Abstract
Background
To evaluate the efficacy and toxicity of a combination chemotherapy consisting of gemcitabine, carboplatin, and docetaxel (GCD) in patients with advanced urothelial carcinoma (UC) as a phase II trial.
Materials and methods
Patients with metastatic or locally advanced unresectable UC were eligible for this trial. All enrolled patients were considered to be “unfit” for cisplatin-based chemotherapy, or to have methotrexate, vinblastine, doxorubicin, cisplatin (MVAC)-refractory UC. The chemotherapy regimen consisted of gemcitabine 1000 mg/m2 on days 1 and 8, and carboplatin (with a target area under the curve of 5) and docetaxel 70 mg/m2 on day 1; this was repeated every 21 days.
Results
Thirty-five patients were enrolled, with a median age of 68 years. A total of 89 cycles were administered (median, 2 cycles). Major toxicities were Grade 3/4 neutropenia in 28 (80.0%) patients and Grade 3/4 thrombocytopenia in 18 (51.5%). An objective response rate (ORR) was 11 of 21 patients (52.4%), including a complete response in 1 (4.8%). The median overall survival (OS) was 13.1 months (1-year survival rate, 60%) and the median progression-free survival (PFS) was 5.0 months. Among 16 patients who had previously received MVAC, the ORR, the median PFS, the median OS and 1-year survival rate was 56.3%, 5.0 months, 12.6 months and 54%, respectively.
Conclusions
GCD chemotherapy is active and well tolerated as a first- or second-line therapy for patients with advanced UC. Response rate, duration and survival did not differ between those with and without a history of MVAC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1990s, the most common first-line systemic chemotherapy for patients with advanced and/or unresectable urothelial carcinoma (UC) was combination therapy with cisplatin, methotrexate, doxorubicin, and vinblastine (MVAC) [1]. In Phase III studies of MVAC therapy in patients with advanced UC, the objective response rate (ORR) was 35–45%, and the median overall survival (OS) was 12 months [1, 2]. Although the frequency of response to MVAC therapy was promising, the duration of response was short, and the survival rate at 5 years after initiating the therapy was only 3.2%. In addition, treatment with MVAC is associated with substantial toxicity, including myelosuppression, mucositis, nephrotoxicity, and neuropathy. Therapy-related mortality rates range from 2 to 4% [2, 3]. Recently, Phase II and Phase III studies found that gemcitabine–cisplatin (GC) combination therapy was effective and comparable to MVAC in the treatment of patients with advanced UC [4, 5]. Compared with MVAC therapy, the safety profile of GC therapy was better and the ORR was similar (49 and 46% for GC therapy and MVAC, respectively). However, the therapeutic application of GC to patients with renal impairment is difficult because of the renal toxicity of cisplatin. Since the impairment of renal function is common in patients with advanced UC, it is necessary to develop more active and less toxic treatments.
Carboplatin was developed with the intent of providing an efficacy that is similar to that of cisplatin and has less renal toxicity. Carboplatin seldom causes renal impairment and has an established formula (Calvert formula) that allows for the accurate dosing of the drug on the basis of renal function. The latter has made this agent an attractive alternative to cisplatin.
Docetaxel is also an active single agent in previously-treated patients with UC. In a Phase II study of docetaxel therapy in patients with advanced or metastatic UC relapsing or refractory to no more than one prior cisplatin-containing treatment regimen, the ORR rate was 13.3%, and the median OS was 9 months [6]. Furthermore, docetaxel and gemcitabine combination therapy has also been reported to be active and well tolerated in elderly patients with advanced non-small-cell lung cancer [7, 8].
Our study was designed to evaluate the safety and efficacy of combination chemotherapy consisting of gemcitabine, carboplatin, and docetaxel (GCD) in patients with metastatic and/or locally advanced unresectable UC who had previously undergone MVAC chemotherapy or were not suitable for cisplatin therapy.
