Abstract
Widespread generalist predators may affect declining keystone prey populations. However, this phenomenon is not well understood. In this paper, we assessed whether the abundance and population growth of European rabbits Oryctolagus cuniculus, a keystone prey species in Mediterranean Iberia, was related to the abundance and diet of red foxes, Vulpes vulpes, a widespread generalist predator. In a locality in central Spain, where rabbit population abundance declined, we estimated rabbit abundance during almost 3 years and determined fox abundance and diet during two concurrent years. We calculated a fox predation index (percentage of consumed rabbit biomass × fox abundance) to assess the importance of rabbits to foxes. We employed a multi–model approach to explain rabbit abundance and population growth. Foxes consumed between 60 and 99 % rabbit biomass in their diets, and this was independent of rabbit abundance. Periods of higher fox predation index coincided with lower rabbit density and vice versa. Two models best explained rabbit abundance and four rabbit population growth. They included the fox predation index and its interaction with rabbit abundance during the previous month. Altogether, fox predation, intraspecific density dependence, and their interaction partly explained rabbit population dynamics. We conclude that in order to propel the recovery of the rabbit in Iberia, it is essential to better understand the role of these factors in driving the abundance of the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European rabbit (Oryctolagus cuniculus L.), a lagomorph native to the Iberian Peninsula, is the most preferred prey of foxes (Vulpes vulpes L.) in central–southern Iberia (Díaz-Ruiz et al. 2013). Rabbits are the main prey item for foxes and other predators because of its considerable size and usual high abundance (Delibes and Hiraldo 1981; Ferrer and Negro 2004; Delibes-Mateos et al. 2008a). Rabbits in this part of the world are thus considered a keystone species i.e., a species that maintains the structure and integrity of the community, and is therefore a species of high conservation concern (Delibes-Mateos et al. 2007; Wagner 2012). Rabbits create open areas, promote soil fertility and increase plant growth and diversity (Gómez-Sal et al. 1999; Willot et al. 2000). In addition, rabbit latrines provide food for many invertebrates (Verdú and Galante 2004) and rabbit burrows provide nest sites and shelter for vertebrates and invertebrates (Gálvez et al. 2009). The rabbit is also of high economic importance in Iberia since it is one of the most hunted small game species in Spain and Portugal (Delibes-Mateos et al. 2008a).
In recent decades, most Iberian rabbit populations have decreased drastically due to the impact of viral diseases such as myxomatosis and rabbit hemorrhagic disease (hereafter RHD), alongside with habitat loss and overhunting (Delibes-Mateos et al. 2009). Because of these declines, the rabbit is now classified as a “Vulnerable” species in the Spanish Red List (Villafuerte and Delibes-Mateos 2007). Understanding which factors may hinder the recovery of the rabbit in the Iberian Peninsula, is crucial to propose mitigating actions (Delibes-Mateos et al. 2009, 2014a, b). In particular, assessing the role of predators on rabbit populations is fundamental given that predation is one of the main processes structuring animal communities (Sinclair and Krebs 2002).
Predation effects on a prey species are often difficult to quantify (Valkama et al. 2005), and predation consequences on ecosystem functioning are often not well understood. However, baseline information on predator abundance and prey consumption can be used to quantify predation intensity (Korpimäki et al. 1991), and the relationship between predation intensity, prey abundance and its population growth employed to estimate the effects of predation on a prey (Sinclair et al. 1998, 2006). Here, we assessed whether predation by the main predator of the rabbit in Iberia, the red fox, was related to the abundance and population growth of the rabbit populations in a locality in central Spain.
The red fox is considered to be one of the most widespread generalist vertebrate predators in the world (Macdonald and Reynolds 2004). Red foxes are known to limit prey abundance, especially when prey populations are already declining or at low densities (Saunders et al. 2010). Studies demonstrating fox effects on rabbit abundance were mostly from Australia where rabbits were introduced and are considered pests (Pech et al. 1992; Banks 2000). In Iberia, however, foxes are often assumed to affect the abundance and population growth of rabbit populations (Villafuerte et al. 1996; Calzada 2000; Delibes-Mateos et al. 2008b), though there is no empirical data to adequately demonstrate this. In central Spain, most small game hunters argue that foxes negatively impact rabbits and partridges; hence foxes are often controlled in hunting estates (Delibes-Mateos et al. 2013). But, the effects of fox predation on Iberian rabbit population dynamics are not well understood (Norbury and Jones 2015), with no evidence that rabbit populations increase with fox control (Díaz-Ruiz and Ferreras 2013).
