Abstract
Lynch syndrome is a cancer-predisposing syndrome inherited in an autosomal-dominant manner, wherein colon cancer and endometrial cancer develop frequently in the family, it results from a loss-of-function mutation in one of four different genes (MLH1, MSH2, MSH6, and PMS2) encoding mismatch repair proteins. Being located immediately upstream of the MSH2 gene, EPCAM abnormalities can affect MSH2 and cause Lynch syndrome. Mismatch repair proteins are involved in repairing of incorrect pairing (point mutations and deletion/insertion of simple repetitive sequences, so-called microsatellites) that can arise during DNA replication. MSH2 forms heterodimers with MSH6 or MSH3 (MutSα, MutSβ, respectively) and is involved in mismatch-pair recognition and initiation of repair. MLH1 forms a complex with PMS2, and functions as an endonuclease. If the mismatch repair system is thoroughly working, genome integrity is maintained completely. Lynch syndrome is a state of mismatch repair deficiency due to a monoallelic abnormality of any mismatch repair genes. The phenotype indicating the mismatch repair deficiency can be frequently shown as a microsatellite instability in tumors. Children with germline biallelic mismatch repair gene abnormalities were reported to develop conditions such as gastrointestinal polyposis, colorectal cancer, brain cancer, leukemia, etc., and so on, demonstrating the need to respond with new concepts in genetic counseling. In promoting cancer genome medicine in a new era, such as by utilizing immune checkpoints, it is important to understand the genetic and genomic molecular background, including the status of mismatch repair deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is fundamentally a genetic disease, and mutations (pathogenic variants) are pivotal to its etiology and progression. Carcinogenesis develops by accumulation of numerous genetic and epigenetic abnormalities [1,2,3,4]. Therefore, cancer has the following characteristics: sustained proliferative signaling, evasion of growth suppressors, resistance cell death, replicative immortality, angiogenesis induction, and activation of invasion and metastasis [5]. Therefore, elucidation of its etiology and development of therapeutic measures is essential [6]. Although rare, hereditary (familial) cancer syndromes are observed in cancers derived from any organ. In individuals with hereditary cancer syndrome, the initial cancer-causing mutation is inherited through the germline and therefore, is already present in every cell of the body. Lynch syndrome (MIM# 120435) is a highly penetrant autosomal-dominant syndrome characterized by several individuals in the family affected with colorectal cancer (CRC) or extracolonic tumors of the endometrium, stomach, small bowel, ureter, renal pelvis, ovary, and hepatobiliary tract [7]. Lynch syndrome occurs due to loss-of-function of the mismatch repair mechanism for genomic replication errors. This article outlines the basis of molecular genetics involved in Lynch syndrome.
DNA repair system
Large numbers of cell division are required to produce an individual with an estimated 37 trillion cells from a single-cell zygote. The frequency of replication errors is 10−10 per base of DNA per cell division, and in an estimated 1015 cell divisions during an individual’s lifetime replication errors cause thousands of new DNA mutations in the genome in every cell. Eukaryotes possess multiple repair systems to avoid replication errors (Table 1). Protecting integrity through genome repair prevents cancer development and progression by genomic abnormalities. Genes encoding molecules involved in genome repair are referred to as DNA repair genes, and as “caretaker tumor suppressor genes”.
The mismatch repair system was recognized in 1961, with proposal that the correction of DNA base pair mismatches within recombination intermediates is the basis for gene conversion [8]. Elucidation of the mismatch repair system was followed by fundamental research based on Escherichia coli [9]. The methyl-directed pathway depends on the products of four E. coli mutator genes: mutH, mutL, mutS, and uvrD [10,11,12]. Inactivation of any of these genes increases the generation of mutations in the E. coli cell by 50- to 100-fold, indicating the importance of this pathway in mutation avoidance and genetic stability. The reduction in mutability afforded by the E. coli methyl-directed system has been attributed to its role in the strand-specific elimination of DNA errors (Table 2) [6, 13,14,15,16,17,18]. Research on the mismatch repair system has advanced extensively and has clarified its mechanism and role as an essential mechanism for maintaining genome integrity in organisms and involved in predisposition to cancer development.
Genes responsible for Lynch syndrome
Lynch syndrome (alias: hereditary nonpolyposis colorectal cancer—HNPCC) is an autosomal-dominant inherited disorder caused by germline mutations in DNA mismatch repair (MMR) genes. Patients with Lynch syndrome are at an increased risk of developing tumors from a young age and throughout their lifetime. Most of them suffer from multiple synchronous and/or metachronous primary tumors. Colorectal cancer and endometrial cancer (female) are well known in the tumor spectrum of Lynch syndrome. In addition, patients with Lynch syndrome have high potential for developing cancer of the urinary tract, the stomach, the small intestine, the biliary tract, the skin, the brain, and others.
Many human mismatch repair (MMR) proteins are known, and several encoding genes have been isolated so far. Currently, four types of MMR genes, MLH1 (MIM# 120436), MSH2 (MIM# 609309), MSH6 (MIM# 600678), and PMS2 (MIM# 600259), are used in the clinic applications related to Lynch syndrome. An outline of the responsible genes is shown in Table 3 and Fig. 1. The EPCAM, which encodes a cell adhesion molecule, is not an MMR gene. However, structural abnormality in EPCAM may cause Lynch syndrome, because it is adjacent to the MSH2 gene [19].