Materials and methods
Patients
Thirty-five patients who were treated between April 2002 and March 2008 at the Akita University Hospital were enrolled in this study. All of them had histologically confirmed UC that was either metastatic or locally advanced and unresectable. In addition, patients eligible for this study had to meet at least one of the following three criteria: (1) Patients were considered to be “unfit” for administration of cisplatin [e.g., MVAC, high-dose-intensity MVAC (HD-MVAC)] because of advanced age and/or renal dysfunction (serum creatinine greater than 1.2 mg/dL). (2) Patients had had to discontinue first-line chemotherapy with MVAC or HD-MVAC because of tumor progression or unacceptable toxicity. (3) The disease had relapsed in patients after first-line chemotherapy with MVAC or HD-MVAC. Prior cytotoxic treatment and local radiation were permitted. Eastern Cooperative Oncology Group (ECOG) performance scores were 0, 1, or 2 for all patients. Patients should have recovered from any effects of a major surgery, and at least 4 weeks should have elapsed since completion of chemotherapy. Written informed consent was obtained in all cases. The study was approved by the institutional ethical review board of Akita University Graduate School of Medicine.
Treatment plan
Table 1 outlines the treatment schedule and dose of the GCD regimen. Docetaxel infusion preceded the infusion of carboplatin, which was infused on day 1. The carboplatin infusion was followed by gemcitabine infusion. The starting dose level (level 0) was maintained in patients whose neutrophil count at the nadir was 500/mm3 or greater, and whose platelet count at the nadir was 50,000/mm3 or greater. In patients with a neutrophil nadir of <500/mm3 and/or a platelet count nadir <50,000/mm3, the dose was reduced by one dose level for the next cycle. If the neutrophil nadir was less than 500/mm3, or if the platelet count nadir was less than 50,000/mm3 after a reduction in dose level (−1), then subsequent cycles were started at a lower dose level (−2). Gemcitabine was administered at day 8 only if the neutrophil count was 1000/mm3 or greater and if the platelet count was 100,000/mm3 or greater. The dose could not be escalated once it was reduced.
Assessment of response and adverse events
Radiographic analyses of tumor size and tumor burden, and disease staging were performed at baseline and after every second cycle, or as indicated clinically. Responses to GCD combination therapy were determined using the definitions according to RECIST version 1.0 [9]. The duration of response was determined from the date of the observed response to the date of disease progression, or the last contact with the patient. Survival duration was measured from the initiation of the first cycle of GCD chemotherapy until death or the last contact with the patient.
The severity of adverse events was graded according to NCI-CTCAE version 2.0.
Statistical analysis
OS and progression-free survival (PFS) were plotted by the Kaplan–Meier method, and differences between groups were calculated by the log-rank test. Comparison of response rate between groups was performed using Fisher’s exact test. Statistical analysis was carried out using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients characteristics
From April 2002 to March 2008, 35 patients were enrolled in this study. A total of 14 patients were treated with radiation combination therapy. Demographic and baseline characteristics of the patients are presented in Table 2. The median age was 68 years (range 41–83 years). ECOG performance status was 0 in 30 of 35 patients (85.7%), and 1 in the other 5 patients (14.3%). Twenty-six patients (74.3%) had previously undergone chemotherapy (neoadjuvant and/or adjuvant chemotherapy), which included HD-MVAC in 24 patients, and MVAC in 2 patients. Twelve patients had prior total cystectomy, and 7 patients had prior nephro-ureterectomy. Metastasis to at least 1 region outside of the urothelial tract was observed in all 35 patients (100.0%). The most frequent site of metastasis was the lymph nodes (17 of 35 patients; 43.6%).
Treatment administered
All the 35 patients received a total of 89 cycles of chemotherapy (median of 2 cycles; range 1–8 cycles). No dose reductions were required in 13 of 26 patients (50.0%) at the beginning of the 2nd cycle.
