Abstract
Background
Fluorescence imaging by means of Indocyanine green (ICG) has been applied to intraoperatively determine the perfusion of the anastomosis. The purpose of this Individual Participant Database meta-analysis was to assess the effectiveness in decreasing the incidence of anastomotic leak (AL) after rectal cancer surgery.
Methods
We searched PubMed, Embase, Cochrane Library and ClinicalTrial.gov, EU Clinical Trials and ISRCTN registries on September 1st, 2019. We considered eligible those studies comparing the assessment of anastomotic perfusion during rectal cancer surgery by intraoperative use of ICG fluorescence compared with standard practice. We defined as primary outcome the incidence of AL at 30 days after surgery. The studies were assessed for quality by means of the ROBINS-I and the Cochrane risk tools. We calculated odds ratios (ORs) using the Individual patient data analysis, restricted to rectal lesions, according to original treatment allocation.
Results
The review of the literature and international registries produced 15 published studies and 5 ongoing trials, for 9 of which the authors accepted to share individual participant data. 314 patients from two randomized trials, 452 from three prospective series and 564 from 4 non-randomized studies were included. Fluorescence imaging significantly reduced the incidence of AL (OR 0.341; 95% CI 0.220–0.530; p < 0.001), independent of age, gender, BMI, tumour and anastomotic distance from the anal verge and neoadjuvant therapy. Also, overall morbidity and reintervention rate were positively influenced by the use of ICG.
Conclusions
The incidence of AL may be reduced when ICG fluorescence imaging is used to assess the perfusion of a colorectal anastomosis. Limitations relate to the consistent number of non-randomized studies included and their heterogeneity in defining and assessing AL. Ongoing large randomized studies will help to determine the exact role of routine ICG fluorescence imaging may decrease the incidence of AL in surgery for rectal cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The leak of a colorectal or coloanal anastomosis is one of the most dreaded complications after rectal surgery, being associated with increased mortality and morbidity [1], as well as poor oncologic outcome [2]. Different from intraperitoneal anastomotic leak, the risk of a definitive stoma due to impossible rescue of the anastomosis or impaired bowel function are relevant and dramatically affect quality of life.
A high variability in studies reporting anastomotic leak (AL) is a consequence of the lack of a standardized definition [3]. In rectal surgery the incidence of AL is reported up to 19% [3, 4]. In the REAL score database, collecting 26 different series for a total of 9735 cases, the occurrence of AL after surgery for rectal cancer was 9.7% [5]. Not surprisingly, the Association of Coloproctology of Great Britain and Ireland (ACPGBI) sets new techniques to reduce AL as a research priority [6].
Many different factors have been considered influencing the risk of AL, including gender, age, comorbidities, American Society of Anaesthesiologists (ASA) score, obesity and malnutrition, tobacco, immunosuppression, alcohol abuse, preoperative chemotherapy and radiotherapy, advanced tumour stage, low anastomoses and operative time [7], but adequate perfusion of the anastomosis is surely essential for optimal healing and AL prevention [8, 9]. Therefore, it is likely that intraoperative assessment of bowel ischemia and manoeuvres to optimize anastomotic perfusion may reduce the event of AL.
Recently, the introduction of fluorescence imaging technology seems to cover the need for a reliable intraoperative predictive test to be used for large bowel resections. Previous studies and meta-analysis showed a potential benefit especially in reducing anastomotic leak in rectal cancer surgery [10]. Nevertheless, most of those studies were either underpowered or did not take into sufficient consideration possible confounding factors.
The aim of the present study was to perform a systematic review of the literature available on this topic, including RCTs, prospective cohort studies, prospective and retrospective case–control studies, to structure an Individual Participant Data (IPD) database for an objective analysis of incidence of AL comparing the use of Indocyanine Green (ICG) fluorescence imaging with standard practice in patients undergoing rectal cancer surgery.
Materials and methods
An IPD meta-analysis (IPD-MA) of studies published up to September 1st, 2019, comparing ICG fluorescence to a control group in rectal cancer surgery was performed. The FLUREAL (FLUorescence to prevent REctal Anastomotic Leak) study was registered with PROSPERO (CRD42019121390). We reviewed the literature systematically following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of IPD checklist (PRISMA-IPD) guidelines [11].
