Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common progressive hereditary kidney disease. In 85–90 % of cases, ADPKD results from a mutation in the PKD1 gene, and the other 10–15 % of the cases are accounted for by mutations in PKD2. PKD1 and PKD2 encode polycystin-1 and polycystin-2. Polycystin-1 may be a receptor that controls the channel activity of polycystin-2 as part of the polycystin signaling complex. ADPKD is characterized by the progressive development of fluid-filled cysts derived from renal tubular epithelial cells that gradually compress the parenchyma and compromise renal function. In recent years, considerable interest has developed in the primary cilia as a site of the proteins that are involved in renal cystogenesis. The pathological processes that facilitate cyst enlargement are hypothesized to result from two specific cellular abnormalities: (1) increased fluid secretion into the cyst lumen and (2) inappropriately increased cell division by the epithelium lining the cyst. Since there is no clinically approved specific or targeted therapy, current practice focuses on blood pressure control and statin therapy to reduce the cardiac mortality associated with chronic kidney disease. However, recent advances in our understanding of the pathways that govern renal cystogenesis have led to a number of intriguing possibilities in regard to therapeutic interventions. The purpose of this article is to review the pathogenesis of renal cyst formation and to review novel targets for the treatment of ADPKD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common progressive hereditary kidney disease, and its incidence is 1 per 500–1000 persons in the general population [1]. It is caused by a germline mutation in PKD1 (85–90 %) or PKD2 (10–15 %) [2]. ADPKD is characterized by the slow development over a period of decades of large fluid-filled cysts in the kidneys [3]. The cysts result in dramatic enlargement of the kidneys, and, more importantly, they severely compromise the functional integrity of the remaining normal parenchyma. Cyst initiation and expansion is a complex process characterized by abnormalities in tubular cell proliferation, fluid secretion, extracellular matrix formation, and cell polarity [4].
Clinically significant impairments of renal function in ADPKD usually occur by late middle-age, and end-stage kidney disease (ESKD) requiring renal replacement therapy occurs in approximately 50 % of patients by 70 years of age [5]. Renal function begins to decline in the fourth decade of life, when the glomerular filtration rate (GFR) begins to decrease by 4.4–5.9 ml/min/year [5]. Although GFR is routinely used as a biomarker of renal disease progression, since it does not decrease substantially until extensive and irreversible damage to the noncystic parenchyma occurs, it is necessary to identify more reliable biomarkers to follow the progression of this disease [6]. The decreases in GFR in ADPKD are inversely proportional to kidney size and cyst volume as assessed by the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) [7, 8]. Because ultrasound measurements cannot detect changes in kidney size over short time intervals, magnetic resonance (MR) imaging with or without gadolinium contrast enhancement has become the gold standard for assessing changes in kidney volume, and thus in the prognosis [9].
There are no clinically approved specific or targeted therapies for ADPKD. Many drugs are currently in the clinical trial stage, and angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), vasopressin V2 receptor antagonists, somatostatin, and mammalian target of rapamycin (mTOR) inhibitors continue to be evaluated in clinical trials [10]. However, since no one drug alone would appear to be fully capable of slowing the progressive cyst growth that occurs in ADPKD, combinations of approaches to the treatment of ADPKD still require further study. This review article focuses on recent advances in research on the pathogenesis of ADPKD and on its potential treatments.
Genetic background
In 85–90 % of cases, ADPKD results from a mutation in PKD1 (polycystic kidney disease 1, ch16p13.3, 46 exons) [11], and the other 10–15 % of the cases are accounted for by mutations in PKD2 (polycystic kidney disease 2, ch4q21, 15 exons) [12]. Given the large database, molecular diagnostics are now becoming an option in situations in which imaging studies are inconclusive, in situations in which patients wish to know their genetic predisposition, or to evaluate potential kidney donors [13].