Adverse effects
The overall safety of the treatment regimen was evaluated according to the frequency and severity of treatment-related adverse events. Frequencies of Grade 1 to Grade 4 adverse events are shown in Table 3. Neutropenia was the most frequently observed Grade 3/4 adverse event with the GCD regimen. Twenty-eight of 35 patients (80.0%) experienced Grade 3/4 neutropenia. The incidence rate of Grade 3/4 adverse effects in 11 patients with impaired renal function was not different from that in 24 patients with normal renal function. For example, Grade 3/4 neutropenia was noted in 65.7 and 83.3%, and Grade 3/4 anemia in 45.5 and 41.7%, respectively. Similarly, the incidence rate of Grade 3/4 adverse effects in patients over 75 years was not different from that in patients aged 75 years or younger. For example, Grade 3/4 neutropenia was observed in 77.7 and 77.0%, and Grade 3/4 anemia in 33.3 and 46.2%, respectively. No toxicity-related deaths were encountered in this phase II study.
Tumor response
A total of 35 patients could be assessed for response. Excluding the 14 patients who had received combination radiation therapy, the ORR was 52.4% (11 of 21 patients), including a complete response (CR) in 1 (4.8%), and a partial response (PR) in 10 (47.6%). Another 9 patients (42.9%) had stable disease. The response rate (CR + PR) among patients with lymph node, lung, liver, and soft tissue metastases was 41.2, 12.5, 50.0, and 50.0%, respectively. Among 16 patients who had previously received MVAC or HD-MVAC, the ORR was 56.3% (9 patients), including a CR in 1 (6.3%), and a PR in 8 (50.0%). The ORR in patients previously treated with MVAC or HD-MVAC was not different from that in patients without previous chemotherapy (p = 0.53).
Survival
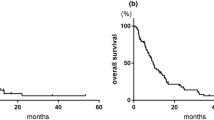
The outcome of GCD combination chemotherapy on overall survival duration (i.e., time from the first treatment with the study drugs to death due to any cause) was determined for the 35 enrolled patients (Fig. 1). Among the 21 patients without combination radiation therapy, the median OS was 13.1 months [95% confidence interval (CI) 11.4–14.7 months], with a 1-year survival of 60%, and a 2-year survival of 24%. The median PFS was 5.0 months (95% CI 2.3–7.7 months). In the 16 patients who had previously received MVAC or HD-MVAC, the median OS was 12.6 months (95% CI 8.9–16.3 months), with a 1-year survival rate of 54%, and a 2-year survival rate of 22% (Fig. 2). The median PFS was 5.0 months (95% CI 3.2–6.8 months) (Fig. 3).
Finally, the OS in patients who had previously been treated with MVAC or HD-MVAC was not different from that of patients without such a history (p = 0.13).
Discussion
Despite significant progress with combination chemotherapy, advanced UC remains a fatal disease for the vast majority of patients with metastatic or unresectable disease. The two active agents—paclitaxel and gemcitabine—have been reported to have favorable toxicity profiles and a potentially synergistic interaction with platinum [10, 11]. Therefore, these two drugs have led to the development of taxane- and gemcitabine-based doublets with cisplatin or carboplatin [12–15]. Although GC combination chemotherapy appears to be well tolerated, the complete response rate and survival with this combination is not superior to that of MVAC, and the response duration is short [16]. Furthermore, patients with advanced UC are often considered to be “unfit” for cisplatin-based chemotherapy because they are often at an advanced age, and have impaired renal function (although judgements to this effect and the criteria for “unfit” may often be based on the physician’s discretion or preference). We sought to enhance the treatment efficacy by developing regimens incorporating three active agents to build on taxan- and gemcitabine-based chemotherapy [16, 17], while reducing toxicity by the application of carboplatin.
With a median of two courses, the present GCD chemotherapy was well tolerated despite the fact that a number of patients were elderly (mean 68 years), and 26% were over 75 years in age. While Grade 3/4 myelosuppression was often encountered, the frequencies of hematologic toxicity were similar to those seen with GC combination chemotherapy [5]. In spite of the high incidence of Grade 3/4 toxicity, there was no drug toxicity-related death. Furthermore, the incidence rate of Grade 3/4 adverse effects was not distinctively high in elderly patients, or in patients with impaired renal function. Therefore, we believe that the GCD regimen was well tolerated and safe, even in elderly patients and those with impaired renal function.