In 1993, two research groups independently isolated MSH2, a human mismatch repair gene that is highly homologous to the mutator phenotype gene, mutS of E. coli [20, 21]. Genomic MSH2 covers approximately 73 kb and contains 16 exons and is mapped to chromosome 2p22-p21 [22, 23]. In 1994, as the second responsible gene of Lynch syndrome, MLH1, the E. coli mutL homologue, was isolated from 3p22.2 according to the mapping in the previous year [24, 25]. Human MLH1 consists of 19 coding exons spanning approximately 100 kb and is highly conserved in especially in exons 1–7 [26]. In 1995, mismatch binding factors were found as the 100 kDa MSH2 or as heterodimers of the 160 kDa polypeptide called GTBP (for G/T binding protein). Using sequence analysis, GTBP was recognized as a new member of the MutS homologue [27, 28]. MSH6 (GTBP) was first reported by Japanese researchers as a gene responsible for Lynch syndrome [29, 30]. In 1994, a germline deletion of the PMS2 was also identified in families with Lynch syndrome. Moreover, additional deletions in tumor samples with microsatellite instability (MSI)-high showed the presence of two-hits [31], indicating that there are pseudogenes corresponding to the PMS2, and that careful consideration is required for genetic testing [31, 32].
Structure and function of MMR proteins
Each MMR protein encoded by the corresponding MMR gene has a unique function in repairing replication errors. Therefore, MMR proteins possess unique functional domains. When mutations of MMR genes occur in the DNA site corresponding to the functional domain, DNA repair function may be impaired. Schematic representations of MLH1, MSH2, MSH6, and PMS2 proteins are shown in Fig. 2 [33,34,35,36,37]. Both MLH1 and PMS2 have an ATP binding domain and require ATP molecules for the endonuclease function.
Many human MMR-related proteins have been identified as homologues of E. coli MMR proteins (Table 4) [21,22,23,24,25,26,27,28, 38,39,40,41,42,43,44,45,46,47,48]. These include human homologues of MutS, MutL, ExoI, DNA polymerase δ (pol δ), proliferating cellular nuclear antigen (PCNA), replication factor (RFC), and DNA ligase I. Although, MutS and MutL proteins of E. coli form homodimers and perform DNA repair functions, functional heterodimer formation is necessary in humans. MSH2 heterodimerizes with MSH6 or MSH3 to form MutSα or MutSβ, respectively. These are involved in the mismatch-pair recognition and initiation of repair [49,50,51,52,53]. In particular, MutSβ recognizes the insertion/deletion loop. On the contrary, MLH1 heterodimerizes with PMS2, PMS1, or MLH3 to form MutLα, MutLβ, MutLγ, respectively [36, 37, 39, 50, 51, 53,54,55,56,57,58,59]. MutLα is a latent endonuclease, that forms a complex with MutS heterodimer, and breaks one chain of the heteroduplex DNA strand with mismatch pairs [57]. The DQHA(X)2E(X)4E motif of PMS2 is probably involved in this nick forming function. MutLβ is one of the endonucleases acting on single-strand breaks in DNA, but its specific function is still unclear. MutLγ is an endonuclease targeting single-strand breaks in supercoiled DNA and plays an important role in meiosis [60,61,62].
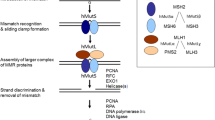
Mechanisms of mismatch repair
The mismatch repair (MMR) system consists of sequential steps for the recognition, removal, and re-synthesis of the mismatch site in DNA. This system that maintains DNA fidelity is well conserved from E. coli to eukaryotes. A schematic diagram of the pathway is shown in Fig. 3 [52, 57, 59, 61,62,63,64,65,66,67,68,69,70,71,72]. Base–base mismatches in double-strand DNA are recognized by MutSα (heterodimer of MSH2-MSH6). MutSα binds as a sliding clamp around the double-strand DNA. In this step, MSH2 requires ATP for sliding of the MutSα clamp on the double-strand DNA [73]. The ATP-activated state of MutSα can interact with MutLα (heterodimer of MLH1-PMS2 and forms a tetrameric complex) [74,75,76]. The tetrameric complex slides up and down the double-strand DNA and searches a single-strand DNA gap on the nascent (daughter) strand that recruits proliferating cell nuclear antigen (PCNA) and Replication factor C (RFC). MutLα can incise the nascent (daughter) strand upon activation by PCNA [57, 77]. Then, exonuclease 1 (Exo 1) is recruited and removes the nascent (daughter) strand around the error region. The re-synthesis step is accomplished by DNA polymerase (Polδ or Polε) and Ligase 1.
MSH2 and MLH1 each have an ATPase domain whose product functions in a biological reaction by ATP-hydrolysis (Fig. 2). An ATP-hydrolysis reaction is necessary when MutSα recognizes a mismatch site or when MutLα forms a nick in the DNA strand [78,79,80,81]. Therefore, it is presumed that completion of the MMR pathway requires consumption of some energy.
