Abstract
Background
The aim of this study was to evaluate the efficacy and toxicities of second-line chemotherapy regimens with docetaxel and gemcitabine (GD), or paclitaxel and gemcitabine (GP) for advanced or metastatic urothelial carcinoma (UC) that did not respond to first-line platinum-based chemotherapy.
Methods
From 2002 to 2017, 78 patients with metastatic UCs that progressed after platinum-based chemotherapy were treated with either GD (n = 41) or GP (n = 37). We compared these two different regimens by analyzing their efficacy and toxicities in a retrospective manner.
Results
Of the 78 patients enrolled in this study, it was possible to determine treatment efficacy in 70; the proportion of patients with objective response and disease control were 8.6 (9/70) and 54.3% (38/70), respectively. The median progression-free survival and overall survival in the total population (GP and GD) were 3.5 (95% CI 0.6–53.3) and 9.6 months (95% CI 1.2–53.3), respectively. There was no significant difference between the two regimens (GD or GP) regarding survival outcomes. Treatment-related adverse events were mostly manageable, but one patient died as a result of febrile neutropenia. The presence of liver metastasis and anemia (Hb < 10.0 g/dl) was prognostic factors for worse survival.
Conclusions
Combination chemotherapy with either GP or GD was a favorable and well-tolerated second-line treatment regimen for patients with advanced or metastatic UC following the failure of a platinum-based regimen. Further study using a large prospective cohort is needed to identify patients who will benefit from second-line combination therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin-based chemotherapy is regarded as the gold standard for treatment of patients with advanced or metastatic urothelial carcinoma (UC) [1]. The regimen of methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) has been widely used as first-line chemotherapy [2]. More recently, combination therapy with gemcitabine and cisplatin (GC) has become standard first-line chemotherapy [3, 4]. However, there is no standard second-line treatment for patients who experience cancer progression after platinum-based chemotherapy until the recent approval of pembrolizumab as a second-line treatment drug for metastatic urothelial carcinoma [5, 6]. Although many regimens that include docetaxel, paclitaxel, gemcitabine, and vinflunine have been used in a second-line setting, as yet no randomized phase III trials with adequate comparator arms have been conducted to assess the true value and overall survival (OS) benefits of second-line combination therapy [7,8,9,10].
Previous studies showed that treatment with a single agent, such as docetaxel or paclitaxel, yielded modest effects, but combination therapy of paclitaxel or docetaxel with gemcitabine (GP or GD) showed better results [8, 11,12,13]. Progression-free survival (PFS) and OS in a randomized phase III trial for GP-treated patients were reported as 3.1 and 8.0 months, respectively [8]. In another study, Naiki et al. [14] reported that PFS and OS in GD-treated patients were 4.4 and 10.8 months, respectively. In a KEYNOTE trial, treatment with pembrolizumab prolonged the OS of bladder cancer patients by about 3 months over single-agent chemotherapy using paclitaxel, docetaxel, or vinflunine [6]. This is probably because single-agent chemotherapy is recommended in National Comprehensive Cancer Network and European Association of Urology guidelines in a second-line setting.
So far, there have been no trials to compare the efficacy between GP and GD as second-line treatments. In our hospital, we have used either GD or GP to treat patients who experience tumor progression after platinum-based combination chemotherapy since 2002. In the present study, we retrospectively evaluated the efficacy and toxicity of GD and GP regimens as a second-line chemotherapy for advanced or metastatic UC patients. Furthermore, we analyzed the factors which affected the survival time and treatment-related adverse events of the patients who received GD or GP combination therapy in a retrospective manner.
Patients and methods
Patients eligible for the present study had histologically confirmed metastatic UC of the urinary bladder or upper urinary tract. Patients who experienced tumor recurrence or progression after cisplatin-based chemotherapy, with or without radical surgery (cystectomy or nephroureterectomy), were included. Patients who developed recurrence or progressed within 12 months after the receipt of platinum-based neoadjuvant or adjuvant chemotherapy were also included. Patients who had received prior second-line chemotherapy were excluded from the present study. Between September 2002 and December 2017, 189 patients with advanced or metastatic UC had received the first line, platinum-based chemotherapy. Seventy-eight out of 189 (41.2%) patients were eligible for treatment with the second line GD/GP therapies. Further inclusion criteria were ECOG performance status ≤ 2, life expectancy ≥ 12 weeks, white blood cell count ≥ 3,000/µl, and adequate hepatic function. Paclitaxel and docetaxel were used in Osaka City University hospital under the approval of institutional review board (no. 139 and 371, respectively) until the approval by Ministry of Health, Labor and Welfare in 2014 in Japan. Written informed consent was obtained from all patients before they received this therapy. This study was approved by the institutional review board at Osaka City University Hospital in accordance of with the Declaration of Helsinki (No. 3959).