In the present study, the ORR was 52.4%, with a CR rate of 4.8%. The response rate in this study was relatively lower than that of the paclitaxel–carboplatin–gemcitabine combination therapy reported by Hussain et al. (52.4 vs. 68%) [16]. One possible reason for this difference in response rate was that our study included a larger number of patients with a previous history of chemotherapy with MVAC or HD-MVAC (60 vs. 11% in the study by Hussain et al. [16]). On the other hand, the median OS was 13.1 months in our study; this result is similar to that reported with GC combination therapy (12–14 months) [4, 15, 18] and MVAC combination therapy (12 months) [19, 20]. It is notable that in all of the reported studies the patients were chemo-naive. Considering that the present study included 26 (74.3%) patients who had a previous history of chemotherapy with MVAC or HD-MVAC, the GCD regimen appears to be at least as effective as the MVAC or GC regimen. Comparing patients who received radiation therapy with GCD chemotherapy with those who received GCD chemotherapy alone, the patients who received radiation had a better survival rate (Fig. 1). One possible reason for this difference might be the radiosensitization effect of docetaxel and carboplatin; this could also be the reason why the 14 patients (40.0%) who received radiation therapy plus GCD chemotherapy had only 1 target lesion that could be controlled with chemoradiation therapy.
As a second-line chemotherapy for prior MVAC-treated metastatic UC, the results of the present study indicate that the GCD chemotherapy is effective with outcomes that are comparable to those seen in patients without any history of chemotherapy. Of the 16 patients who could be assessed for response to therapy, the ORR was 56.3%, and the median OS was 12.6 months (Figs. 2, 3). Currently, there is no established second-line chemotherapy for patients who experience clinical failure or recurrence after platinum-based chemotherapy, including MVAC and GC. There have been a number of recent reports of clinical trials that have been performed to address this issue [6, 21–28]. McCaffrey et al. [6] described the results of a Phase II trial that used docetaxel monotherapy and showed that the ORR was 13.3%, and the median OS was 5.1 months. Gemcitabine monotherapy has been shown to provide a 22–23% ORR, with 5–9 months median OS, and 3.1–3.8 months median PFS [22, 23]. In trials of paclitaxel plus carboplatin/cisplatin combination therapy, the ORR was 16–36%, the median OS was 6–10.3 months, and the median PFS was 4–6.2 months [15–18]. In trials of gemcitabine plus docetaxel/paclitaxel combination therapy, the ORR was 17–27%, and the median PFS was 7.7–14.4 months [21, 28]. The 53.0% ORR and 13.1 months median OS achieved in our study seems to be comparable with the results reported in these previous reports. Therefore, we believe that our GCD triplet combination may possess substantial activity against advanced UC that has recurred after failure of platinum-containing regimens, especially MVAC and HD-MVAC. However, this remains merely a possibility at present and whether the current GCD regimen is also effective against advanced UC after treatment failure or recurrence following GC chemotherapy should be further investigated. Furthermore, because the duration of PFS in the present study remained rather short at 5 months, the development of a more active and durable regimen requiring the aid of new active agents may be required.
In summary, GCD chemotherapy was effective in patients with advanced UC, even in those with a history of MVAC or HD-MVAC treatment. The regimen was feasible in elderly patients as well as in patients with impaired renal function. However, the short duration of PFS remains a major problem.
