary sodium concentration/plasma sodium concentration)/(u-Cr concentration/s-Cr concentration)] × 100.
The eGFR was used as a marker of renal function and was calculated using the simplified MDRD equation modified by the appropriate coefficient for Japanese populations by sex as follows: eGFR = 194 × Cr−1.094 × Age−0.287 (female: ×0.739) (ml/min/1.73 m2) [15].
A sandwich immunoassay was used to measure the value of urine copeptin (Copeptin, human—EIA Kit, Bachem Americas, Inc. Torrance, CA, USA), plasma copeptin (Copeptin, human—EIA Kit, Peninsula Laboratories International, Inc. San Carlos, CA, USA) and urine cAMP levels (Cyclic AMP Direct EIA kit, Arbor Assays, Inc., Ann Arbor, MI, USA), respectively.
TKV was measured by performing standard abdominal MRI without the use of intravenous contrast on T2-weighted images. TKV was obtained by calculating using a standard formula: Renal volume = π/6 × length × width × depth [16]. Length and width were obtained from longitudinal images acquired in planes ranging from sagittal to coronal, whereas depth was obtained from transverse images of the mid-kidney acquired in the plane perpendicular to the longitudinal plane. Because height was the best reference for TKV, we also examined with height-adjusted TKV (htTKV) (cm3/m) using ellipsoid methods as mentioned above.
Statistical analysis
Because it is impossible to test the aliquot sample from 24-hour urine collection, we evaluated not only u-cAMP and u-copeptin but also u-cAMP divided by u-Cr (u-cAMP/u-Cr) and u-copeptin/u-Cr. To test the correlations between u-cAMP, u-cAMP/u-Cr, u-copeptin and u-copeptin/u-Cr [17], all variables were logarithmically normalized [11, 18]. Variables are expressed as median with interquartile range (IQR) for nonparametric data. Pearson’s correlation coefficients were used to examine the associations among selected clinical variables and variables representing disease severity. R 2.14.0 was used to determine the outcome measure [19]. Two-tailed p values of <0.05 were considered to indicate a statistically significant difference.
Results
Patients’ characteristics (Table 1)
This study involved 50 patients with ADPKD (26 men and 24 women; mean age: 49.3 years). Table 1 shows the subjects’ baseline characteristics. The median eGFR, cystatin C and TKV were 53.2 ml/min/1.73 m2 (interquartile range: IQR; 29.4–68.45), 1.12 mg/l (IQR; 0.95–2.01), and 1138.1 ml (IQR; 814.7–2065.0), respectively. At baseline, eGFR and TKV showed significant mutual inverse correlation (R = −0.524, p < 0.001). Of all included patients, 45 patients (90 %) take antihypertensive agents.
The efficacy of u-cAMP and u-cAMP/u-Cr in detecting ADPKD progression
The median urinary cAMP level was 1184.75 (IQR; 639.50–1216.78) pmol/L. Table 2 lists results of the associations of urine cAMP and u-cAMP/u-Cr levels with physiological parameters and measures of ADPKD disease severity. Neither u-cAMP nor u-cAMP/u-Cr showed significant correlation with TKV (u-cAMP; R = −0.104, p = 0.482, u-cAMP/u-Cr; R = −0.006, p = 0.967), htTKV (u-cAMP; R = −0.184, p = 0.215, u-cAMP/u-Cr; R = −0.035, p = 0.817), or eGFR (u-cAMP; R = 0.224, p = 0.126, u-cAMP/u-Cr; R = 0.077, p = 0.602). Although u-cAMP was significantly correlated with u-OSM (R = 0.520, p < 0.001), no significant association was found between u-cAMP and s-OSM (R = −0.217, p = 0.144). In addition, there was no significant correlation between u-cAMP/u-Cr and u-OSM (R = −0.193, p = 0.254) or s-OSM (R = −0.094, p = 0.528). Examining u-cAMP and other markers of disease severity for any association, we found that u-cAMP was positively correlated with u-Cr (R = 0.595, p < 0.001), NAG (R = 0.465, p < 0.001) and inversely correlated with FENa (R = −0.460, p = 0.001). There was no significant association between u-cAMP/u-Cr and any parameter without u-copeptin/u-Cr (Table 3).