Relationship between MMR system and DNA damages
Depending on the DNA damage pattern, specific mismatch repair molecules and complexes are involved (Fig. 4) [49, 63, 65, 82,83,84,85]. The MutSα (heterodimer of MSH2-MSH6) contributes to mismatch recognition by single nucleotide substitution (e.g., G:T mismatch pair) and recognition of small insertion–deletion loops (IDL, e.g., error of the repeat number in adenine clusters), whereas MutSβ (heterodimer of MSH2-MSH3) contributes to the repair of small loops and relatively large damages up to about 10 nucleotide loops. Recently, the function of MutSβ has attracted attention for its biological characteristics and as a prognostic factor of elevated microsatellite instability at selected tetranucleotide (EMAST) colorectal cancer, which shows instability in the repeat sequence of the tetranucleotides [86,87,88,89,90]. The clinical characteristics are presumed to involve the MSH3 deficiency state.
MutL function mainly involves MutLα, a heterodimer of MLH1 and PMS2. However, MutLγ, a heterodimer of MLH1 and MLH3, is involved in repair in the case of instability greater than a trinucleotide repeat.
EPCAM as the gene responsible for Lynch syndrome
EPCAM is located at 2p21 adjacent to the MSH2 on the 5’ upstream, and encodes the EpCAM protein, expressed on the membrane of cells in epithelial tissues and plasma cells, and is deeply involved in the function of cell–cell interaction [91, 92]. Although EPCAM is not directly responsible for Lynch syndrome, it has a positional feature, as it is located 17 kb upstream of MSH2. Monoallelic cis-deletions of the last exons of EPCAM result in loss of its polyadenylation, transcriptional read-through into MSH2 with mosaic promotor methylation, and the generation of fused EpCAM–MSH2 transcripts (Fig. 5) [19]. The cis-deleted alleles inhibit MSH2 expression, and finally causes Lynch syndrome in 1–3% of the affected families [19, 93].
In addition, biallelic inactivation of EPCAM is responsible for congenital tufting enteropathy (CTE, MIM# 613217) with an estimated incidence of one in 50,000–100,000 births in Western Europe [94,95,96]. CTE presents within the first months of life with severe chronic watery diarrhea and growth restriction. EPCAM abnormalities responsible for CTE are usually missense mutations, nonsense mutations, minute insertions/deletions, and splicing errors, which differ in type from extensive deletions that cause the EPCAM-associated Lynch syndrome [97]. Interestingly, in this case, one gene causes two unrelated genetic gastrointestinal disorders to be associated with different types of abnormalities.
Constitutional mismatch repair deficiency syndrome
Constitutional mismatch repair deficiency syndrome (CMMR-D) is caused by biallelic homozygous or compound heterozygous pathogenic germline mutations of MMR genes, and is a distinct childhood cancer preposition syndrome (MIM# 276300) with an autosomal recessive inheritance [98]. This condition was clarified from two different reports on children from consanguineous marriages within families with Lynch syndrome and MLH1 germline mutations who developed malignancies in early childhood (age range 14 months to 6 years) [99, 100]. In 1959, a condition strongly suspected as CMMR-D was reported by Turcot et al. [101] in two siblings with numerous colorectal adenomatous polyps, colorectal carcinoma and malignant brain tumors. Later, this condition was considered as a subtype of familial adenomatous polyposis (FAP) called Turcot’s syndrome [102].
In biallelic germline mutation carriers of MMR genes, hematological malignancies, brain/central nervous system (CNS) tumors and Lynch syndrome-associated carcinomas develop frequently [98]. In the gastrointestinal tract, bowel adenomatous polyposes are often observed as premalignant lesions that require differential diagnosis from FAP. The median age at diagnosis of hematological malignancies and brain/CNS tumors was, respectively, 6.6 (age range 1.2–30.8) and 10.3 (age range 3.3–40) years. However, Lynch syndrome-associated tumors developed later [median age at diagnosis 21.4 years (age range 11.4–36.6)], and are mostly colorectal cancers [103]. Various non-neoplastic features are related to CMMR-D including Cafe au lait spots (NF1 like), skin hypopigmentation, mild defects in immunoglobulin class switching recombination, agenesis of the corpus callosum, cavernous brain hemangioma, capillary hemangioma of the skin, combination of various congenital malformations, and Lupus erythematosus.
Lynch syndrome-associated tumors from patients with CMMR-D are considered to represent the characteristics of the DNA replication error as in the cases with Lynch syndrome. Thus, they often present with MSI-H findings, but not necessarily in all cases [103].
Genetic testing for Lynch syndrome
In order to select high-risk individuals with Lynch syndrome from among patients with colorectal cancer and to increase the efficiency of detecting germline mutations, microsatellite instability (MSI) testing and/or immunohistochemical staining (IHC) of MMR proteins is recommended as universal tumor screening, and should be conducted first [104,105,106]. The MSI testing facilitates easy identification of events in which genetic integrity has been damaged due to repair failures of DNA replication errors using simple repeated microsatellite sequences [107,108,109,110,111]. Five types of repeat-markers including mononucleotide and dinucleotide repeats have been used, but recently mononucleotide repeat-markers have been preferred. Cases with different numbers of repeats between normal tissue-derived DNA and cancer-derived DNA are considered as positive [112]. If two of the five markers show instability, the tumor is evaluated as MSI-high (MSI-H). The results of MSI-H colorectal cancer are shown in Fig. 6. If one of the markers shows instability, the tumor is considered as MSI-low (MSI-L). If positive markers are not observed, the mismatch repair system is evaluated to be proficient and is called MS-stable (MSS).
