Abstract
Background
Retrospective evaluation of the effectiveness and adverse events (AEs) of a sequential chemotherapy regimen using gemcitabine + carboplatin (GCarbo) followed by GCarbo + docetaxel (GCarboD) for advanced upper-tract urothelial carcinoma (UTUC).
Methods
We treated 56 patients with advanced UTUC. Mean patient age was 68.9 years, creatinine clearance was 51.2 mL/min, and the observation period was 20 months. Patients received two courses of GCarbo comprising 800 mg/m2 gemcitabine on days 1, 8, and 15, and carboplatin at an area under the curve of four on day 2. If this regimen was effective, we administered two more courses of GCarbo; if the regimen was ineffective, we switched to two courses of GCarboD (70 mg/m2).
Results
Complete (n = 3) and partial response (PR; n = 25) were achieved after GCarbo. Mean response duration was 9.7 months. Two of 17 cases achieved PR after GCarboD treatment (mean duration, 31.5 months). Median survival was 14.0 months with the GCarbo/GCarboD regimen. Responders to GCarbo therapy survived significantly longer. AEs with the GCarbo regimen included 31 instances of G3/4 blood toxicity and 8 instances of G3/4 urticaria; however, there were only 6 instances of G3/4 gastrointestinal complications. AEs with the GCarboD regimen included 16 instances of blood toxicity and 8 instances of gastrointestinal complications. Neither regimen resulted in G3/4 renal toxicity.
Conclusions
GCarbo and GCarboD chemotherapy may be administered safely to patients with advanced UTUC, with or without renal dysfunction. Response to GCarbo was high (50.0 %) whereas GCarboD was of limited effectiveness for non-responders to GCarbo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upper-tract urothelial carcinoma (UTUC) is a relatively rare and aggressive disease accounting for 10 % of renal malignancies and 5 % of urothelial carcinomas (UC) [1, 2]. Although radical nephroureterectomy (RNU) and bladder cuff resection are the standard treatment for non-metastatic UTUC, patients often develop local or metastatic recurrence, so the prognosis is very poor [3–7].
Despite recent developments in systematic chemotherapy for advanced UC, successful outcomes after treatment for metastasis or the local recurrence of UTUC remain elusive [3–7]. Tanaka et al. [3] described the prognostic factors and reported the oncological outcomes for 204 Japanese UTUC patients who experienced disease recurrence after RNU. Of these, 132 (64.7 %) underwent systemic chemotherapy, of whom 116 (87.9 %) underwent cisplatin-based chemotherapy. However, 1 and 3-year overall and cancer-specific survival were only 53.6 and 54.4 % and 12.9 and 13.3 %, respectively, with most patients succumbing to UTUC within 3 years, even though systemic chemotherapy was administered after relapse.
According to the 2014 European Association of Urology (EAU) guidelines [8], UTUCs are urothelial tumors; therefore, platinum-based chemotherapy is expected to lead to similar results to those observed for bladder cancer. However, not all patients receive this treatment owing to the risks of comorbidity and impaired renal function after radical surgery.
Kaag et al. [9] reported a decrease of 24 % in mean estimated glomerular filtration rate (eGFR) for 388 patients undergoing nephroureterectomy for UTUC with a cut-off value of 60 mL/min/1.73 m2. Of these, 49 % were eligible for chemotherapy before surgery; however, only 19 % remained eligible postoperatively.
Although cisplatin-based chemotherapy is effective, its nephrotoxic properties render it unsuitable for patients with renal dysfunction. Carboplatin is an alkylating anticancer agent that is less nephrotoxic than cisplatin; therefore, carboplatin-containing chemotherapeutic agents are presumed to be useful for treatment of patients with advanced UC and renal impairment. The combination of gemcitabine and carboplatin (GCarbo) was developed for patients with metastatic UC of the bladder who were unfit for the cisplatin-based therapy and has subsequently been adopted by other investigators for the treatment of such patients [10–17].