PKD1 and PKD2 encode polycystin-1 and polycystin-2, respectively. As shown in Fig. 1, polycystin-1 comprises 4302 amino acids, and it has a large extracellular domain, 11 transmembrane spanning segments, and a short cytoplasmic tail [14, 15]. The extracellular NH2-terminal domain contains a distinct combination of protein motifs involved in protein–protein and protein–carbohydrate interactions. The region that consists of the last five transmembrane spans of polycystin-1 shares sequence homology with polycystin-2 [16]. Polycystin-2 consists of 968 amino acids, and it has 6 transmembrane domains [17]. It is a permeable Ca2+-non-selective cation channel that belongs to the transient receptor potential (TRP) cation channel subfamily [18]. The last five transmembrane spans in polycystin-2 bear a strong TRP channel signature, and the region between S5 and S6 contains the putative pore region [13]. The cytoplasmic tail of polycystin-2 contains a Ca2+ binding site and a coiled coil domain that is responsible for numerous protein–protein interactions [19]. Polycystin-1 and polycystin-2 interact through their respective C-termini [20]. The interaction depends on the integrity of the coiled coil domain in the C-terminus of polycystin-1 and has led to the hypothesis that polycystin-1 is a receptor that controls the channel activity of polycystin-2 as part of the polycystin signaling complex.
Both polycystin proteins have complex subcellular localizations. Polycystin-1 is found in the basolateral plasma membrane domain of polarized epithelial cells, where it participates in both intercellular adherence junctions and focal adhesion complexes with the underlying basement membrane [21]. In addition, a cleavage product of polycystin-1 that includes the C-terminal tail is capable of translocating to the nucleus and regulating gene transcription [22, 23]. Most of the polycystin-2 protein is concentrated in intracellular compartments, where it appears to play a role in regulating the release of Ca2+ from intracellular stores. Its role as a cation channel is consistent with the fact that it is a family of the ion channels [24]. Polycystin-2 may also play a role in cell proliferation and differentiation by regulating the cell cycle [25]. Both polycystin-1 and polycystin-2 are localized to the primary cilia that grace the apical surfaces of most polarized epithelial cell types. These non-motile, chemo- and mechano-sensory structures seem to be critically intertwined in the pathophysiology of renal cystic disease.
Mechanisms of cyst formation and enlargement in ADPKD
In ADPKD patients, every cell carries a mutated allele of either PKD1 or PKD2. However, cysts develop in only a small fraction of the nephrons, and they are thought to originate from clonal growth of single cells within the tubules [26]. A somatic mutation or insufficient expression of the wild-type allele is thought to initiate renal cyst formation. Cyst formation begins in a small fraction of renal tubular cells in which the levels of polycystin-1 and polycystin-2 drop below a critical threshold [27]. A somatic “second-hit” mutation, loss of heterozygosity, or haploinsufficiency may account for the mosaic nature of cyst formation [28, 29]. Cystic epithelial cells are characterized as being incompletely differentiated and persistently proliferative, and aberrant proliferation of tubular epithelial cells is thought to cause the wall of the tubule to expand to form a mural pocket. Most cysts that expand to approximately 2 mm in diameter detach from the parent tubule and become isolated fluid-filled sacs lined by an epithelial cell layer [30]. These isolated cysts continue to expand in size as a result of a combination of mural epithelial cell proliferation and transepithelial fluid secretion.
Recent evidence indicates that a significant reduction of functional polycystin-1 expression below a critical threshold level is sufficient to result in cyst formation in some situations [27, 31]. A unique chimeric animal model has been produced by mosaic embryos combining Pkd −/− cells with wild-type cells to form kidney cysts whose severity was proportional to the degree of the Pkd −/− contribution to the mosaic animal [32]. Interestingly, the cysts contained both Pkd −/− and wild-type cells in the early stages, but the Pkd −/− cells replaced the wild-type cells over time by inducing apoptosis (Fig. 2). These findings suggest the possibility that cellular loss of polycystin-1 results in cyst formation by both expansion of the null cell mass and induction of programmed cell death in surrounding normal cells.