Treatment schedule and evaluation
GD-treated patients received 70 mg/m2 of docetaxel on day 1 and 1000 mg/m2 of gemcitabine on days 1 and 8 of each 21-day cycle. GP-treated patients received 200 mg/m2 of paclitaxel on day 1 and 1000 mg/m2 of gemcitabine on days 1, 8, and 15 of each 28-day cycle. Between September 2002 and January 2010, GP was selected as a standard regimen for second-line treatment in our hospital, but GD became the standard after 2010, because we observed relatively high incidences of neuro-toxic adverse events in GP-treated patients. Radiographic examinations (computed tomography, magnetic resonance imaging, or bone scan) were performed every 2–3 cycles of treatment, and tumor response and disease progression were measured according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver 1.0, since many cases were evaluated before 2009. Using medical records, OS and PFS were measured until death or progression, respectively. Complete response (CR) was defined as disappearance of all target lesions, and partial response (PR) was defined as a decrease of at least 30% in the sum of the diameters of the target lesions. Progressive disease (PD) was defined as an increase of at least 20% in the sum of the diameters of the target lesions, or the appearance of one or more new lesions. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Adverse events were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) ver 4.0. Eight out of 78 patients were excluded from this analysis, since only non-measurable lesions, such as bone or lymph node metastasis with ≥ 10 to < 15 mm short axis, existed in these patients.
Risk factors
There are no universal risk factors for patients with metastatic urothelial cancer; however, Eastern Cooperative Oncology Group performance status (PS) more than 0, hemoglobin level less than 10 g/dL, the presence of liver metastasis, and shorter time from prior chemotherapy were included as risk factors in the previous reports by Bellmunt et al. [6, 16] and Sonpavde et al. [15]. We classified the patients according to the number of risk factors as 0, 1 or 2, or more.
Statistical analysis
The OS and PFS curves were analyzed using the Kaplan–Meier method, and the significance of differences between curves was tested using the log-rank test. To assess the prognostic factors which affect survival outcomes, we also conducted analyses on the baseline characteristics of the patients. A two-sided p value of < 0.05 was considered to be statistically significant. Statistical analysis was carried out using GraphPad Prism ver 7.
Results
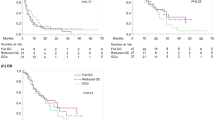
Between September 2002 and December 2017, a total of 78 patients received either GD or GP treatment as second-line chemotherapy for advanced or metastatic UC. Characteristics for all patients are shown in Table 1 and correspond to patient status at the start of GD or GP treatment. Of the 78 patients enrolled in this study, 70 could be evaluated to determine treatment efficacy. The median number of cycles of treatment was 2 (range 1–13). Patient responses to treatment are listed in Table 2. One patient (1.4%) had CR, 5 patients (7.1%) had PR, and objective response rate (ORR) was 8.6% (6/70). The median PFS and OS in the total population were 3.5 (95% CI 0.6–53.3) and 9.6 months (95% CI 1.2–53.3), respectively (Fig. 1). No significant difference was observed in the number of risk factors between GP and GD treatment groups (Supplemental Table 1). The median OS of GD and GP was 9.4 (95% CI 0.6–37.0) and 9.8 (95% CI 1.2–53.3) months, respectively. There was no significant difference between the two regimens regarding response parameters (CR, PR, SD or PD) and survival outcomes.
Prognostic factors
Analyses on the baseline characteristics of the patients were performed to assess the prognostic factors which affect the survival outcomes. Results are shown in Table 3.
Site of metastases
OS in patients with hepatic or visceral metastasis [5.7 (95% CI, 1.6–16.2) vs. 2.3 (95% CI, 0.6–40.2)] was significantly shorter than that in those without hepatic or visceral metastasis [10.3 (95% CI, 1.6–53.3) vs. 15.0 (95% CI, 2.6–53.3)].