References
Saxman SB, Propert KJ, Einhorn LH et al (1997) Long-term follow-up of a Phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 15:2564–2570
Stadler WM, Kuzel TM, Rahgavan D et al (1997) Metastatic bladder cancer: advances in therapy. Eur J Cancer 33:523–526
Sternberg CN, Yagoda A, Scher HI et al (1989) Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelial: Efficacy and patterns of response and relapse. Cancer 64:2448–2458
Kaufman D, Raghavan D, Carducci M et al (2000) Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol 18:1921–1927
von der Maase VDH, Hansen SW, Roberts JT et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068–3077
MaCafferey JA, Hilton S, Mazumdar M et al (1997) Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 15:1853–1857
Hainsworth JD, Erland JB, Barton JH et al (2003) Combination treatment with weekly docetaxel and gemcitabine for advanced non-small-cell lung cancer in elderly patients and patients with poor performance status: results of Minnie Pearl Cancer Research Network phase II trial. Clin Lung Cancer 5:33–38
Hainsworth JD, Spigel DR, Farley C et al (2007) Weekly docetaxel versus docetaxel/gemcitabine in the treatment of elderly or poor performance status patients with advanced nonsmall cell lung cancer. Cancer 110:2027–2034
Therasse P, Arbucl SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Jekunen AP, Christen RD, Shalinsky DR et al (1994) Synergistic interaction between cisplatin and taxol in human ovarian carcinoma cell in vitro. Br J Cancer 69:299–306
Peters GJ, Bergman AM, Ruiz van Haperen VW et al (1995) Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol 22:72–79
Dreicer R, Monala J, Roth BJ et al (2000) Phase II study of cisplatin and paclitaxel in advanced carcinoma of the urothelium: an Eastern Cooperative Oncology Group Study. J Clin Oncol 18:1058–1061
Zielinski CC, Schnack B, Grbovic M et al (1998) Paclitaxel and carboplatin in patients with metastatic urothelial cancer: results of a phase II trial. Br J Cancer 78:370–374
Bellmunt J, de Wit R, Albanell J et al (2001) A feasibility study of carboplatin with fixed dose of gemcitabine in ‘unfit’ patients with advanced bladder cancer. Eur J Cancer 37:2212–2215
von der Maase H, Hansen SW, Roberts JT et al (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 17:3068–3077
Hussain M, Vaishampayan U, Du W et al (2001) Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol 19:2527–2533
Hoshi S, Ohyama C, Ono K et al (2004) Gemcitabine plus carboplatin, and gemcitabine, docetaxel and carboplatin combined chemotherapy regimens in patients with metastatic urothelial carcinoma previously treated with a platinum-based regimen. Int J Clin Oncol 9:125–129
Moore MJ, Winquist EW, Murray N et al (1999) Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer: a phase II trial of the national cancer institute of Canada clinical trials group. J Clin Oncol 17:2876–2881
Logothetis CJ, Dexeus FH, Finn L et al (1990) A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 8:1050–1055
Loehrer PJ Sr, Einhorn LH, Elson PJ et al (1992) A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 7:1066–1073
Sternberg CN, Calabro F, Pizzocaro G et al (2001) Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. J Clin Oncol 92:2993–2998
Lorusso V, Pollera CF, Autimi M et al (1998) A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Eur J Cancer 34:1208–1212
Akaza H, Naito S, Usami M et al (2007) Efficacy and safety of gemcitabine monotherapy in patients with transitional cell carcinoma after cisplatin-containing therapy: a Japanese experience. Jpn J Clin Oncol 37:201–206
Uhm JE, Lim HY, Kim WS et al (2007) Paclitaxel with cisplatin as salvage treatment for patients with previously treated advanced transitional cell carcinoma of the urothelial tract. Neoplasia 9:18–22
Kouno T, Ando M, Yonemori K et al (2007) Weekly paclitaxel and carboplatin against advanced transitional cell cancer after failure of a platinum-based regimen. Eur Urol 52:1115–1122
Soga N, Onishi T, Arima K et al (2007) Paclitaxel carboplatin chemotherapy as a second-line chemotherapy for advanced platinum resistant urothelial cancer in Japanese cases. Int J Urol 14:828–832
Vaishampayan UN, Faulkner JR, Small EJ et al (2005) Phase II trial of carboplatin and paclitaxel in cisplatin-pretreated advanced transitional cell carcinoma. Cancer 104:1627–1632
Dreicer R, Manola J, Schneider DJ et al (2003) Phase II trial of gemcitabine and docetaxel in patients with advanced carcinoma of the urothelium. Cancer 97:2743–2747
Conflict of interest
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tsuruta, H., Inoue, T., Narita, S. et al. Combination therapy consisting of gemcitabine, carboplatin, and docetaxel as an active treatment for advanced urothelial carcinoma. Int J Clin Oncol 16, 533–538 (2011). https://doi.org/10.1007/s10147-011-0224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0224-4