In this paper, we first examine rabbit consumption by foxes and estimate fox abundance changes in a locality where an overall decline in rabbit abundance had been recorded for a three–year period. We also employed a smaller concurrent two–year dataset, to analyse and discuss the potential role of fox predation on the rabbit population. Finally, we discuss how our results can be utilised to better understand predator–prey relationships in a context of biodiversity conservation.
Materials and methods
Study area
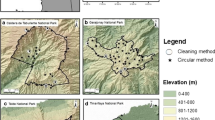
We carried out our fieldwork in a property located in the Albacete province (central Spain, Fig. 1). The climate is Mediterranean, characterized by mild wet winters and warm dry summers. The landscape is typical of most rural Mediterranean areas (Blondel and Aronson 1999), occupied by Mediterranean scrubland (e.g., with rosemary Rosmarinus officinalis L., kermes oaks Quercus coccifera L., thyme Thymus sp.), pastures (mainly grasses), ‘dehesas’ (savanna–like formations combining pasture with intermittent cereal cultivation in park–like woodlands of mainly oaks Quercus ilex ssp. rotundifolia Lam., Blondel and Aronson 1999), and croplands (mainly cereals and vineyards Vitis vinifera L.). Besides agriculture, other sources of income included horse breeding and hunting of small game, such as rabbits. During the study, a gamekeeper, who was responsible for regulating hunting and controlling predators, was employed in the property during the study except for the last two months. Predator control is generally undertaken in a non–systematic way in small game hunting estates (Delibes-Mateos et al. 2013) and unfortunately, for our study site no data on the intensity of fox control was available.
Location of the study area in central Spain. The transect for spotlight fox counts is depicted as a black thick line within this study area. The location of the sampling grid for cleared–plot rabbit pellet counts is shown as a dark grey point. The circle in light grey colour shows the area where red fox scats were collected during transects on foot
Because our study locality contained gentle slopes and ecotones between Mediterranean scrubland areas and pastures or cropland, it was highly favorable for rabbits and foxes (Lombardi et al. 2003; Macdonald and Reynolds 2004). Highest rabbit densities were usually found along the ecotones between open scrub and cereal crops; areas of softer soil of greater ease for building rabbit warrens. Rabbit densities in the study area ranged from low to high densities for Iberia (Fernandez-de-Simon et al. 2011a; Delibes-Mateos et al. 2014b). Fox abundance was considered as very low to intermediate for this region (see Results; Sobrino et al. 2009; Fernandez-de-Simon 2013).
Rabbit and fox abundance estimation
To estimate rabbit abundance we conducted cleared–plot pellet counts during a period of three years (summer 2006–summer 2009). Cleared–plot pellet counts is a cost–efficient method that is widely used in rabbit studies; it provides one of the most reliable estimates of rabbit abundance (Fernandez-de-Simon et al. 2011a, b; Ferreira et al. 2014). Within a 40 × 70 m grid, we set 40 circular (0.5 m2) plots distributed regularly in the area with the highest rabbit abundance that was accessible to foxes living in the surroundings (see below, Fig. 1). Every month, we obtained a pellet count index corrected for pellet persistence (hereafter N, pellets m−2 day−1; see Fernandez-de-Simon et al. 2011a, b). We also calculated the monthly rabbit population growth by using N from consecutive months (Sinclair et al. 2006) as follows:
where T is the number of days between visits and 30.42 is the average length of a calendar month in days.