Search strategy
We searched systematically Embase, PubMed and Cochrane Library electronic databases up to September 1st, 2019. The search strings are indicated in Online Appendix 1.
Inclusion and exclusion criteria
We selected all studies comparing the intraoperative use of ICG fluorescence imaging with standard practice for assessment of perfusion of the anastomosis after trans-abdominal rectal cancer surgery on human patients, regardless of the operative approach. The incidence of AL at 30 days after surgery represented the primary outcome. RCTs, prospective cohort studies, prospective and retrospective case–control studies were searched. Articles not mentioning anastomotic leak after rectal cancer surgery, overlapping studies, case reports, case series with less than 20 patients treated for rectal cancer, reviews, consensus statements and opinion articles were excluded. Articles not published in English were also excluded.
Study selection and data extraction
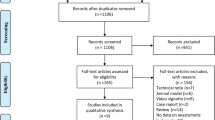
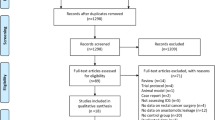
Two reviewers (MAB, NV) performed the search independently; a third author (AA) arbitrated any disagreements weather to include or exclude selected studies. Studies and results were input into a standardized database, while duplicates removed. We searched manually the reference lists of the included studies. Only the data of patients who had undergone rectal cancer surgery followed by an anastomosis were included. Figure 1 illustrates a flowchart of the extraction process.
All the authors of the eligible studies deemed suitable were contacted up to four times by e-mail; an electronic spreadsheet was sent praying to complete it with their data in an anonymous way. All the data in spreadsheet format returned were merged into a single database for analysis. In five cases authors did not reply [12,13,14,15,16], while in a sixth [17] the local policy required IRB which was not received in time.
Outcomes of interest
Outcomes of interest were chosen by the proposing investigators based on the related literature. Primary outcome was the incidence of AL at 30 days after surgery. Data regarding how intraoperative leak tests were performed, how the anastomosis was treated in case of failure (suture or redone) and the ways to assess postoperatively a possible anastomotic leak were recorded.
Secondary outcomes were morbidity, defined as any kind of complication, reintervention, defined as the occurrence of any second or subsequent surgical procedure, both within 60 days from the index surgery and hospital stay, defined as the length of the inpatient stay calculated from the day of admission and the day of discharge.
Subgroup analyses were planned for gender, age > 65 years, body mass index (BMI) ≥ 25 kg/m2, tumour distance ≤ 6 cm from anal verge, anastomotic distance ≤ 6 cm from anal verge and preoperative radiotherapy (RT) and/or chemotherapy (CT).
We also collected data for preoperative characteristics of patients such as cardiovascular diseases, steroid therapy, tobacco use and intraoperative characteristics of patients such as operative time, type of anastomosis, protective stoma, pT, pN, pM and stage, in order to verify symmetry.
Quality assessment
All studies fulfilling the selection criteria for this review were assessed for methodological quality and risk of bias. Table 1 shows individual scores of quality assessment items per study, assessed using ROBINS-I tool [18] and Cochrane risk tool [19]. As most of the series included data of colonic and rectal lesions in a way that could not be distinguished, a specific enquiry was sent to authors asking to restrict to rectal lesions only the data provided in the database of their published series.
Statistical analyses
All analyses were performed according to original treatment allocation (intention-to-treat analysis). The categorical variables were described as absolute/relative frequencies while the continuous ones as the median and interquartile range (IQR). The association between any categorical risk factor and AL occurrence was analysed using Fisher’s exact test; the Mann–Whitney test was used for continuous predictors. A whole series of univariate binary logistic models was used to estimate the potential role on AL occurrence (dependent variable) of every risk factor (independent variables). All reported P-values were obtained by the two-sided exact method at the conventional 5% significance level. Data analysis was performed as of October 2019 by using R 3.6.1 (R Foundation for Statistical Computing, Vienna-A, https://www.R-project.org).
Ongoing trials
Ongoing trials were assessed searching ClinicalTrial.gov register, EU Clinical Trials Register and the UK ISRCTN Registry for the terms “indocyanine” OR “fluorescence” AND “rect*”. Only studies dealing with the issue of anastomotic perfusion were selected.