We have previously reported the feasibility and effectiveness of carboplatin-based combination chemotherapy for patients aged ≥70 years with advanced bladder cancers [18]. However, there is currently no established chemotherapy regimen for advanced UTUC. Although gemcitabine + cisplatin or methotrexate, vinblastine, doxorubicin, cisplatin (M-VAC) may be promising for advanced UTUC and bladder cancer, renal dysfunction is common among patients with advanced UTUC and cisplatin is often unsuitable for such patients. Here we retrospectively evaluated the effectiveness and adverse events (AEs) of a sequential chemotherapy regimen using GCarbo followed by GCarbo + docetaxel (GCarboD) for advanced UTUC.
Materials and methods
Study design and population
Fifty-six patients (37 men and 19 women; mean age 68.9 years; age range 43–89 years) with advanced UTUC were enrolled in this study from September 2004 to December 2012. Eligible patients had been histologically proved to have recurrent or metastatic UC. Twenty-three cases after RNU and 33 inoperable cases were enrolled. Nine patients of those enrolled had received chemotherapy with M-VAC before enrolling in this study. The study protocol was approved by the ethics committee of Hirosaki University (Hirosaki, Japan) and all patients provided written informed consent before administration of chemotherapy.
Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, adequate bone marrow function (absolute neutrophil count of ≥1,500/mm3 and platelet count of ≥100,000/mm3), and adequate hepatic function (total bilirubin level <1.5 mg/dL). eGFR was calculated by use of the Cockcroft–Gault equation. Patients were excluded from this study if they had central nervous system metastasis, mental retardation, or a severe bacterial infection. Patients were judged as unfit for cisplatin-based chemotherapy if they met at least one of five criteria:
-
1.
ECOG performance status of 2;
-
2.
creatinine clearance <60 mL/min;
-
3.
grade 2 or higher hearing loss;
-
4.
grade 2 or higher neuropathy; or
-
5.
New York Heart Association class III heart failure [19].
Treatment schedule
The therapeutic regimen comprised two lines:
-
1.
GCarbo therapy as first-line therapy, with two courses as a set; and
-
2.
GCarboD therapy as second-line therapy if the response to the first-line therapy was insufficient.
GCarbo therapy comprised 800 mg/m2 gemcitabine on days 1, 8, and 15 and carboplatin at an area under the curve of 4 according to the Calvert formula [20] on day 2. If this regimen was effective, another two courses of GCarbo therapy were administered. If this regimen did not induce reduction in tumor size, however, the treatment was switched to GCarboD therapy, which comprised 800 mg/m2 gemcitabine on days 1 and 8, 70 mg/m2 docetaxel on day 1, and carboplatin (area under the curve of 4) on day 2.
Patient evaluation
Baseline evaluations included complete history and physical examinations, assessment of ECOG performance status, abdominal and pelvic computed tomography (CT) or magnetic resonance imaging, and chest radiography or CT. Tumor response was assessed by use of the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Toxicity was evaluated before each treatment cycle and graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
End points and statistical analysis
The end points of this study were overall survival (OS) and toxicity. OS was defined as the time from the start of treatment to death from any cause. For surviving patients, outcomes were determined on the last follow-up day. Data were analyzed by use of SPSS statistical software (version 18.0; SPSS, Chicago, USA). Correlations between survival and subgroup classification were identified by use of the log-rank test. Statistical significance was set at p < 0.05.
Results
Patient characteristics
Demographic and baseline characteristics of the patients are presented in Table 1. The median age was 68.9 years (range 43–89 years). Average creatinine clearance was 51.2 mL/min (range 11.6–99.3 mL/min). ECOG performance status was 0 for 41 of 56 patients (73.2 %) and 1 for the other 15 (26.8 %). Nine (16.1 %) patients had previously received M-VAC chemotherapy. The mean observation period was 20.0 (range 3–62) months. There were 23 cases with recurrent disease after nephroureterectomy and 33 cases with unresectable tumors. Metastasis to at least one region outside the urothelial tract was observed for 51 (91.1 %) patients. The most frequent sites of metastasis were the lymph nodes (47 of 56 patients; 83.9 %); 38 (67.9 %) patients were deemed unfit for cisplatin-based chemotherapy.