The pathological processes that facilitate cyst enlargement are hypothesized to result from two specific abnormalities: (1) increased fluid secretion into the cyst lumen and (2) inappropriately increased cell division by the epithelium lining the cyst. The increased secretion might be expected to increase hydrostatic pressure inside the cyst and encourage expansion, while the increase in cell proliferation would simultaneously induce de novo cyst formation. A previous study demonstrated that the rate of fluid secretion into the cyst lumen is directly proportional to the amount of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel present in the apical membrane [33]. Since the CFTR chloride channel is activated by elevation of the cytosolic level of cyclic AMP [34], excessive tubular epithelial cell proliferation and fluid secretion may be under the control of inappropriately elevated cyclic AMP.
Ciliopathies and ADPKD
Recent accumulating evidence has led to the emergence of cilia as central to the pathogenesis of ADPKD (Fig. 3). One of the first discoveries that associated primary cilia with cystic disease was that the Tg737 gene, whose function is disrupted in the Oak Ridge Polycystic Kidney mouse (Tg737orpk) [35], is in an ortholog of the Chlamydomonas intraflagellar transport protein 88 (IFT88) [36]. Although the IFT88 mutant mice were already known to exhibit developmental defects that included cystic kidneys, the evidence that a gene involved in ciliary assembly could cause such defects was new and important.
GFP-tagged versions of the mammalian polycystin-1 and polycystin-2 orthologs were localized to the ciliated tips of Caenorhabditis elegans male sensory neurons, and subsequently both polycystins were co-localized with the primary cilium in human kidney cell lines [37]. Interestingly, in addition to the polycystins, the protein responsible for autosomal recessive PKD, fibrocystin, and the proteins responsible for the recessively inherited nephronophthises, have also been found to co-localize with the cilia or basal body [38]. Ciliary polycystins may play a part in coordinating the cellular response to changes in extracellular fluid flow. It has been suggested that polycystin-1 and polycystin-2 may mediate this process, at least in part, by forming a mechanosensory complex that responds to shear stress by increasing cytoplasmic calcium [39, 40].
The polycystins may also govern cellular processes via the JAK/STAT [41, 42], p53 [32], mTOR [43], NFAT/AP-1 [44], or Wnt signaling pathways [45, 46]. If the polycystins are mutated, then presumably one or more of these cellular signaling functions is compromised, and cystogenesis ensues. Proteins involved in key developmental signaling pathways, including those of the Wnt, hedgehog, and planar cell polarity pathways, are physically located in the cilia, lending further support to the hypothesis that the cilia somehow regulate proliferation by sensing the extracellular environment [47].
Diagnosis of ADPKD
Family history
Based on its autosomal dominant mode of inheritance, the morbidity risk of siblings and offspring of an ADPKD patient is 50 %. Family history reveals whether there is a risk. Further examinations of each person with a 50 % risk of developing ADPKD should be standard procedure. In the absence of a ADPKD patient in a family, the probability of a positive diagnosis is much lower. The exception is when a de novo mutation occurs, because even though the patients do not inherit the disease, they can transmit it [48].
Diagnostic imaging tests
It is debatable as to when the first ultrasonography examination should be performed. No lower age limit has been set, but ultrasonography in the first year of life is well known to be of little diagnostic value. Cysts may be already present in the first year of life, and even in fetal life [49], but the absence of cysts does not necessarily exclude the disease. The relationship between the number and size of the cysts and patient age makes diagnosing ADPKD in children difficult. The first ultrasonography should be performed between the ages of 20 and 30 years old, when the high probability of a positive diagnosis is accompanied by potential prophylaxis or treatment of the complications.
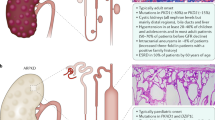
The diagnosis of ADPKD is made on the basis of a family history and imaging findings. The diagnostic criteria for ADPKD in Japan are listed in Table 1. The Research Committee for Progressive Renal Failure of the Ministry of Health, Labour, and Welfare has recently published clinical practice guidelines for ADPKD [50]. The Ravine diagnostic criteria [51] are shown in Table 2 and are commonly applied to the diagnosis of ADPKD. A positive family history remains relevant. The Ravine criteria are applicable only to people with a family history of type 1 ADPKD. For type 2 ADPKD, the criteria listed in Table 3 are of limited value. Their sensitivity for persons < 30 years old is only 67 % [51], and the different criteria as shown in Table 3 have been suggested [52]. As shown in Table 3, a diagnosis of type 2 ADPKD in a patient < 14 years of age is unlikely. On the other hand, the criteria in Table 3 offer almost 100 % certainty of diagnosis in persons > 30 years old [53].