Results
Study selection
Figure 1 presents the results of literature search and selection process of eligible studies in a PRISMA flow diagram format. We identified 947 studies and 7 registered protocols of studies [20,21,22,23,24,25,26]. The full text of 15 published studies was selected [12,13,14,15,16,17, 27,28,29,30,31,32,33,34,35] to which the protocol of 5 eligible ongoing trials was added [20,21,22, 25, 26].
We received reply from 9 different authors of the 20 contacted. The analysis included two randomized trial [26, 35] and seven non-randomized studies [20, 27,28,29,30, 33, 34], two of them being multicentric [20, 26].
Study characteristics
A four hundred and fourteen patients from two randomized trials, 452 patients from three prospective series and 564 from 4 non-prospective studies were included, for a total of 1330 patients. The analysis included 862 individuals in the ICG group and 468 in the control group. The sample size within the different studies ranged from 25 to 422 patients. Table 2 reports differences in AL definitions.
Four of the included studies were retrospective [27,28,29, 34], and all were single-centre studies, except one which included patients recruited at 3 different centres [34]. Three studies are prospective [20, 30, 33], one of them include patients recruited at 3 different centres [33]. One study is a monocentric RCT [26] and one a multicentric RCT [35]. Two studies [28, 29] included individuals undergoing robotic rectal resections. The evaluation of the risk of bias in non-randomized studies (ROBINS-I) reported in all the studies a moderate risk [18].
Characteristics of patients are reported in Table 3.
Pooled trials were comparable for gender, BMI, cardiovascular disease, steroid therapy, tobacco use, pT and pN stage, neoadjuvant therapy. Median tumour distance from the anal verge was 9 cm (7–13 cm) in the ICG group and 10 cm (7–13 cm) in the control group (p = 0.374). Median anastomotic distance was 6 cm (4–9 cm) in the ICG group and 6 cm (4–9 cm) in the control group (p = 0.711). The anastomosis was redone in 13 cases (2.0%) in the ICG group and in 1 case (0.2%) in the control group (p = 0.011). A protective stoma was performed in 405 cases (47.0%) in the ICG group and in 261 cases (55.8%) in the control group (p = 0.003). Median operative time was 216 (172–281) min in the ICG group and 190 (158–257) min in the control group (p < 0.001). Median hospital stay was 7 (6–10) days in the ICG group and 8 (7–13) days in the control group (p < 0.001). A reintervention was necessary in 25 cases (2.9%) in the ICG group and in 29 cases (6.2%) in the control group (p = 0.005). A readmission was necessary in 35 cases (4.1%) in the ICG group and in 12 cases (2.6%) in the control group (p = 0.166).
Primary outcome assessment
Primary outcome was the incidence of AL at 30 days after surgery. The surgeon varied the site of anastomosis after injection of ICG and observation of hypoperfusion in 11.3% (4–23%) of the procedures, while performed a new anastomosis in 13 of the 649 cases in the ICG group (2.0%).
The overall incidence of AL was 6.7%. The assessment of ICG perfusion resulted in an 89.7% reduction in the odds of AL (OR 0.341; 95% CI 0.220–0.530; p < 0.001) (Table 4), and a lower incidence of AL compared with control group (4.2% vs 11.3%, respectively).
Subgroup analyses
Subgroup analysis is detailed in Table 5. ICG perfusion assessment resulted in a 72.5% reduction in the odds of AL among male gender patients (p = 0.001), an 83.3% reduction in patients ≥ 65 years old (p = 0.004), a 95.6% reduction in patients with BMI ≥ 25 kg/m2 (p < 0.001), a 71.4% reduction in patients with tumour distance ≤ 6 cm from the anal verge (p = 0.074), a 71.9% reduction in patients with anastomotic distance ≤ 6 cm from the anal verge (p = 0.004) and a 72.1% reduction in patients who had undergone neoadjuvant therapy (p = 0.059).