Treatment administered
All 56 patients received GCarbo chemotherapy, with an average of 2.2 (range 1–4) treatment courses, and 17 patients received GCarboD chemotherapy with an average of 2.1 (range 1–5) treatment courses.
Adverse effects
The frequencies of grade 3 or higher AEs are shown in Table 2. Grade 3 or higher AEs occurred among 36 (64.3 %) patients who received GCarbo therapy. With regard to AEs with the GCarbo regimen, there were 31 (55.4 %) instances of G3/4 blood toxicity and 8 (14.3 %) instances of G3/4 urticaria; however, there were only 6 (10.7 %) instances of G3/4 gastrointestinal AEs. For the GCarboD regimen, there were 16 (94.1 %) instances of blood toxicity, 8 (47.1 %) instances of gastrointestinal AEs, and 1 (5.9 %) instance of urticaria. There was no occurrence of G3/4 renal toxicity after either regimen.
Tumor response
The response of the 56 patients who received GCarbo therapy was 50 %. Of these, 3 (5.4 %) achieved complete response (CR) and 25 (44.6 %) achieved partial response (PR). The average response duration was 9.7 months (range 2–53 months). Of the patients with M-VAC resistance, three achieved PR. The response of the 17 patients who received GCardoD therapy was only 11.8 %, with an average response duration of 31.5 months (Table 3).
Survival
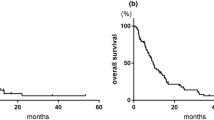
The outcome of GCarbo and GCarboD chemotherapy with regard to OS duration (time from the first treatment with the study drugs to death from any cause) was determined for the 56 enrolled patients. The median OS was 14.0 months, and 1, 3, and 5-year OS were 52.7, 34.1, and 20.4 %, respectively (Fig. 1a). Of these 56 patients, 28 (50.0 %) were responsive to GCarbo therapy and 28 (50.0 %) were non-responsive. OS for the GCarbo responders was significantly longer than for non-responders (28.0 vs 9.0 months, respectively; log-rank test, p = 0.0084; Fig. 1b). Median OS for patients treated with GCarboD was 12.0 months (Fig. 1c).
Discussion
According to the 2014 EAU guidelines, UTUCs are urothelial tumors; results for platinum-based chemotherapy are therefore expected to be similar to those for bladder cancer [8]. Cisplatin-based chemotherapy is, therefore, widely used for management of metastatic or locally advanced UTUC. However, the prognosis for patients with UTUC remains very poor and there is currently no strong evidence regarding the effectiveness of chemotherapy against advanced UTUC [3–7].
Because cisplatin is closely associated with nephrotoxicity, eligibility for cisplatin-based therapy is highly dependent on kidney function. Kaag et al. [9] reported a mean eGFR decrease of 24 % after nephroureterectomy for UTUC. If cut-off value of 60 mL/min/1.73 m2 was used, 49 % of patients were eligible for cisplatin before surgery; however, only 19 % remained eligible postoperatively.
The combination of gemcitabine and carboplatin (GCarbo) was developed for patients with metastatic UC of the bladder who were deemed unfit for cisplatin-based therapy, and the treatment was subsequently adopted for treatment of such patients [10–17]. Therefore, we inferred that this evidence indicates that GCarbo can be used for treatment of advanced UTUC.
Docetaxel is an active single agent for patients previously treated with UC. In a phase II study of docetaxel therapy for patients with advanced or metastatic UC who had relapsed or become refractory to no more than one previous cisplatin-containing treatment regimen, ORR was 13.3 % and median OS was 9 months [21]. Furthermore, combination GCarboD therapy has also been reported to be active and well tolerated by patients with advanced urothelial carcinoma [15, 22]. Tsuruta et al. reported use of GCarboD for advanced or metastatic UC patients who had relapsed or were considered unsuitable for cisplatin. ORR was 52.4 % and median OS was 13.1 months. [22] We therefore concluded that GCarboD was more active than docetaxel monotherapy.