The efficacy of u-copeptin and u-copeptin/u-Cr in detecting ADPKD progression
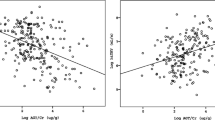
The median urinary copeptin level was 12.19 (IQR; 6.91–22.32) pmol/L. As with u-cAMP, we evaluated not only c-copeptin, but also u-copeptin divided by u-Cr (u-copeptin/u-Cr). We could not find any sex-based difference in u-copeptin (men: median R = 11.076 ng/ml and IQR 4.436–20.823; women: median 12.721 ng/ml and IQR 9.254–24.826; p = 0.319). However, median u-copeptin/u-Cr was significantly higher in women (median 0.219, IQR 0.188–0.387) than in men (median 0.144, IQR 0.095–0.257) (p = 0.020). Only in cases with preserved blood plasma (n = 18), we examined the plasma copeptin values. We could show the positive-correlation between u-copeptin and plasma copeptin but without significant relationship (p = 0.198) mainly because of a small number cases with preserved blood plasma (Fig. 1a). Although there are no significant correlations between plasma copeptin and eGFR (R = −0.245, p = 0.227), there are significant correlations between plasma copeptin and htTKV (R = 0.458, p = 0.019) and TKV (R = 0.465, p = 0.017).
Scatter plots for correlation between u-copeptin/u-Cr and markers of disease severity. a plasma copeptin: R = 0.318 (95 % CI; −0.174 to 0.684) p = 0.198. b TKV: R = 0.351 (95 % CI; 0.075–0.578) p = 0.014. c htTKV: R = 0.383 (95 % CI; 0.107–0.604) p = 0.008. d eGFR: R = −0.304 (95 % CI; −0.542 to −0.022) p = 0.036. e s-OSM: R = 0.306 (95 % CI; 0.020–0.545) p = 0.037. f u-OSM: R = −0.333 (95 % CI; −0.593 to −0.010) p = 0.044
Although u-copeptin was not significantly correlated with TKV (R = 0.261, p = 0.073), htTKV (R = 0.261, p = 0.076), and eGFR (R = −0.153, p = 0.301), urinary copeptin/u-Cr was positively associated with TKV (R = 0.351, p = 0.014, Fig. 1b), htTKV (R = 0.383, p = 0.008, Fig. 1c), and negatively associated with eGFR (R = −0.304, p = 0.036, Fig. 1d). With respect to osmolality, there were no significant differences between u-copeptin and s-OSM (R = 0.198, p = 0.183) or u-OSM (R = 0.200, p = 0.234). On the other hand, u-Copeptin/u-Cr was associated with s-OSM (R = 0.306, p = 0.037, Fig. 1e) and u-OSM (R = −0.333, p = 0.044, Fig. 1f).
U-copeptin was significantly correlated with u-cAMP (R = 0.527, p < 0.001) and u-cAMP/u-Cr (R = 0.361, p = 0.012). U-copeptin/u-Cr was also significantly correlated with u-cAMP/u-Cr (R = 0.460, p = 0.001).
Examining the association between u-copeptin and other markers of disease severity, we found that u-copeptin was significantly correlated with u-Cr (R = 0.324, p = 0.025) and NAG (R = 0.465, p < 0.001). U-copeptin/u-Cr was significantly inversely correlated with u-Cr (R = −0.370, p = 0.010).
Discussion
The relationship between renal function, TKV and cAMP, plasma copeptin in ADPKD patients
In ADPKD, disease progression causes decreased urinary concentrating capacity [12]. Binding of AVP to the V2R at collecting duct cells causes an increase in intracellular cAMP, which then leads to proliferation of epithelial cells and fluid secretion into cysts. However, the use of u-cAMP as a marker of AVP activity remains controversial [17]. Hypertonic saline infusion does not affect u-cAMP in patients with ADPKD [20]. In this study, we failed to show statistical correlations between u-cAMP, u-cAMP/u-Cr and markers of disease severity including TKV, htTKV and eGFR. Therefore, we regretfully conclude that cAMP is not useful as a surrogate marker of ADPKD disease progression.
Plasma copeptin and GFR were significantly associated in ADPKD patients, but not in renal donors without ADPKD [13]. Several previous studies reported that in ADPKD patients, plasma copeptin level is associated with rate of kidney function decrease [12, 21–23]. As indicated in this study, it was also reported that a higher TKV was independently associated with higher plasma copeptin levels [11]. Boertien WE et al. [10] reported that the higher the baseline copeptin concentration, the more measured GFR decreased and the more TKV increased, independent of age, sex, and kidney risk factors.
Validity of u-copeptin as a surrogate marker of ADPKD
Analysis of urine can offer a non invasive means to detect changes in the expression of proteins [24]. In contrast to other body fluids such as serum or plasma, urinary proteins do not undergo detectable degradation by endogenous proteases after voiding, thus minimizing the bias introduced by preanalytical sample handling [24].