Baseline characteristics
There was no significant difference between the primary sites (bladder or upper urinary tract, p = 0.4186) or the level of Eastern Cooperative Oncology Group Performance Status (ECOG-PS) (0 vs. 1, p = 0.1362). OS was not affected by either the neutrophil to lymphocyte ratio (NLR) or time from completion or discontinuation of the previous chemotherapy regimen (p = 0.9476 and 0.3556, respectively). However, those with anemia (Hb < 10) had significantly shorter OS than those who did not [4.6 (95% CI 0.6–15.6) vs. 12.0 (95% CI 1.6–53.3) months, respectively; p < 0.0001]. Risk factors included ECOG performance status above 0, the presence of liver metastasis, Hb < 10 g/dl, and a time, since the completion or discontinuation of the previous therapy of less than 3 months [15, 16]. A Kaplan–Meier curve of the OS in patients according to the risk factors is shown in Fig. 2. The OS in patients with two or more risk factors was significantly worse than that in those with one or no risk factors (Table 3).
Treatment-related adverse events
Treatment-related toxicities are listed in Table 4. Toxicities were mainly hematological, and the most frequent grade 3–4 toxicities were anemia [n = 22 (28.2%)], thrombocytopenia [n = 29 (37.2%)], and neutropenia [n = 55 (70.5%)], with 3 (3.8%) episodes of febrile neutropenia. A treatment-related death occurred in one patient who received GP therapy. Non-hematological toxicities were mild, and included neuropathy [n = 14 (17.9%)] and liver dysfunction [n = 26 (33.3%)]. Neutropenia was more frequently observed in GD-treated patients (p = 0.0407), but the incidence of neuropathy was significantly higher in GP-treated patients (p = 0.0023).
Discussion
In the present study, we reported the outcomes of combination chemotherapy using GP or GD as a second-line treatment in patients with metastatic UC. Both regimens achieved similar OS with tolerable toxicities observed in the majority of patients.
Several studies have shown treatment effects of GP combination therapy in a second-line setting for the treatment of advanced or metastatic UC after platinum-based chemotherapy. However, the responses to treatment have been variable [17,18,19,20,21]. There are a number of different factors that may explain these differences. One possible explanation is differences in the doses of gemcitabine and paclitaxel. In a previous study by Fechner et al., the dose of paclitaxel and gemcitabine was 120 and 1250 mg/m2, respectively, and the median OS was 9 months, which is relatively shorter than in other studies. In the present study, we used 200 mg/m2 of paclitaxel and 1000 mg/m2 of gemcitabine. Another explanation is that the proportion of visceral metastasis depended on the reports.
Docetaxel is an active single agent for treating UC patients who were previously treated with first-line chemotherapy. In a phase 2 study of docetaxel therapy for patients with metastatic UC who failed to respond to or relapsed after one prior cisplatin-containing regimen, the median OS was 9 months (95% CI 6–12) [22]. Kim et al. also reported the results from a phase 2 study in which weekly docetaxel as second-line chemotherapy was administered to patients with metastatic UC. Docetaxel (30 mg/m2) was administered on days 1 and 8 every 21 days, and the median OS was 8.3 months (95% CI 5.9–10.6) [23]. Recently, there have been several reports on docetaxel-based chemotherapy regimens in a second-line setting [7, 14, 24]. Kakutani et al. reported the outcome of docetaxel, ifosfamide and cisplatin chemotherapy with a median OS of 8.5 months (95% CI 6.5–18.8 months) [25]. Furthermore, combination of ramucirumab, a human IgG1 VEGFR-2 antagonist, with docetaxel treatment demonstrated superior PFS period over chemotherapy in patients with platinum-refractory metastatic UC [26]. Although ramucirumab has not yet been approved for the treatment of metastatic UC in Japan, this positive result is likely to permit its approval. Therefore, the combination of ramucirumab and docetaxel could become an important option for second-line therapy, or even third-line therapy after pembrolizumab [27].