Secondary outcomes
The overall incidence of short-term morbidity, assessed including 862 patients in the ICG group and 468 patients in the control group, was 26.1%. The assessment of tissue perfusion by means of ICG resulted in a 32.1% reduction in the odds of AL (OR 0.638; 95% CI 0.497–0.821; p = 0.001), and a lower morbidity rate compared with control group (23.0% vs 31.8%, respectively). On the same dataset the incidence of reintervention was 4.1%. The assessment of tissue perfusion by means of ICG resulted in a 72.5% reduction in the odds of AL (OR 0.452; 95% CI 0.262–0.782; p = 0.005), and a lower reintervention rate compared with the control group (2.9% vs 6.2%, respectively).
The median hospital stay, assessed including 846 patients in the ICG group and 465 patients in the control group, was 7 (6–10) days in the ICG group and 8 (7–13) days in the control group (p < 0.001). Considering a threshold of less than 8 days of hospital stay the ICG perfusion group shows a shorter hospital stay in comparison with control group, although this difference is not statistically significative (96.8% vs 94.8%, respectively; OR 0.606; 95% CI 0.345–1.063; p = 0.1).
Ongoing trials
We found 7 ongoing RCTs searching ClinicalTrial.gov register, EU Clinical Trials Register and the UK ISRCTN Registry. Table 6 reports characteristics of the identified trials. The incidence of AL is the primary outcome in all the studies.
Discussion
The current systematic review and IPD analysis show that when used intraoperatively the imaging provided by ICG fluorescence has the potential to reduce the risk of AL in surgery for cancer of the rectum. However, the interpretation of these results should take into account the bias deriving by a vast majority of non-randomized studies among those included in the analysis.
Numerous investigations have evaluated the application of ICG fluorescence in colorectal surgery, but the majority of them consist of small sample series. Recently, a multicentre retrospective study [34] and finally a prospective multicentre study [33] and a randomized multicentre study [27] have appeared in the literature. We thought this could be a confusing situation, masking the differences between the groups and preventing the analysis of the subgroups. Therefore, we opted for an IPD meta-analysis project in order to look for differences even in groups of subsets.
ICG fluorescence appears to be an important aid in identifying hypoperfusion of colon segments in the anastomosis, thus suggesting a variation in the surgical program, broadening the margins of resection or indicating the need for revision and/or new anastomosis. A change in the expected level of anastomosis was decided at 11.3% (4–23%), in line with the decrease in the incidence of AL reported in the ICG group.
The possibility of verifying from patient to patient the composition of the groups allows to draw some conclusions on the real symmetry of the two groups. For instance, we could notice that the groups are symmetric for distance of anastomosis from the anal verge, while there is a lower incidence of protective stomas in the ICG group. Furthermore, we could perform analyses of large subgroups that allow us to conclude that the use of ICG is important regardless of gender, age, BMI and anastomotic distance from the anal verge.
We recognize the limitations of this work. We assessed the quality of the studies by means of the ROBINS-I tool [18], resulting all in a moderate risk of bias. Furthermore, all but five studies were retrospective [27, 29, 31, 32, 34] and in all these studies, data related to the use of ICG and those representing the control group were collected in different timeframes. In addition, it should be considered the risk of publication bias in trials investigating, whether fluorescence imaging has a role on the incidence of AL. Further limitations, such as the non-unique definition of AL and how to assess it, both radiologically and clinically, as well as differences in surgical procedure and employment of the ICG should also be considered. ICG fluorescence was injected before the anastomosis was formed in all the studies included, except the one by Kudszus et al. [32]. However, the variations in the way to use it may have played a role in the modification of the surgical program. Kim et al., Rosati et al., Ris et al., Watanabe et al. and Boni et al. [12, 27, 33,34,35], evaluated the perfusion of the anastomosis with ICG after completing the surgical resection. Jafari et al. [29], assessed the right transition point under white light; therefore, following the injection of ICG, the point of transection was reviewed in 19% of cases. Furthermore, in few cases, imaging by ICG fluorescence has also been used after the anastomosis was performed [32]. Moreover, the quantitative assessment of a suitable or unsuitable pre-anastomotic perfusion is not well determined, mostly due to the fact that most real-time imaging systems do not have the capability of assessing tissue perfusion. However, some experimental studies have been published that evaluate the quantification of fluorescence in animal models [36]. A technical improvement will certainly benefit the final result of the ICG application, making it possible to define different degrees of infusion. Ultimately, we did not consider in the analysis the data of the seven eligible studies that were excluded as not corresponding to the inclusion criteria, i.e. participants affected by rectal cancer. Therefore, we are not able to compare results with participants whose tumour was located above the rectosigmoid junction.