Renal dysfunction is common among patients with advanced UTUC; thus, cisplatin is often unsuitable. In this study, we retrospectively evaluated the effectiveness and instances of AEs for a sequential chemotherapy regimen of GCarbo followed by GCarboD for advanced UTUC.
Several sequential types of chemotherapy for UTUC have been reported. Galsky et al. reported that they conducted a phase II trial of dose-dense doxorubicin plus gemcitabine then paclitaxel plus carboplatin for 25 patients with advanced urothelial carcinoma and impaired renal function. In their study, there were five complete responses and nine partial responses, so the overall response was 56 % and median survival was 15 months [23]. Boukovinas et al. reported use of sequential gemcitabine and cisplatin then docetaxel for treatment of 38 patients with advanced urothelial carcinoma. In their study, 5 patients had complete response (13.1 %) and 16 patients had partial response (42.1 %). Median overall survival was 13 months [24]. Artz et al. reported sequential chemotherapy with docetaxel and methotrexate then gemcitabine and cisplatin for 13 patients with metastatic urothelial cancer. In their study, overall response was 54 % and median OS was 13.6 months [25].
In our study, 3 (5.4 %) patients achieved CR and 25 (44.6 %) achieved PR by use of the GCarbo regimen, and the mean duration of the response was 9.7 months (range 2–53 months). PR was achieved for three (33.3 %) of nine M-VAC-resistant cases. GCarboD treatment was administered to 17 cases and yielded two (11.8 %) PRs; the mean duration of response was 31.5 months. Median survival was 14 months for GCarbo/GCarboD regimens, with 1, 3, and 5-year survival of 50.8, 34.1, and 20.4 %, respectively. Compared with previous studies, we believe that overall response and median survival in our study were acceptable.
Our study had several limitations. First, the patient cohort was relatively small. Second, the study was a retrospective, single-group study. Third, in this study there were 18 patients (32.1 %) deemed fit for cisplatin. It is believed that carboplatin-based chemotherapy does not compare with cisplatin-based chemotherapy with regard to survival time [26]. We conducted carboplatin rather than cisplatin-based chemotherapy because our patients were likely to be elderly and more seriously ill, because of metastatic disease, and, compared with cisplatin, it was easier to adjust the dose of carboplatin on the basis of renal function or general condition.
Although the patient cohort was relatively small and the study was preliminary, the results suggest that GCarbo/GCarboD sequential chemotherapy may be safe and effective for treatment of advanced UTUC. An acceptable response (50.0 %) against advanced UTUC was achieved by use of the GCarbo regimen, even among M-VAC-resistant cases. The median OS of 14 months may be acceptable compared with OS survival outcomes in previous studies. However, the effectiveness of GCarboD was limited for GCarbo non-responders.