The diagnosis of ADPKD may also be established based on other imaging techniques, including computed tomography (CT) and MRI. Imaging techniques other than ultrasonography are more sensitive, and thus the Ravine criteria cannot be applied. Two or more renal cysts, usually measuring <1 cm in diameter, are found in approximately 17 % of healthy persons between 18 and 29 years old [54]. If all cysts measuring <1 cm in diameter as presented in Table 3 were eliminated, the Ravine criteria could be used in CT diagnosis. It is worth mentioning that separate MR criteria for persons with a positive ADPKD family history have been worked out, and they are shown in Table 4 [54].
Genetic screening
A mutation of the PKD1 or PKD2 genes is the cause of ADPKD. These genes are located on chromosome 16 and 4, respectively. The hereditary nature of the disease makes diagnosis based on genetic tests theoretically possible irrespective of the patient’s age. However, genetic analysis is not a standard procedure in practice, and it is mainly performed on young persons when ultrasonography provides insufficient information, and if there are additional recommendations, such as a desire to be a kidney donor. The size and complexity of the PKD1 gene hamper genetic evaluation of ADPKD [48, 55].
The diagnosis of ADPKD depends on direct or indirect detection of a mutation. For instance, indirect detection is performed by the DNA linkage analysis method. The fundamental drawback of this method is the need to test at least 4 ADPKD patients and many healthy members in the same family. Direct detection techniques of mutation, used when only a proband’s blood sample is available, are free of the limitation, and their sensitivity for detection of an individual mutation exceeds 60 %. These tests, although expensive, are commercially available [48, 55]. More than 200 mutations of the two genes have been described to date.
Rossetti et al. [56] have recently developed and validated a strategy for analyzing both the PKD1 and PKD2 genes by using next-generation sequencing that involves pooling long-range PCR amplicons and multiplexing bar-coded libraries. They used this strategy to characterize a cohort of 230 ADPKD patients and detected definite and likely pathogenic variants in 115 (63 %) of the 183 patients with typical ADPKD. They also identified atypical mutations, a gene conversion, and one missed mutation resulting from an allele dropout, and they characterized the patterns of deep intronic variations of both genes. They concluded that their strategy is a model for future genetic characterization of large ADPKD populations.
Prognosis of ADPKD
Although the clinical manifestations of PKD1 and PKD2 overlap completely, type 1 ADPKD is associated with more severe disease than type 2 ADPKD, with bigger kidneys and earlier onset of ESKD. Half of type 1 ADPKD patients require renal replacement therapy by the age of 54, and half of type 2 ADPKD patients by the age of 73 [57]. However, ADPKD is characterized by high variability of the disease progression. In the available data, proper assessment of the stage of the disease advancement and the disease progression is of crucial importance in the diagnostic process.
ADPKD can be diagnosed based on the number and volume of cysts, but they are also indicators of disease progression. The CRISP Study was created to identify markers of disease progression [58], and the results revealed a correlation between changes in total kidney volume (TKV) and declines in GFR [59]. The latest data from the CRISP Study showed that a height-adjusted TKV (htTKV) at baseline of 600 cm3 or greater successfully predicted the risk of developing renal insufficiency in ADPKD patients within an 8-year follow-up period with 75 % accuracy in the study cohort [60]. In the CRISP Study the htTKV increased each year, but for the first 3 years GFR remained unchanged. A decline in GFR by 10.6 and 22.3 % below baseline was then noted at 6 and 8 years, respectively. Tokiwa et al. [61] have recently reported that a multiple linear regression analysis of data obtained from 73 Japanese ADPKD patients showed that the changes in GFR per year was significantly and independently inversely correlated with the change in TKV per year.