Despite these limitations, the present study suggests that ICG offers a tangible benefit in rectal cancer surgery by reducing the incidence of AL and, consequently, reducing morbidity and the rate of reoperation.
Conclusions
Despite the significant limitations due to the intrinsic heterogeneity of the available studies, this type of IPD meta-analysis can allow us to draw some conclusions. Fluorescence imaging of the ICG appears to be a promising tool that is most likely useful in clinical practice, from a potential reduction in the rate of AL in individuals undergoing resection of the rectum for cancer, compared to standard practice. The advantage seems independent of gender, age, BMI and anastomotic distance from the anal border. However, other larger, prospective and randomized studies on the topic will help to determine whether the occurrence of AL may be decreased by the routine use of ICG fluorescence imaging during surgery for rectal cancer.
References
Vallance A, Wexner S, Berho M, Cahill R, Coleman M, Haboubi N, Heald RJ, Kennedy RH, Moran B, Mortensen N, Motson RW, Novell R, O'Connell PR, Ris F, Rockall T, Senapati A, Windsor A, Jayne DG (2017) A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis 19:O1–O12
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102:462–479
Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, Senagore AJ, International Anastomotic Leak Study G (2008) Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg 32:1147–1156
T Pinkney group TESoCc (2017) The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: an international snapshot audit. Colorectal Dis. https://doi.org/10.1111/codi.13646
Arezzo A, Migliore M, Chiaro P, Arolfo S, Filippini C, Di Cuonzo D, Cirocchi R, Morino M (2019) The REAL (REctal Anastomotic Leak) score for prediction of anastomotic leak after rectal cancer surgery. Tech Coloproctol 23:649–663
Tiernan J, Cook A, Geh I, George B, Magill L, Northover J, Verjee A, Wheeler J, Fearnhead N (2014) Use of a modified Delphi approach to develop research priorities for the association of coloproctology of Great Britain and Ireland. Colorectal Dis 16:965–970
Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, Margolin DA, Martz JE, McLemore EC, Molena D, Newman MI, Rafferty JF, Safar B, Senagore AJ, Zmora O, Wexner SD (2016) Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg 20:2035–2051
Rutegard M, Rutegard J (2015) Anastomotic leakage in rectal cancer surgery: the role of blood perfusion. World J Gastrointest Surg 7:289–292
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Blanco-Colino R, Espin-Basany E (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22:15–23
Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF (2015) Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 313:1657–1665
Kim JC, Lee JL, Yoon YS, Alotaibi AM, Kim J (2016) Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot 12:710–717
Martin-Perez B, Otero-Piñeiro A, DeLacy-Oliver B, Pena-Lopez R, Arroyave MC, Fernandez-Hevia M, Lacy A (2017) Transanal total mesorectal excision for rectal cancer: assessment with indocyanine green. Surg Endosc Other Intervent Techn 31:S204
Moore C, Turner J, Naddell C, Okonkwo A, Childs E, Clark C (2016) Short term outcomes in laparoscopic colorectal surgery with and without the use of fluorescent angiography. Surg Endosc Other Intervent Tech 30:S347
Quartey B, Chinn B, Wilkins K, Notaro J, Alva S, Saleem A, Rampakakis E, Starker P (2016) Real time intraoperative assessment of colonic perfusion in colon and rectal surgery. Dis Colon Rectum 59:e339–e340
Ramphal W, Crolla RMPH, Gobardhan PD, Wijsman JH, Van Der Schelling GP, Schreinemakers JMJ (2017) Colorectal perfusion with indocyanine green in colonic resection performed in robotic surgery: reducing the risk of anastomotic leakage? Surg Endosc Other Intervent Tech 31:S4
Mizrahi I, Abu-Gazala M, Rickles A, Fernandez L, Petrucci A, Wolf J, Sands D, Wexner SD (2017) Indocyanine green fluorescence angiography for low anterior resection: results of a comparative cohort study. Colorectal Dis 19:37
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Foo (2016) The impact of indocyanine green-enhanced fluorescence imaging on bowel transection in left-sided colorectal resection. https://ClinicalTrials.gov/show/NCT02669485