Immunohistochemical staining of MMR proteins can reveal damaged molecules using specific antibodies. Staining with four antibodies—MLH1, MSH2, MSH6, and PMS2—can predict the gene causing Lynch syndrome, because the mismatch repair proteins form heterodimeric complexes (Table 5) [113,114,115,116,117,118,119,120].
For MSI testing, sensitivity ranged from 66.7 to 100.0% and specificity ranged from 61.1 to 92.5%, whereas for IHC staining, sensitivity ranged from 80.8 to 100.0% and specificity ranged from 80.5 to 91.9% [121].
Approximately 10–15% of sporadic colorectal cancers show MSI-H findings. The cause is mostly the loss of MSH1 protein due to methylation of the MLH1 gene promoter region. About half of MSI-H sporadic colorectal cancers show BRAFV600E mutation, which is not detected in colorectal cancers from patients with Lynch syndrome. MLH1 methylation analysis and BRAF V600E mutation testing in colorectal cancers can reduce the number of samples and simplify the genetic testing for Lynch syndrome, leading to cost and time savings [35, 122].
Final genetic testing for Lynch syndrome is performed using DNA sequencing in selected cases excluding sporadic colon cancer from all colorectal cancers. For a long time, genetic testing has mainly been performed using Sanger sequencing, and multiplex ligation-dependent probe amplification (MLPA) has been adopted for a wide range of abnormalities such as large deletions/insertions [123]. Clinical genetics is currently transitioning from phenotype-directed single gene testing to multigene panels [124]. Multigene panel testing using next generation sequencing for hereditary colorectal cancer has been evaluated as a feasible, timely, and cost-effective approach compared to single gene testing [125]. Previously, the distribution of germline mutations in MMR and EPCAM genes in Lynch syndrome was thought to predominantly occur in MSH2 and MLH1, and less frequently in MSH6 and PMS2. As a result of multigene panel testing without universal tumor screening, Espenschied et al. reported that MSH6 mutations were the most frequent, followed by PMS2, MSH2, MLH1, and EPCAM (Table 6a) [123, 126,127,128]. About 12% of individuals carrying MMR gene mutations have breast cancer alone. Moreover, even MMR gene mutation carriers do not necessarily meet the criteria for Lynch syndrome or the BRCA1/BRCA2 testing criteria. However, MSH6 and PMS2 germline pathogenic variants are associated with an increased risk for breast cancer [126, 129]. Figure 2 shows the gene-specific distributions of germline variants by the types of abnormalities in mismatch repair genes. Most MSH2, MLH1, and MSH6 pathogenic variants were truncated types such as nonsense mutations or frameshift mutations (Table 6b) [130]. A wide range of rearrangements were detected at 10, 7, and 10% for MSH2, MLH1 and PMS2, respectively. Therefore, selection of an appropriate analysis method is required for genetic testing.
Effectiveness of immune check point blockades and a hypermutable state (high tumor mutational burden)
As cancer cells escape the host immune system by suppressing T cell activation, they have immunosuppressive functions attributed to immune checkpoint molecules. The immune checkpoint molecules include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1, CD279) [131, 132], which were found to negatively control the immune system [133, 134]. In human cancer treatment, anti-PD-1 antibody was found to be effective for non-small cell lung cancer, malignant melanoma, and renal cell cancer, and was also clinically applicable in safety [135]. The clinical efficacy of PD-1 inhibitor was found to be higher in mismatch repair-defective colorectal and non-colorectal cancers compared to proficient-mismatch repair cancers [136]. According to recent findings, high tumor mutational burden (TMB) is an excellent biomarker for predicting the efficacy of immune checkpoint inhibitors (ICIs) [137, 138], and the group of colorectal cancer patients with the biological characteristics of deficient mismatch repair (dMMR) has a significantly better response to ICIs than those with proficient mismatch repair (pMMR) [136, 139]. In gastrointestinal cancer, the state of microsatellite instability high (MSI-H) state has been shown to correlate well with high TMB based on an analysis of many cancer genomes [140]. The microsatellite instability (MSI) testing is used as a standard biomarker to predict the response of ICIs [141, 142].
Future directions
The long-term and detailed research on two families with familial accumulation of various cancers conducted 100 years ago has subsequently led to the establishment of Lynch syndrome. On the other hand, mismatch repair genes have been elucidated as part of the genome integrity system in E. coli and yeast. These researchers worked together to understand the clinical, genetic, and molecular biology aspects of Lynch syndrome. With its natural history and molecular biological characteristics clarified, pre-symptomatic diagnosis by genetic testing for at-risk persons in the family and appropriate medically actionable interventions, such as early diagnosis, are becoming possible.
The development of ICIs is a major milestone in the treatment of Lynch syndrome, where the associated cancers are with almost MSI-H. These studies have shown new possibilities for the treatment of familial (hereditary) tumor syndrome. In future, we hope that advances in the integrated understanding of the clinical and molecular biology of Lynch syndrome will lead to the development of new effective treatments.
Change history
31 July 2019
In the original publication, part d of Figure 2 was mistakenly not included.