Unfortunately, there is no literature concluding how copeptin is cleared from the body. Copeptin has a molecular weight of 5 kDa [6] and consequently is subjected to glomerular filtration. Taking the undeniable effect of urine volume into account, we evaluated not only u-copeptin but also u-copeptin/u-Cr, as has been done with albuminuria. Results of this study and previous reports suggest that decreased renal clearance may lead to higher plasma copeptin values and, consequently, higher u-copeptin/u-Cr values.
With respect to TKV, urinary copeptin and urinary copeptin/u-Cr correlated with TKV in this study, although these correlations were moderate. We therefore chose htTKV as a surrogate marker, as it has recently been shown to be a strong predictor of the development of stage 3 and 4 KDOQI CKD within 8 years in ADPKD patients [25]. In this study, a linear model to predict htTKV achieved a high accuracy.
It is a clinically accepted fact that patients with ADPKD already have decreased urinary concentrating capacity [26] at a young age [27], and that plasma osmolality is maintained within the normal range at the cost of higher plasma copeptin and AVP levels [18]. Boertien et al. [10] show that in relatively early stage ADPKD, plasma copeptin levels, as a marker for AVP, are not associated with plasma osmolality. However, in another study involving different populations, the same group found a significant association between plasma copeptin level and plasma osmolality [11]. On the other hand, two other studies performed in patients without ADPKD investigating the association between plasma osmolality and plasma copeptin level showed that in accordance with normal physiology, the higher the plasma osmolality, the higher the plasma copeptin level [21, 28]. Given these previous observations, it is difficult to conclude whether or not there is statistical relationship between plasma osmolality and plasma copeptin level in ADPKD. Our data indicated that u-copeptin/u-Cr was significantly positively associated with s-OSM and negatively associated with u-OSM. Previous report that maximal urinary concentration capacity was lower in ADPKD patients [18] may be consistent with our data of inversely significant correlation between u-copeptin/u-Cr and u-OSM.
Although several reports evaluate urine copeptin in model animals [29, 30], as far as we know, ours is the first report indicating the usefulness of u-copeptin as a valuable novel biomarker to identify ADPKD disease progression. Unlike previous studies involving plasma copeptin in patients in a relatively early phase of disease [18], ours enrolled ADPKD patients with CKD stage ≤4. As mentioned above, the novel treatment of V2R antagonist for ADPKD with CKD stage ≤4 began in Japan under national health insurance in 2014. Therefore, it may be possible to use u-copeptin/u-Cr as a therapeutic response evaluation of this therapy.
We acknowledge that this study has limitations. First, little is known about the freeze–thaw durability of urine copeptin. Second, patients were allowed to drink ad libitum in this study. After 14 h of water deprivation, ADPKD patients tended to have higher plasma osmolality and significantly higher plasma AVP and copeptin levels, whereas u-OSM was similar in ADPKD patients and controls [18]. Differences in hydration status between individuals may be lead to variability in urine copeptin concentration. We do not yet know how urine copeptin values might change following water load or deprivation. On the other hand, it is known from previous studies that plasma copeptin values can decrease very quickly after a water load [6], suggesting extrarenal clearance as the predominant clearance mechanism [10]. The relationship between u-copeptin and limitations on liquid intake should be the subject of a future investigation. Third, we do not know about u-copeptin values in healthy controls and patients with CKD other than ADPKD. It was reported that plasma copeptin is unspecific for ADPKD and mostly shows considerable overlap with healthy controls [31]. Therefore, from this study, one may not be able to conclude whether or not urine copeptin is a specific marker for ADPKD, or a marker for chronic kidney disease progression in general. Fourth, mainly because of a small number cases with preserved blood plasma (n = 18), we could not show the significant correlations between u-copeptin and plasma copeptin. The relationship between plasma copeptin and u-copeptin will be one of the agenda to be examined in the future. Fifth, no sex-based differences in u-copeptin values were found in this study. On the other hand, it was reported that plasma copeptin concentration was higher in men than in women [11]. Unfortunately, at present, we cannot determine the cause of this outcome. Hereafter, we should explore the root causes of this difference. Sixth, as a matter of course, we should perform validation analysis. Replication of our findings must precede their clinical usage.
In conclusion, our results suggest that u-copeptin/u-Cr might be a convenient and easily measured surrogate marker to help predict disease progression in ADPKD. It is tempting to hypothesize that, in ADPKD patients, a u-copeptin increase could be used as a surrogate marker to predict the treatment efficacy with respect to renoprotection.
References
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–301.