Hospital OU, Oulu Uo, District CFH, Hospital TU, Hospital TU, Helsinki HDo, Uusimaa, Hospital PTC, Seinäjoki Central Hospital S, Finland (2018) Indocyanine green fluorescence imaging in prevention of colorectal anastomotic leakage. https://ClinicalTrials.gov/show/NCT03602677
University A, Hospital AAU (2015) The Role of Indocyanine Green (ICG) Fluorescence imaging on anastomotic leak in robotic colorectal surgery. https://ClinicalTrials.gov/show/NCT02598414
University Hospital G, Kanker KOT (2019) Assessment of graft perfusion and oxygenation for improved outcome in esophageal cancer surgery. https://ClinicalTrials.gov/show/NCT03587532
Zhang Z, Hospital PUMC, Hospital BCY, Institute C, Hospital CAoMS, Hospital CPG, Hospital PUPs, Hospital BC, Hospital B, Xinhua Hospital SJTUSoM, Hospital R, Hospital R, University F, Hospital GPPs, Southern Medical University C, University FHoCM, University FHoJ, University TFAHwNM, Hospital FMUU, University FAHoCM, Shanghai General Hospital SJTUSoM, Hospital BF (2019) Perfusion outcomes with near infrared-indocyanine green imaging system in laparoscopic total mesorectal excision for mid- or low-rectal cancer. https://ClinicalTrials.gov/show/NCT04012645
Armstrong G, Croft J, Corrigan N, Brown JM, Goh V, Quirke P, Hulme C, Tolan D, Kirby A, Cahill R, O'Connell PR, Miskovic D, Coleman M, Jayne D (2018) IntAct: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis 20:O226–o234
Rybakow (2017) A study of perfusion of colorectal anastomosis using FLuorescence AnGiography (FLAG-trial). https://ClinicalTrials.gov/show/NCT03390517
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2017) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc Other Intervent Tech 31:1836–1840
Ciarleglio FA, Brolese A, Marcucci S, Valduga P, Beltempo P, Prezzi C, Bondioli P, Berlanda G (2017) Preliminary results on application of da vinci fluorescence imaging vision system with indocyanine green (ICG) in robotic colo rectal surgery. Surg Endosc Other Intervent Tech 31:S470
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27:3003–3008
Keller D, Ibarra S, Flores JR, Haas EM (2016) Impact of fluorescence angiography on clinical and financial outcomes in colorectal surgery: A case matched series. Gastroenterology 150:S1242
Kin C, Vo H, Welton L, Welton M (2015) Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 58:582–587
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck's Archiv Surg 395:1025–1030
Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali V, Douissard J, Cunningham C, Lindsey I, Guy R, Jones O, George B, Morel P, Mortensen NJ, Hompes R, Cahill RA (2018) Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg 105:1359–1367
Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki C, Endo I (2019) Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc 34:202
De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise P, Vignali A, Rosati R (2019) Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc 34:53
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102:e169–176
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr Alessio Pigazzi received research support from Novadaq, Inc and is a consultant for Intuitive Surgical. Alberto Arezzo, Marco Augusto Bonino, Frédéric Ris, Luigi Boni, Elisa Cassinotti, Dominic Chi Chung Foo, Nga Fan Shum, Alberto Brolese, Francesco Ciarleglio, Deborah S Keller, Riccardo Rosati, Paola De Nardi, Ugo Elmore, Uberto Fumagalli, Mehraneh Dorna Jafari, Evgeny Rybakov, Mikhail Alekseev, Jun Watanabe, Nereo Vettoretto, Roberto Cirocchi, Roberto Passera, Mario Morino declare that they have no conflict of interest.
Ethical approval
Ethical approval is not needed as this study corresponds to a meta-analysis of studies already published.
Informed consent
Informed consent is not needed as this study corresponds to a meta-analysis of studies already published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arezzo, A., Bonino, M.A., Ris, F. et al. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: an individual participant data analysis. Surg Endosc 34, 4281–4290 (2020). https://doi.org/10.1007/s00464-020-07735-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07735-w