Abbreviations
- MMR:
-
Mismatch repair
- MSI:
-
Microsatellite instability
- PCNA:
-
Proliferating cellular nuclear antigen
- RFC:
-
Replication factor
- CTE:
-
Congenital tufting enteropathy
- CMMR-D:
-
Constitutional mismatch repair deficiency
- CNS:
-
Central nervous system
- IHC:
-
Immunohistochemical staining
- MLPA:
-
Multiple ligation-dependent probe amplification
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- PD-1:
-
Programmed cell death protein 1
- TMB:
-
Tumor mutational burden
- ICI:
-
Immune checkpoint inhibitor
References
Vogelstein B, Fearon ER, Hamilton SR et al (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319:525–532
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Bodmer W, Bishop T, Karran P (1994) Genetic steps in colorectal cancer. Nature Genet 6:217–219
Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87:159–170
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Tamura K, Utsunomiya J, Iwama T et al (2004) Mechanism of carcinogenesis in familial tumors. Int J Clin Oncol 9:232–245
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348:919–932
Holliday R (1964) A mechanism for gene conversion in fungi. Genet Res 5:282–304
Modrich P (2016) Mechanisms in E. coli and mismatch repair. Angew Chem Int Ed Engl 55:8490–8501
Nevers P, Spats HC (1975) Escherichia coli mutants uvr D and uvr E deficient in gene conversion of lambda-heteroduplexes. Mol Gen Genet 139:233–243
Rydberg B (1978) Bromouracil mutagenesis and mismatch repair in mutator strains of Escherichia coli. Mutat Res 52:11–24
Glickman BW, Radman M (1980) Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci USA 77:1063–1067
Lauhe RS, Su SS, Morich P (1987) Requirement for d(GATC) sequences in Escherichia coli mutHLS mismatch correction. Proc Natl Acad Sci USA 84:1482–1486
Su SS, Morrich P (1986) Escherichia coli mutS-encoded protein binds to Mismatched DNA base pairs. Proc Natl Acad Sci USA 83:5057–5061
Meselson M (1988) Methyl-directed repair of DNA mismatches. In: Low KB (ed) Recombination of the genetic material. Academic Press, San Diego, pp 91–113
Modrich P (1989) Methyl-directed DNA mismatch correction. J Biol Chem 264:6597–6600
Grilley M, Holmes J, Yashar B et al (1990) Mechanisms of DNA-mismatch correction. Mutat Res 236:253–267
Modrich P (1991) Mechanisms and biological effects of mismatch repair. Annu Rev Genet 25:229–253
Ligtenberg MJL, Kuiper RP, Chan TL et al (2009) Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3-prime exons of TACSTD1. Nature Genet 41:112–117
Fishel R, Lescoe MK, Rao MRS et al (1993) The human mutator gene homolog MSH2 and its association. Cell 75:1027–1038
Leach FS, Nicolaides NC, Papadopoulos N et al (1993) Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75:1215–1225
Kolodner RD, Hall NR, Lipford J et al (1994) Structure of the human MSH2 locus and analysis of two Muir-Torre kindreds for msh2 mutations. Genomics 24:516–526
Fishel R, Ewel A, Lee S et al (1993) Binding of mismatched microsatellite DNA sequences by the human MSH2 protein. Science 266:1403–1405
Papadopoulos N, Nicolaides NC, Wei Y-F et al (1994) Mutation of a mutL homolog in hereditary colon cancer. Science 263:1625–1629
Bronner CE, Baker SM, Morrison PT et al (1994) Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368:258–261
Han H-J, Maruyama M, Baba S et al (1995) Genomic structure of human mismatch repair gene, hMLH1, and its mutation analysis in patients with hereditary non-polyposis colorectal cancer (HNPCC). Hum Molec Genet 4:237–242
Drummond JT, Li G-M, Longley MJ et al (1995) Isolation of an hMSH2-p160 heterodimer that restores DNA mismatch repair to tumor cells. Science 268:1909–1912
Palombo F, Gallinari P, Iaccarino I et al (1995) GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science 268:1912–1914
Miyaki M, Konishi M, Tanaka K et al (1997) Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nature Genet 17:271–272
Akiyama Y, Sato H, Yamada T et al (1997) Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res 57:3920–3923
Nicolaides NC, Papadopoulos N, Liu B et al (1994) Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371:75–80
De Vos M, Hayward BE, Picton S et al (2004) Novel PMS2 pseudogenes can conceal recessive mutations causing a distinctive childhood cancer syndrome. Am J Hum Genet 74:954–964
Ban C, Juno M, Yang W (1999) Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell 97:85–97
Tran PT, Liskay RM (2000) Functional studies on the candidate ATPase domains of Saccharomyces cerevisiae MutLalpha. Mol Cell Biol 20:6390–6398
Räschle M, Dufner P, Marra G et al (2002) Mutations within the hMLH1 and hPMS2 subunits of the human MutLalpha mismatch repair factor affect its ATPase activity, but not its ability to interact with hMutSalpha. J Biol Chem 277:21810–21820
Guerrette S, Acharya S, Fishel R (1999) The interaction of the human MutL homologues in hereditary nonpolyposis colon cancer. J Biol Chem 274:6336–6341
Kondo E, Horii A, Fukushige S (2001) The interaction domains of three MutL heterodimers in man: hMLH1 interacts with 36 homologous amino acid residues within hMLH3, hPMS1 and hPMS2. Nucleic Acids Res 29:1695–1708