The roles of doublet or triplet combinations remain unproven, given the activity of various single agents in the salvage setting. However, combination chemotherapy exhibited improved OS compared with patients enrolled in trials of single-agent chemotherapy as salvage therapy which is reported to be associated with greater toxicity [12]. In this study, incidences of hematological adverse events were higher than those in the previous reports. However, neutropenia was easily managed with granulocyte growth factors, and none of the patients needed routine granulocyte-colony stimulating factor. No patients had severe neuropathy.
In this study, liver and visceral metastasis were experienced in 19.2 and 57.5% of patients, respectively, which is relatively higher than in the previous reports [14, 28]. In the present study, the ORR (8.6%) and median PFS (3.5 months) were modest, but combination chemotherapy yielded favorable outcomes when considering the relatively longer OS (9.6 months) and manageable adverse events.
The present study had several limitations. First, this study was retrospectively designed to compare the two different regimens. Secondly, the cohort was relatively small and it was a single-group study. However, despite the small cohort size, this study was able to show favorable outcomes of second-line chemotherapy treatment using either GD or GP.
In a KEYNOTE-045 trial, pembrolizumab was associated with significantly longer overall survival as second-line therapy for platinum-refractory advanced UC, and has been approved for the treatment of patients with UC who failed to respond to platinum-based chemotherapy [6]. In this trial, pembrolizumab was compared with single-agent chemotherapy with paclitaxel, docetaxel, or vinflunine, and with regard to OS, treatment with pembrolizumab yielded almost 3 months elongation over single-agent chemotherapy (10.3 vs. 7.4 months). Although there have been no randomized trials to compare the survival benefit between combination chemotherapy and checkpoint inhibitors for metastatic UC patients, pembrolizumab has become the gold standard as a second-line therapy for metastatic UC. However, combination chemotherapy could become a third-line therapy after the failure of checkpoint inhibitors.
Conclusions
Both GD and GP were well-tolerated and effective regimens for patients with advanced or metastatic UC that progressed after platinum-based chemotherapy.
References
Logothetis CJ, Dexeus FH, Finn L et al. (1990) A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol 8(6):1050–1055. https://doi.org/10.1200/JCO.1990.8.6.1050
Saxman SB, Propert KJ, Einhorn LH et al. (1997) Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 15(7):2564–2569. https://doi.org/10.1200/JCO.1997.15.7.2564
von der Maase H, Hansen SW, Roberts JT et al. (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18(17):3068–3077. https://doi.org/10.1200/JCO.2000.18.17.3068
von der Maase H, Sengelov L, Roberts JT et al. (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23(21):4602–4608. https://doi.org/10.1200/JCO.2005.07.757
Ortmann CA, Mazhar D (2013) Second-line systemic therapy for metastatic urothelial carcinoma of the bladder. Future Oncol 9(11):1637–1651. https://doi.org/10.2217/fon.13.139
Bellmunt J, de Wit R, Vaughn DJ et al. (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. https://doi.org/10.1056/NEJMoa1613683
Dreicer R, Manola J, Schneider DJ et al. (2003) Phase II trial of gemcitabine and docetaxel in patients with advanced carcinoma of the urothelium: a trial of the Eastern Cooperative Oncology Group. Cancer 97(11):2743–2747. https://doi.org/10.1002/cncr.11413
Albers P, Park SI, Niegisch G et al. (2011) Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99]. Ann Oncol 22(2):288–294. https://doi.org/10.1093/annonc/mdq398
Kobayashi K, Matsuyama H, Shimizu K et al. (2016) Clinical significance of a second-line chemotherapy regimen with paclitaxel, ifosfamide and nedaplatin for metastatic urothelial carcinoma after failure of cisplatin-based chemotherapy. Jpn J Clin Oncol 46(8):775–780. https://doi.org/10.1093/jjco/hyw071
Bellmunt J, Fougeray R, Rosenberg JE et al. (2013) Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol 24(6):1466–1472. https://doi.org/10.1093/annonc/mdt007