To estimate fox abundance, we conducted spotlight counts along a 15–km–transect driven at night, starting at least one hour after sunset. Surveys were undertaken for three consecutive nights, unless prevented by meteorological conditions or logistic constraints. Our fox transect was as close as 300 m from the rabbit abundance sampling grid (Fig. 1) to allow us to detect changes in fox abundance that may prey upon our monitored rabbit population. To account for seasonal changes in fox abundance (Reynolds and Tapper 1995), we conducted spotlight count surveys twice a year: in winter–spring (March–May), and in summer (July) during 2007–2009. We used the mean number of individual red foxes seen per km and night in each period as a red fox abundance index (hereafter RF, Pech et al. 1992; Sobrino et al. 2009).
Fox diet assessment
We examined changes in fox diet during a two–year period (March 2007–June 2009) from scats collected along transects (2.4–9.0 km long) walked every month (Fig. 1). Transects, though not standardised, traversed land features (e.g., tracks, paved roads, water streams, fences, rabbit warrens, etc.; Webbon et al. 2004) where foxes regularly deposited scats during territory marking. Fox scats were identified by their scent, size, shape, thickness and by the presence of fox hairs, which appear in scats because of grooming (Delibes-Mateos et al. 2008c and references therein). We analyzed a total of 342 fox scats. In April 2007, December 2007 and March 2009 no scats were collected due to logistic constraints. In our analyses we excluded May 2009, since fewer than 5 scats were available for that month. For the remaining months, at least 5 scats were available for analysis (Fig. S1 in Electronic Supplementary Material (ESM)); this sample size was considered adequate since only a small number of food items were consumed (mainly rabbits, see below, Table 1).
Scats were dried at 60 °C for a period of 48 h and then weighted to the nearest 0.001 g using a digital scale. The scats were then soaked overnight in water, after which we teased them apart over a 0.5 mm sieve (Reynolds and Aebischer 1991). We classified remains found in each scat into 10 food classes: (a) rabbit, (b) hare, (c) undetermined lagomorph, (d) small mammals, (e) carrion (wild ungulates, livestock and mammalian carnivores), (f) birds and eggs, (g) reptiles and amphibians, (h) arthropods, (i) fruits and seeds, and (j) others (rare items or impossible to determine). The dry weight of the remains of each food class in each scat was estimated as the product of the percent volume of each prey item and the scat’s dry weight (Delibes-Mateos et al. 2008c). We calculated the consumed biomass of each item by multiplying the dry weight of the item in the scats by the coefficient of digestibility specific to the food class. The latter is the ratio of the fresh ingested food/dry weight of the scats produced (Reynolds and Aebischer 1991), obtained from 10–day food trials using two captive red foxes (one male, one female) at the IREC–UCLM experimental facility (Table 1; Fernandez-de-Simon 2013). For arthropods, and reptiles/amphibians, we used the coefficients of digestibility reported by Reynolds and Tapper (1995) and Sarmento (1996), respectively. To assess the contribution made by rabbit to fox diet during each month, we estimated the percentage of rabbit biomass consumed. We also calculated a monthly red fox predation index (hereafter RFI) as the product of monthly rabbit consumption (percentage of consumed rabbit biomass) and the fox abundance index (RF, see also Korpimäki et al. 1991). We derived a winter RF for the period December–May, and summer RF for the period June–November.