Reenan RA, Kolodner RD (1992) Isolation and characterization of two Saccharomyces cerevisiae genes encoding homologs of the bacterial HexA and MutS mismatch repair proteins. Genetics 132:963–973
Li GM, Modrich P (1995) Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc Natl Acad Sci USA 92:1950–1954
Johnson RE, Kovvali GK, Guzder SN et al (1996) Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem 271:27987–27990
Umar A, Buermeyer AB, Simon JA et al (1996) Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell 87:65–73
Tishkoff DX, Boerger AL, Bertrand P et al (1997) Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci USA 94:7487–7492
Longley MJ, Pierce AJ, Modrich P (1997) DNA polymerase delta is required for human mismatch repair in vitro. J Biol Chem 272:10917–10921
Schmutte C, Marinescu RC, Sadoff MM et al (1998) Human exonuclease I interacts with the mismatch repair protein hMSH2. Cancer Res 58:4537–4542
Tishkoff DX, Amin NS, Viars CS et al (1998) Identification of a human gene encoding a homologue of Saccharomyces cerevisiae EXO1, an exonuclease implicated in mismatch repair and recombination. Cancer Res 58:5027–5031
Lin YL, Shivji MK, Chen C et al (1998) The evolutionarily conserved zinc finger motif in the largest subunit of human replication protein A is required for DNA replication and mismatch repair but not for nucleotide excision repair. J Biol Chem 273:1453–1461
Gu L, Hong Y, McCulloch S et al (1998) ATP-dependent interaction of human mismatch repair proteins and dual role of PCNA in mismatch repair. Nucleic Acids Res 26:1173–1178
Zhang Y, Yuan F, Presnell SR et al (2005) Reconstitution of 5′-directed human mismatch repair in a purified system. Cell 122:693–705
Genschel J, Littman SJ, Drummond JT et al (1998) Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J Biol Chem 273(31):19895–19901
Iyer RR, Pluciennik A, Genschel J et al (2010) MutLalpha and proliferating cell nuclear antigen share binding sites on MutSbeta. J Biol Chem 285(15):11730–11739
Plotz G, Raedle J, Brieger A et al (2003) N-terminus of hMLH1 confers interaction of hMutLalpha and hMutLbeta with hMutSalpha. Nucleic Acids Res 31(12):3217–3226
Dahal BK, Kadyrova LY, Delfino KR et al (2017) Involvement of DNA mismatch repair in the maintenance of heterochromatic DNA stability in Saccharomyces cerevisiae. PLoS Genet 13(10):e1007074
Villahermosa D, Christensen O, Knapp K et al (2017) Schizosaccharomyces pombe MutSα and MutLα maintain stability of tetra-nucleotide repeats and Msh3 of hepta-nucleotide repeats. G3 (Bethesda) 7(5):1463–1473
Prolla TA, Baker SM, Harris AC et al (1998) Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat Genet 18(3):276–279
Jäger AC, Rasmussen M, Bisgaard HC et al (2001) HNPCC mutations in the human DNA mismatch repair gene hMLH1 influence assembly of hMutLalpha and hMLH1-hEXO1 complexes. Oncogene 20(27):3590–3595
Cannavo E, Marra G, Sabates-Bellver J et al (2005) Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res 65(23):10759–10766
Kadyrov FA, Dzantiev L, Constantin N et al (2006) Endonucleolytic function of MutLalpha in human mismatch repair. Cell 126(2):297–308
Peng M, Litman R, Xie J, Sharma S, Brosh RM Jr, Cantor SB (2007) The FANCJ/MutLalpha interaction is required for correction of the cross-link response in FA-J cells. EMBO J 26(13):3238–3249
Pluciennik A, Dzantiev L, Iyer RR et al (2010) PCNA function in the activation and strand direction of MutLα endonuclease in mismatch repair. Proc Natl Acad Sci USA 107(37):16066–16071
Kunkel TA, Erie DA (2005) DNA mismatch repair. Annu Rev Biochem 74:681–710
Li GM (2008) Mechanisms and functions of DNA mismatch repair. Cell Res 18(1):85–98
Kunkel TA, Erie DA (2015) Eukaryotic mismatch repair in relation to DNA replication. Annu Rev Genet 49:291–313
Marti TM, Kunz C, Fleck O (2002) DNA mismatch repair and mutation avoidance pathways. J Cell Physiol 191(1):28–41
Friedberg EC (2003) DNA damage and repair. Nature 421(6921):436–440
Martin SA, Lord CJ, Ashworth A (2010) Therapeutic targeting of the DNA mismatch repair pathway. Clin Cancer Res 16(21):5107–5113
Boland CR, Goel A (2010) Microsatellite instability in colorectal cancer. Gastroenterology 138(6):2073–2087
Fishel R (2015) Mismatch repair. J Biol Chem 290(44):26395–26403
Groothuizen FS, Sixma TK (2016) The conserved molecular machinery in DNA mismatch repair enzyme structures. DNA Repair (Amst) 38:14–23
Hingorani MM (2016) Mismatch binding, ADP-ATP exchange and intramolecular signaling during mismatch repair. DNA Repair (Amst) 38:24–31
Kadyrova LY, Kadyrov FA (2016) Endonuclease activities of MutLα and its homologs in DNA mismatch repair. DNA Repair (Amst) 38:42–49
Peltomäki P (2016) Update on Lynch syndrome genomics. Fam Cancer 15:385–393
Liu D, Keijzers G, Rasmussen LJ (2017) DNA mismatch repair and its many roles in eukaryotic cells. Mutat Res 773:174–187
Lee JB, Cho WK, Park J et al (2014) Single-molecule views of MutS on mismatched DNA. DNA Repair 20:82–93
Plotz G, Raedle J, Brieger A et al (2002) hMutSalpha forms an ATP-dependent complex with hMutLalpha and hMutLbeta on DNA. Neucleic Acids Res 30(3):711–718