Potential therapies for ADPKD
The goal of potential therapies for ADPKD is slowing or preventing the growth of cysts while treating the complications of ADPKD. Changing the natural history of the disease by altering the course of cyst growth has received the most attention recently. The major treatment strategies currently being tested can be divided into two categories: (1) strategies designed to reduce cAMP levels, and (2) strategies designed to inhibit cell proliferation. Several compounds shown to be effective in preclinical models have been tested in clinical trials, and more trials are planned. In addition, a larger number of newer compounds have been developed that are generally designed to implement one or more of these strategies.
Tolvaptan
Arginine vasopressin stimulates cAMP production in the distal nephron and collecting ducts by binding to the vasopressin V2 receptor [62]. Vasopressin V2 receptor antagonists (OPC-31260 and tolvaptan) have been shown to be effective in reducing cAMP levels in kidney epithelial cells and reducing cytogenesis in several PKD models [60, 63, 64]. A number of clinical studies of the effect of tolvaptan in ADPKD have been completed or are currently in progress under the Tolvaptan Efficacy and Safety in Management of PKD and Outcomes (TEMPO) program [65]. The results of Phase 2 studies suggest that tolvaptan is well tolerated in most ADPKD patients at a dose that controls urine osmolality to < 300 mOsm/kg [65], and that water dieresis and thirst are predictable side effects. The results of a large, multicenter, randomized, placebo-controlled trial of tolvaptan in patients with a TKV of > 750 ml will be reported in 2012 [65]. It was recently reported that 51 (81 %) of 63 ADPKD patients had completed 3 years of tolvaptan therapy [66]. The baseline TKV values (control 1422 ml, tolvaptan 1635 ml) and eGFR values (both 62 ml/min/1.73 m2 in that trial) were similar. TKV in the control group increased by 5.8 % per year versus 1.7 % per year in the tolvaptan group (P < 0.001), and the corresponding annualized eGFR decline was −2.1 versus −0.71 ml/min/1.73 m2 (P = 0.01). The results of sensitivity analyses that included censored subjects were similar, whereas the results of the mixed model repeated measures analyses were significant at each year for TKV and nonsignificant for eGFR. The increases in TKV values were correlated with decreasing eGFR values (r = −0.21, P < 0.01). The authors in the TEMPO program concluded that ADPKD cyst growth progressed more slowly in the tolvaptan group than in historical controls. At present analysis of TEMPO3/4 is still underway [67].
Somatostatin
Somatostatin can slow the development and growth of both renal and hepatic cysts (polycystic liver disease and polycystic kidney disease) because of its ability to inhibit cAMP signaling in cholangiocytes and tubular epithelial cells [68]. In a randomized, double-blind, placebo-controlled trial, the long-lasting somatostatin analogue octreotide (< 40 mg every 28 days) was given to ADPKD patients who had polycystic liver disease (PLD) for 1 year, and the increases in liver volume in the octreotide group were significantly smaller than in the placebo group (−4.95 vs. +0.92 %, P = 0.048). Similarly, TKV remained stable in the octreotide group but increased significantly in the control group (+0.25 vs. +8.6 %, P = 0.045) [68]. However, there was no significant difference in loss of GFR between the octreotide group and the control group (−5.1 vs. −7.2 %, P = 0.98). Nevertheless, patients in the octreotide group reported subjective benefits, such as less pain and increased physical activity. Major side effects were diarrhea and impaired glucose tolerance.
mTOR inhibitors
mTOR inhibitors have been shown to inhibit cell proliferation and cyst growth in a number of orthologous and non-orthologous models and were therefore regarded as potential therapeutic agents for ADPKD [69, 70]. The above findings were supported by a retrospective observational study showing that rapamycin was more effective than cyclosporine in limiting native kidney enlargement in ADPKD patients after renal transplantation [71]. In another retrospective study, treatment with the sirolimus regimen for an average of 19.4 months was associated with an 11.9 ± 0.03 % reduction in polycystic liver volume compared to conventional treatment in 7 ADPKD patients who had undergone kidney transplantation [72].