Mochizuki T, Tsuchiya K, Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol. 2013;17:317–26.
Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973;52:2340–52.
Kluge M, Riedl S, Erhart-Hofmann B, Hartmann J, Waldhauser F. Improved extraction procedure and RIA for determination of arginine8-vasopressin in plasma: role of premeasurement sample treatment and reference values in children. Clin Chem. 1999;45:98–103.
de Bree FM, Burbach JP. Structure-function relationships of the vasopressin prohormone domains. Cell Mol Neurobiol. 1998;18:173–91.
Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–9.
Morgenthaler NG, Struck J, Jochberger S, Dunser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–9.
Szinnai G, Morgenthaler NG, Berneis K, Struck J, Müller B, Keller U, et al. Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab. 2007;92:3973–8.
Roussel R, Fezeu L, Marre M, Velho G, Fumeron F, Jungers P, et al. Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab. 2014;99(12):4656–63.
Boertien WE, Meijer E, Li J, Bost JE, Struck J, Flessner MF, et al. Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: results from the CRISP cohort. Am J Kidney Dis. 2013;61:420–9.
Meijer E, Bakker SJ, van der Jagt EJ, Navis G, de Jong PE, Struck J, et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;6:361–8.
Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, et al. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2012;27:4131–7.
Zittema D, Berg EV, Meijer E, Boertien WE, Muller Kobold AC, Franssen CFM, et al. kidney function and plasma copeptin levels in healthy kidney donors and autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephol. 2014;9:1533–62.
Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–7.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Tokiwa S, Muto S, China T, Horie S. The relationship between renal volume and renal function in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2011;15:539–45.
Barash I, Ponda MP, Goldfarb DS, Skolnik EY. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:693–7.
Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, et al. Vasopressin, copeptin and renal concentrating capacity in ADPKD patients without renal impairment. Clin J Am Soc Nephrol. 2012;7:906–13.
R Development Core Team. R. a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2011.
Graffe CC, Bech JN, Lauridsen TG, Pedersen EB. Urinary excretion of AQP2 and ENaC in autosomal dominant polycystic kidney disease during basal conditions and after a hypertonic saline infusion. Am J Physiol Renal Physiol. 2012;302:F917–27.
Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010;77:29–36.
Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL. Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond). 2009;116:257–63.
Przybylowski P, Malyszko J, Malyszko JS. Copeptin in heart transplant recipients depends on kidney function and intraventricular septal thickness. Transplant Proc. 2010;42:1808–11.
Fliser D, Novak J, Thongboonkerd V, Argiles A, Jankowski V, Girolami MA, et al. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007;18:1057–71.
Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, et al. kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86.
Meijer E, Rook M, Tent H, Navis G, van der Jagt EJ, de Jong PE, et al. Early renal abnormalities in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:1091–8.
Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA. The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol. 1994;4:1654–60.
Meijer E, Bakker SJ, de Jong PE, Homan van der Heide JJ, van Son WJ, Struck J, et al. Copeptin, a surrogate marker of vasopressin, is associated with accelerated renal function decline in renal transplant recipients. Transplantation. 2009;88:561–7.
Littlejohn NK, Siel RB Jr, Ketsawatsomkron P, Pelham CJ, Pearson NA, Hilzendeger AM, et al. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am J Physiol Regul Integr Comp Physiol. 2013;304:R818–28.
Hilzendeger AM, Cassell MD, Davis DR, Stauss HM, Mark AL, Grobe JL, et al. Angiotensin type 1a receptors in the subfornical organ are required for deoxycorticosterone acetate-salt hypertension. Hypertension. 2013;61:716–22.
Kistler AD, Serra AL, Siwy J, Poster D, Krauer F, Torres VE, et al. urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS one. 2013;8:e5301.
Acknowledgments
This work was supported in part by a grant for the Progressive Renal Diseases Research Project from the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest
Honoraria: Satoru Muto, Shigeo Horie (Otsuka Pharmaceutical); Subsidies or Donations: Satoru Muto, Haruna Kawano, Shigeo Horie (Otsuka Pharmaceutical); Endowed departments by commercial entities: Shigeo Horie (Otsuka Pharmaceutical); Travel fees, gifts, and others: Satoru Muto, Shigeo Horie (Otsuka Pharmaceutical).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nakajima, A., Lu, Y., Kawano, H. et al. Association of arginine vasopressin surrogate marker urinary copeptin with severity of autosomal dominant polycystic kidney disease (ADPKD). Clin Exp Nephrol 19, 1199–1205 (2015). https://doi.org/10.1007/s10157-015-1101-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1101-7