Plotz G, Piiper A, Wormek M et al (2006) Analysis of the human MutLalpha.MutSalpha. Biochem Biophys Res Commun. 340(3):852–859
Friedhoff P, Li P, Gotthardt J (2016) Protein-protein interactions in DNA mismatch repair. DNA Repair 38:50–57
Jeon Y, Kim D, Martin-Lopez JV et al (2016) Dynamic control of strand excision during human DNA mismatch repair. Proc Natl Acad Sci USA 113(12):3281–3286
Fishel R (1998) Mismatch repair, molecular switches, and signal transduction. Genes Dev 12(14):2096–2101
Ban C, Junop M, Yang W (1999) Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell 97(1):85–97
Spampinato C, Modrich P (2000) The MutL ATPase is required for mismatch repair. J Biol Chem 275(13):9863–9869
Lamers MH, Winterwerp HH, Sixma TK (2003) The alternating ATPase domains of MutS control DNA mismatch repair. ENBO J 22(3):746–756
Kolodner RD, Marsischky GT (1999) Eukaryotic DNA mismatch repair. Curr Opin Genet Dev 9(1):89–96
Peltomäki P (2001) Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet 10(7):735–740
Bellacosa A (2001) Functional interactions and signaling properties of mammalian DNA mismatch repair proteins. Cell Death Differ 8(11):1076–1092
Scmidt MHM, Pearson CE (2016) Disease associated repeat instability and mismatch repair. DNA Repair 38:117–126
Campregher C, Schmid G, Ferk F et al (2012) MSH3-deficiency initiates EMAST without oncogenic transformation of human colon epithelialcells. PLoS One 7(11):e50541. https://doi.org/10.1371/journal.pone.0050541(Epub 2012 Nov 27)
Tseng-Rogenski SS, Chung H, Wilk M et al (2012) Oxidative stress induces nuclear-to-cytosol shift of hMSH3, a potential mechanism for EMAST in colorectal cancer cells. PLoS One 7(11):e50616. https://doi.org/10.1371/journal.pone.0050616(Epub 2012 Nov 30)
Watson MMC, Berg M, Søreide K (2014) Prevalence and implications of elevated microsatellite alterations at selected tetranucleotides in cancer. Br J Cancer 111(5):823–827
Carethers JM, Koi M, Tseng-Rogenski SS (2015) EMAST is a form of microsatellite instability that is initiated by inflammation and modulates colorectal cancer progression. Genes 6(2):185–205
Venderbosch S, van Lent-van Vliet S, de Haan AF et al (2015) EMAST is associated with a poor prognosis in microsatellite instable metastatic colorectal cancer. PLoS One 10(4):e0124538. https://doi.org/10.1371/journal.pone.0124538.eCollection
Dollé E, Theise ND, Schmelzer E et al (2015) EpCAM and the biology of hepatic stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol 308:G233–G250
Huang L, Yang Y, Yang F et al (2018) Functions of EpCAM in physiological processes and diseases. Int J Mol Med 42(4):1771–1785
Kovacs ME, Papp J, Szentirmay Z et al (2009) Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat 30(2):197–203
Sivagnanam M, Mueller JL, Lee H et al (2008) Identification of EpCAM as the gene for congenital tufting enteropathy. Gastroenterol 135(2):429–437
Reifen RM, Cutz E, Griffiths AM et al (1994) Tufting enteropathy: a newly recognized clinicopathological entity associated with refractory diarrhea in infants. J Pediatr Gastroenterol Nutr 18(3):379–385
Goulet O, Salomon J, Ruemmele F et al (2007) Intestinal epithelial dysplasia (tufting enteropathy). Orphanet J Rare Dis 2(1):20
Pathak SJ, Mueller JL, Okamoto K et al (2019) EPCAM mutation update: variants associated with congenital tufting enteropathy and Lynch syndrome. Hum Mutat 40(2):142–161
Wimmer K, Kratz CP, Vasen HFA et al (2014) Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘care for CMMRD’ (C4CMMRD). J Med Genet 51(6):355–365
Ricciardone MD, Ozçelik T, Cevher B et al (1999) Human MLH1 deficiency predisposes to hematological malignancy and neurofibromatosis type 1. Cancer Res 59(2):290–293
Wang Q, Lasset C, Desseigne F et al (1999) Neurofibromatosis and early onset of cancers in hMLH1-deficient children. Cancer Res 59(2):294–297
Turcot J, Despres JP, St Pierre F et al (1959) Malignant tumors of the central nervous system associated with familial polyposis of the colon: report of two cases. Dis Colon Rectum 2:465–468
Hamilton SR, Liu B, Parsons RE et al (1995) The molecular basis of Turcot’s syndrome. N Engl J Med 332(13):839–847
Lavoine N, Colas C, Muleris M et al (2015) Constitutional mismatch repair deficiency syndrome: clinical description in a French cohort. J Med Genet 52(11):770–778
Turcot J, Despres JP, St Pierre F (1959) Malignant tumors of the central nervous system associated with familial polyposis of the colon: report of two cases. Dis Colon Rectum 2(5):465–468
Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009) Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 11(1):35–41
Giardiello FM, Allen JI, Axilbund JE et al (2014) Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multisociety Task Force on colorectal cancer. Am J Gastroenterol 109(8):1159–1179
Syngal S, Brand RE, Church JM et al (2015) ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110(2):223–262
Boland CR, Thibodeau SN, Hamilton SR et al (1998) A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58(22):5248–5257
Ionov Y, Peinado MA, Malkhosyan S et al (1993) Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363(6429):558–561