References
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics. CA Cancer J Clin 59:225–249
Kaag MG, O’Malley RL, O’Malley P et al (2010) Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 58:581–587
Tanaka N, Kikuchi E, Kanao K et al (2013) Patient characteristics and outcomes in metastatic upper tract urothelial carcinoma after radical nephroureterectomy: the experience of Japanese multi-institutions. BJU Int 112:E28–E34
Audenet F, Yates DR, Cussenot O et al (2013) The role of chemotherapy in the treatment of urothelial cell carcinoma of the upper urinary tract (UUT-UCC). Urol Oncol 31:407–413
Hellenthal NJ, Shariat SF, Margulis V et al (2009) Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the upper tract urothelial carcinoma collaboration. J Urol 182:900–906
Youssef RF, Lotan Y, Sagalowsky AI et al (2013) Radical nephroureterectomy for pathologic T4 upper tract urothelial cancer: can oncologic outcomes be improved with multimodality therapy? Int Braz J Urol 39:614–621
Alva AS, Matin SF, Lerner SP et al (2012) Perioperative chemotherapy for upper tract urothelial cancer. Nat Rev Urol 9:266–273
Roupret M, Babjuk M, Comperat E et al (2013) European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 63:1059–1071
Kaag MG, O’Malley RL, O’Malley P et al (2010) Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 58:581–587
Bellmunt J, Ribas A, Eres N et al (1997) Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 80:1966–1972
Bamias A, Moulopoulos LA, Koutras A et al (2006) The combination of gemcitabine and carboplatin as first-line treatment in patients with advanced urothelial carcinoma. A Phase II study of the Hellenic Cooperative Oncology Group. Cancer 106:297–303
De Santis M, Bellmunt J, Mead G et al (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: eORTC study 30986. J Clin Oncol 30:191–199
Park JH, Lee SW, Kim HS et al (2013) Combination of gemcitabine and carboplatin as first line treatment in elderly patients or those unfit for cisplatin-based chemotherapy with advanced transitional cell carcinoma of the urinary tract. Cancer Chemother Pharmacol 71:1033–1039
Koie T, Ohyama C, Hashimoto Y et al (2013) Efficacies and safety of neoadjuvant gemcitabine plus carboplatin followed by immediate cystectomy in patients with muscle-invasive bladder cancer, including those unfit for cisplatin: a prospective single-arm study. Int J Clin Oncol 18:724–730
Hoshi S, Ohyama C, Ono K et al (2004) Gemcitabine plus carboplatin; and gemcitabine, docetaxel, and carboplatin combined chemotherapy regimens in patients with metastatic urothelial carcinoma previously treated with a platinum-based regimen: preliminary report. Int J Clin Oncol 9:125–129
Xu N, Zhang XC, Xiong JP et al (2007) A phase II trial of gemcitabine plus carboplatin in advanced transitional cell carcinoma of the urothelium. BMC Cancer 7:98
Hoschke B, May M, Seehafer M et al (2004) Our experience with 23 consecutive patients on gemcitabine/carboplatin chemotherapy for treatment of metastasized transitional cell carcinoma of the urothelium. Int J Urol 11:461–466
Yoneyama T, Tobisawa Y, Yoneyama T et al (2014) Carboplatin-based combination chemotherapy for elderly patients with advanced bladder cancer. Int J Clin Oncol (in press)
Galsky MD, Hahn NM, Rosenberg J et al (2011) Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 29:2432–2438
Calvert AH, Newell DR, Gumbrell LA et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
McCaffrey JA, Hilton S, Mazumdar M et al (1997) Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 15:1853–1857
Tsuruta H, Inoue T, Narita S et al (2011) Combination therapy consisting of gemcitabine, carboplatin, and docetaxel as an active treatment for advanced urothelial carcinoma. Int J Clin Oncol 16:533–538
Galsky MD, Iasonos A, Mironov S et al (2007) Phase II trial of dose-dense doxorubicin plus gemcitabine followed by paclitaxel plus carboplatin in patients with advanced urothelial carcinoma and impaired renal function. Cancer 109:549–555
Boukovinas I, Androulakis N, Vamvakas L et al (2006) Sequential gemcitabine and cisplatin followed by docetaxel as first-line treatment of advanced urothelial carcinoma: a multicenter phase II study of the Hellenic Oncology Research Group. Ann Oncol 17:1687–1692
Artz A, Stadler WM, Vogelzang NJ et al (2005) A phase II trial of sequential chemotherapy with docetaxel and methotrexate followed by gemcitabine and cisplatin for metastatic urothelial cancer. Am J Clin Oncol 28:109–113
Dogliotti L, Carteni G, Siena S et al (2007) Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol 52:134–141
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yoneyama, T., Imai, A., Hatakeyama, S. et al. Sequential chemotherapy using gemcitabine + carboplatin followed by gemcitabine + carboplatin + docetaxel for advanced upper-tract urothelial cancer. Int J Clin Oncol 20, 1179–1184 (2015). https://doi.org/10.1007/s10147-015-0846-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0846-z