Nevertheless, the results of recent clinical trials using mTOR inhibitors in ADPKD patients have been disappointing. In the SIRENA crossover study, 21 patients with an eGFR > 40 ml/min were randomized to 6-month treatment with sirolimus (starting dose 3 mg/day; targeted blood trough level 10–15 ng/ml) or conventional therapy [73]. No significant difference between TKV or eGFR on the sirolimus regimen and conventional treatment were found in the 15 patients who completed the study, although cyst volume increased less and renal parenchymal volume increased after treatment with sirolimus [73]. This was an explorative study with a relatively small sample size and short follow-up. The short duration of the trial did not allow the demonstration of beneficial effects on GFR. Moreover, the statistical power was not sufficient to evaluate the effects of sirolimus therapy. A study by Serra et al. [74] in 100 early-stage ADPKD patients (eGFR > 70 ml/min/1.73 m2) showed that sirolimus therapy (target dose, 2 mg/day) for 18 months did not significantly alter TKV or eGFR compared with the control group, which received standard care. The median increase in TKV over 18 months in the control group was 99 cm3 [74]. The authors reported the limitation that their trial was not designed to assess the effect of sirolimus on the GFR. As expected, the GFR declined only slightly in the control group, but it remained stable in the sirolimus group.
HALT-PKD studies
Standard blood pressure control remains a practical recommendation for PKD patients, not only to protect their kidneys but to limit the extrarenal complications of ADPKD. Seventy-one hypertensive ADPKD patients had a greater annual increase in TKV than normotensive ADPKD patients [7, 75], and they had higher prevalences of left ventricular hypertrophy, ischemic heart disease, and stroke [76]. Although the renin–angiotensin–aldosterone system is activated in ADPKD patients with cyst expansion, data from late-stage ADPKD patients suggest that the role of ACE inhibitors in slowing kidney disease progression remains uncertain [77]. The potential benefits of rigorous (≤110/75 mmHg) versus standard (≤130/80 mmHg) blood pressure control with a combination lisinopril and telmisartan versus lisinopril monotherapy on kidney disease progression in ADPKD is being assessed in the ongoing HALT-PKD study [75]. TKV and eGFR will be used as outcome measures in that study, which has recruited both early (eGFR > 60 ml/min/1.73 m2)- and advanced (eGFR 25–60 ml/min/1.73 m2)-stage ADPKD patients.
Summary
Family history and ultrasonography are the most important factors in diagnostic evaluations for ADPKD. Early diagnosis of hypertension in ADPKD patients is of great significance. Disease progression is assessed mainly on the basis of repeated measurements of GFR. TKV is a useful marker of early-stage ADPKD. Progress in the treatment of ADPKD will surely serve as a stimulus for establishing reliable and inexpensive methods of early detection of the disease, which will enable treatment before hypertension and renal insufficiency have developed. Based on the results of recent clinical trials, several key issues need to be addressed to facilitate practical use of the compounds which are available.
References
Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4:1140–50.
Norby S, Schwartz M. Possible locus for polycystic kidney disease on chromosome 2. Lancet. 1990;336:323–4.
Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30.
Chang MY, Ong AC. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and treatment. Nephron Physiol. 2008;108:1–7.
Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68.
Lee YR, Lee KB. Reliability of magnetic resonance imaging for measuring the volumetric indices in autosomal-dominant polycystic kidney disease: correlation with hypertension and renal function. Nephron Clin Pract. 2006;103:c173–80.
Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–45.
O’Neil WC, Robbin ML, Bae KT, Grantham JJ, Chapman AB, Guay-Woodford LM, et al. Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Am J Kidney Dis. 2005;46:1058–64.
Bae KT, Tao C, Zhu F, Bost JE, Chapman AB, Grantham JJ, et al. MRI-based kidney volume measurements in ADPKD: reliability and effect of gadolinium enhancement. Clin J Am Soc Nephrol. 2009;4:719–25.
Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–37.
The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–94.
Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–42.
Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–60.
Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–60.
Nims N, Vassmer D, Maser RL. Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease 1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry. 2003;42:13035–48.
Li A, Tian X, Sung SW, Somolo S. Identification of two novel polycystic kidney disease-1-like genes in human and mouse genomes. Genomics. 2003;81:596–608.
Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, et al. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–65.
Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217.
Celic A, Petri ET, Demeler B, Ehrlich BE, Boggon TJ. Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem. 2008;283:28305–12.
Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–83.
Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–64.
Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C-terminus. J Clin Invest. 2004;114:1433–43.
Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69.
Qamar S, Vadivelu M, Sandford R. TRP channels and kidney disease: lessons from polycystic kidney disease. Biochem Soc Trans. 2007;35:124–8.
Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix–loop–helix inhibitor Id2. Nat Cell Biol. 2005;7:1202–12.
Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–16.
Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–77.
Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–87.
Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest. 1997;99:194–9.
Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31:1145–52.
Jiang ST, Chiou YY, Wang E, Lin HK, Lin YT, Chi YC, et al. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol. 2006;168:205–20.
Nishio S, Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, et al. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J Clin Invest. 2005;115:910–8.
Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol. 1998;9:903–16.
Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004;66:964–73.
Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–18.
Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–71.
Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–16.
Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan RS, et al. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–10.
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37.
Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, et al. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–52.
Bhunia AK, Piontek K, Boletta A, Liu A, Qian F, Xu PN, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–68.
Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69.
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–71.
Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, et al. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004;279:55455–64.
Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, et al. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–53.
Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16:349–60.
Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–73.
Pei Y. Diagnostic approach in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2006;1:1108–14.
Shamshirsaz AA, Bekheimia RM, Kamgar M, Johnson AM, McFann K, Cadnapaphornchai M, et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 2005;68:2218–24.
Horie S. ADPKD: molecular characterization and quest for treatment. Clin Exp Nephrol. 2005;9:282–91.
Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic disease 1. Lancet. 1994;343:824–7.
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–301.
Demetriou K, Tziakouci C, Anninou K, Eleftheriou A, Koptides M, Nicolaou A, et al. Autosomal dominant polycystic kidney disease—type 2. Ultrasound, genetic and clinical correlations. Nephrol Dial Transpl. 2000;15:205–11.
Nascimento AB, Mitchell DG, Zhang XM, Kamishima T, Parker L, Holland GA. Rapid MR imaging detection of renal cysts: age-based standards. Radiology. 2001;221:628–32.
Gattone VH 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–6.
Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–16.
Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, et al. Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol. 2012;23:915–33.
Norby S, Schwartz M. Possible locus for polycystic kidney disease on chromosome 2. Lancet. 1990;336:323–4.
Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–57.
Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86.
Tokiwa S, Muto S, China T, Horie S. The relationship between renal volume and renal function in autosomal polycystic kidney disease. Clin Exp Nephrol. 2011;15:539–45.
Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68.
Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–4.
Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–8.
Torres VE. Role of vasopressin antagonists. Clin J Am Soc Nephrol. 2008;3:121218.
Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years’ experience. Clin J Am Soc Nephrol. 2011;6:2499–507.
Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3–4 Study. Am J Kidney Dis. 2011;57:692–9.
Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, et al. Randomized clinical trial of long-acting somatostatin for autosomal polycystic kidney disease and liver disease. J Am Soc Nephrol. 2010;21:1052–61.
Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:sPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transpl. 2006;21:598–604.
Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of pkd1. J Am Soc Nephrol. 2010;21:489–97.
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cytogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–71.
Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–8.
Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol. 2010;21:1031–40.
Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–9.
Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Miller JP, et al. The HALT polycystic kidney disease trials: design and implementation. Clin J Am Soc Nephrol. 2010;5:102–9.
Chang MY, Kuok CM, Chen YC, Ryu SJ, Tian YC, Wu-Chou YH, et al. Comparison of intracerebral hemorrhage and subarachnoid hemorrhage in patients with autosomal-dominant polycystic kidney disease. Nephron Clin Pract. 2010;114:c158–64.
Jafar TH, Stark PC, Schmid CH, Strandgaard S, Kamper AL, Maschio G, et al. The effect of angiotensin-converting enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67:265–71.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mochizuki, T., Tsuchiya, K. & Nitta, K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol 17, 317–326 (2013). https://doi.org/10.1007/s10157-012-0741-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0741-0