Peltomäki P, Aaltonen LA, Sistonen P et al (1993) Genetic mapping of a locus predisposing to human colorectal cancer. Nature 260:810–812
Thibodeau SN, Bren G, Schaid D (1993) Microsatellite instability in cancer of the proximal colon. Nature 260(5109):816–819
Rodriguez-Bigas MA, Boland CR, Hamilton SR et al (1997) A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89(23):1758–1762
Leach FS, Polyak K, Burrell M et al (1996) Expression of the human mismatch repair gene hMSH2 in normal and neoplastic tissues. Cancer Res 56(2):235–240
Thibodeau SN, French AJ, Roche PC et al (1996) Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 56(21):4836–4840
Hendriks Y, Franken P, Dierssen JW et al (2003) Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol 162(2):469–477
de Jong AE, van Puijenbroek M, Hendriks Y et al (2004) Microsatellite instability, immunohistochemistry, and additional PMS2 staining in suspected hereditary nonpolyposis colorectal cancer. Clin Cancer res 10(39):972–980
Lipkin SM, Wang V, Jacoby R et al (2000) MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat Genet 24(1):27–35
Rigau V, Sebbagh N, Olschwang S et al (2003) Microsatellite instability in colorectal carcinoma. The comparison of immunohistochemistry and molecular biology suggests a role for hMSH6 [correction of hMLH6] immunostaining. Arch Pathol Lab Med 127(6):694–700
Hampel H, Frankel WL, Martin E et al (2005) Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 352(18):1851–1860
Hendriks YMC, de Jong AE, Morreau H et al (2006) Dianostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 56(4):213–225
Snowsill T, Coelho H, Huxley N et al (2017) Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess 21(51):1–238
Jin M, Hampel H, Zhou X et al (2013) BRAF V600E mutation analysis simplifies the testing algorithm for Lynch Syndrome. Am J Clin Pathol 140(2):177–183
Lagerstedt-Robinson K, Rohlin A, Aravidis C et al (2016) Mismatch repair gene mutation spectrum in the Swedish Lynch syndrome population. Oncol Rep 36(5):2823–2835
Lorans M, Dow E, Macrae FA et al (2018) Update on hereditary colorectal cancer: improving the clinical utility of multigene panel testing. Clin Colorectal Cancer 17(2):e293–e305. https://doi.org/10.1016/j.clcc.2018.01.001(Epub 2018 Jan 11)
Gallego CJ, Shirts BH, Bennette CS et al (2015) Next-generations sequencing panels for the diagnosis of colorectal cancer and polyposis syndromes: a cost-effectiveness analysis. J Clin Oncol 33(18):2084–2091
Espenschied CR, LaDuca H, Li S et al (2017) Multigene panel testing provides a new perspective on Lynch syndrome. J Clin Oncol 35(22):2568–2575
Yurgelun MB, Kulke MH, Fuchs CS et al (2017) Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol 35(10):1086–1095
Thompson BA, Spurdle AB, Plazzer JP et al (2014) Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet 46(12):107–115
Roberts ME, Jackson SA, Susswein LR et al (2018) MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med 20(10):1167–1174
Plazzer JP, Sijmons RH, Woods MO et al (2013) The InSiGHT database: utilizing 100 years of insights into Lynch syndrome. Fam Cancer 12(2):175–180
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271(5256):1734–1736
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11(11):3887–3895
Tivol EA, Borriello F, Schweitzer AN et al (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3(5):541–547
Nishimura H, Nose M, Hiai H et al (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11(2):141–151
Topalian SL, Hodi S, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520
Snyder A, Makarov V, Merghoub T et al (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189–2199
Rizvi NA, Hellmann MD, Snyder A et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1blockade in non-small cell lung cancer. Science 348(6230):124–128
Yarchoan M, Hopkins A, Jaffee EM et al (2018) Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 377(25):2500–2501
Charmers ZR, Connelly CF, Fabrizio D et al (2017) Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9(1):34. https://doi.org/10.1186/s13073-017-0424-2
Dudley JC, Lin MT, Le DT et al (2016) Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 22(4):813–820
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349):409–413
Acknowledgements
This work was supported in part by a grant from the Japanese Ministry of Education, Science, Sports and Culture of Japan (19K07763).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: In the original publication, Fig. 2d has not been included. Complete Figure 2 is replaced. “MLH1, MSH2, MSH6, PMS2” in legend of figure 2 are made roman. The section, “Effectiveness of immune check point blockades and a hypermutable state (high tumor mutation burden)” is revised to “Effectiveness of immune check point blockades and a hypermutable state (high tumor mutational burden)”.
About this article
Cite this article
Tamura, K., Kaneda, M., Futagawa, M. et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol 24, 999–1011 (2019). https://doi.org/10.1007/s10147-019-01494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01494-y