Sideris S, Aoun F, Zanaty M et al. (2016) Efficacy of weekly paclitaxel treatment as a single agent chemotherapy following first-line cisplatin treatment in urothelial bladder cancer. Mol Clin Oncol 4(6):1063–1067. https://doi.org/10.3892/mco.2016.821
Sonpavde G, Pond GR, Choueiri TK et al. (2016) Single-agent taxane versus taxane-containing combination chemotherapy as salvage therapy for advanced urothelial carcinoma. Eur Urol 69(4):634–641. https://doi.org/10.1016/j.eururo.2015.07.042
Raggi D, Miceli R, Sonpavde G et al. (2016) Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol 27(1):49–61. https://doi.org/10.1093/annonc/mdv509
Naiki T, Kawai N, Hashimoto Y et al. (2014) Gemcitabine and docetaxel, an effective second-line chemotherapy for lung metastasis of urothelial carcinoma. Int J Clin Oncol 19(3):516–522. https://doi.org/10.1007/s10147-013-0574-1
Sonpavde G, Pond GR, Fougeray R et al. (2013) Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol 63(4):717–723. https://doi.org/10.1016/j.eururo.2012.11.042
Bellmunt J, Choueiri TK, Fougeray R et al. (2010) Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28(11):1850–1855. https://doi.org/10.1200/JCO.2009.25.4599
Sternberg CN, Calabro F, Pizzocaro G et al. (2001) Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer 92(12):2993–2998
Meluch AA, Greco FA, Burris HA et al. (2001) Paclitaxel and gemcitabine chemotherapy for advanced transitional-cell carcinoma of the urothelial tract: a phase II trial of the Minnie pearl cancer research network. J Clin Oncol 19(12):3018–3024. https://doi.org/10.1200/JCO.2001.19.12.3018
Kaufman DS, Carducci MA, Kuzel TM et al. (2004) A multi-institutional phase II trial of gemcitabine plus paclitaxel in patients with locally advanced or metastatic urothelial cancer. Urol Oncol 22(5):393–397. https://doi.org/10.1016/j.urolonc.2004.01.002
Li J, Juliar B, Yiannoutsos C et al. (2005) Weekly paclitaxel and gemcitabine in advanced transitional-cell carcinoma of the urothelium: a phase II Hoosier Oncology Group study. J Clin Oncol 23(6):1185–1191. https://doi.org/10.1200/JCO.2005.05.089
Fechner G, Siener R, Reimann M et al. (2006) Randomised phase II trial of gemcitabine and paclitaxel second-line chemotherapy in patients with transitional cell carcinoma (AUO Trial AB 20/99). Int J Clin Pract 60(1):27–31. https://doi.org/10.1111/j.1742-1241.2005.00663.x
McCaffrey JA, Hilton S, Mazumdar M et al. (1997) Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 15(5):1853–1857. https://doi.org/10.1200/JCO.1997.15.5.1853
Kim YS, Lee SI, Park SH et al. (2016) A phase II study of weekly docetaxel as second-line chemotherapy in patients with metastatic urothelial carcinoma. Clin Genitourin Cancer 14(1):76–81. https://doi.org/10.1016/j.clgc.2015.09.008
Gitlitz BJ, Baker C, Chapman Y et al. (2003) A phase II study of gemcitabine and docetaxel therapy in patients with advanced urothelial carcinoma. Cancer 98(9):1863–1869. https://doi.org/10.1002/cncr.11726
Kakutani S, Fukuhara H, Taguchi S et al. (2015) Combination of docetaxel, ifosfamide and cisplatin (DIP) as a potential salvage chemotherapy for metastatic urothelial carcinoma. Jpn J Clin Oncol 45(3):281–285. https://doi.org/10.1093/jjco/hyu201
Petrylak DP, de Wit R, Chi KN et al. (2017) Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 390(10109):2266–2277. https://doi.org/10.1016/S0140-6736(17)32365-6
Yuasa T, Urakami S, Yonese J (2018) Recent advances in medical therapy for metastatic urothelial cancer. Int J Clin Oncol. https://doi.org/10.1007/s10147-018-1260-0
Suyama T, Ueda T, Fukasawa S et al. (2009) Combination of gemcitabine and paclitaxel as second-line chemotherapy for advanced urothelial carcinoma. Jpn J Clin Oncol 39(4):244–250. https://doi.org/10.1093/jjco/hyp003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
Conflict of interest
All authors declared that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takeyama, Y., Kato, M., Nishihara, C. et al. Comparison of efficacy and toxicity of second-line combination chemotherapy regimens in patients with advanced urothelial carcinoma. Int J Clin Oncol 23, 944–950 (2018). https://doi.org/10.1007/s10147-018-1288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1288-1