Data analyses
To understand the feeding ecology of the fox in our study locality, we first investigated the relationship between the percentage of rabbit biomass consumed and rabbit abundance. We then assessed the relationship between the predation index, RFI t (see above) and rabbit abundance index (N t ) and rabbit population growth (NPG t ). Given the time series for rabbit abundance spanned from August 2006 to June 2009, but data of RF (and thus RFI) was collected between April 2007 and May 2009, our analyses only refer to the overlap period of two years. We developed general linear models for (a) N t and (b) NPG t as dependent variables. We considered (i) RFI t , (ii) month, (iii) year, and (iv) the interaction N t–1 × RFI t as independent variables. All models included N t–1 to correct potential temporal autocorrelation. We also run a model with N t–1 only to test whether previous abundance of rabbits alone could also explain rabbit abundance and population growth. In addition, we compared the performance of these models with a null model (intercept only) as an indicator of overall model performance (Burnham and Anderson 2002). In total, 17 models were generated with all possible variable combinations. Models were fitted with a normal error distribution and an identity link function. For model selection we inspected mainly the AICc (Akaike Information Criterion corrected for small sample size), but also D 2 (explained deviance). Given the large number of models tested, we only show those models within ∆ i < 2 (∆ i = difference in AICc with the most parsimonious model). We tested normality, linearity and homocedasticity of model residuals and variables used (Zuur et al. 2007). To meet model assumptions, we transformed, when necessary, either the dependent and/or independent variables with the decimal logarithm transformation (see Zuur et al. 2007). We also tested temporal autocorrelation by a linear model of the consecutive residuals against each other (Breusch–Godfrey test for autocorrelation, Godfrey 1978; Breusch 1979). However, we did not find evidence of temporal autocorrelation. Parameters and probability values of individual variables in the best models are shown. We were more interested in inference than in hypothesis testing so we preferred not to use a critical probability threshold. In this way, models with non–significant variables (P > 0.05) are taken into account and included for inference purposes (see also Burnham and Anderson 2002). We performed these analyses using R version 3.0.1 (R Development Core Team 2013).
Results
Rabbit abundance decreased along three years with maximum in September 2006 (N = 3.53 pellets m−2 day−1) and minimum in March and April 2009 (N = 0.22 pellets m−2 day−1, Fig. 2).
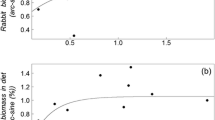
Rabbits were the main prey item consumed by foxes in our study area (Table 1). Monthly rabbit consumption by foxes ranged between 60 and 99 % in biomass terms, and varied independently of rabbit abundance (Fig. 3).
Fox abundance also varied across time, with a minimum in winter–spring 2008, with no foxes observed, to a maximum in winter–spring 2009 (RF = 0.13 foxes km−1, Fig. 4). Predation Index was lowest during winter–spring 2008 (RFI = 0.00 percentage of rabbit biomass × foxes km−1), and highest in winter–spring 2009 (RFI = 11.53 percentage of rabbit biomass × foxes km−1). Predation index was inversely related to rabbit abundance for the overlap period (Fig. 2, see below).
Two models explaining rabbit abundance (log N t ) had ∆ i < 2; these included RFI t (Fig. 5) and the interaction log N t–1 × RFI t (Tables 2 and 3). Four rabbit population growth models also had ∆ i < 2, that included the variable N t–1 (log–transformed, Fig. 6), RFI t and the interaction log N t–1 × RFI t (Tables 2 and 3).
Discussion
Fox predation was related to the decline in rabbit abundance along the two years we were able to obtain simultaneous data on foxes and rabbits. This is in agreement with previous studies conducted in Australia (Pech et al. 1992; Banks 2000), which showed the fox predation had a severe impact on dwindling rabbit populations. In Iberia, Calzada (2000) suggested that foxes could limit and regulate rabbit numbers at low densities within Doñana National Park (SW Spain). In central–southern Spain, Delibes–Mateos et al. (2008b) showed that the removal of foxes was the main management practice that positively contributed to rabbit population change (but see Díaz-Ruiz and Ferreras 2013; Norbury and Jones 2015). Our work here suggests, more specifically, a close relationship between rabbit population trends and fox predation. To our knowledge, this is the first study conducted in the rabbit’s native range that takes into account fox abundance and this species’ diet to measure the impact of fox predation on rabbit abundance and population growth. Although our study was conducted at only one location, this site is potentially representative of many areas in central Iberian Peninsula (e.g., hunting estates of low–to–high rabbit densities, where predator control is carried out, and with other economic activities like agriculture, small game hunting or animal production).
Our findings indicate that, despite the fact that foxes are considered generalist predators capable of consuming a wide range of food items (Macdonald and Reynolds 2004), they may also specialize on more abundant prey items. This has been demonstrated for other parts of the Iberian Peninsula where rabbits are moderately abundant (Díaz-Ruiz et al. 2013). In our study, we showed that high rabbit consumption by foxes occurred independently of rabbit abundance; thus the observed lack of variation in rabbit consumption in our study may indicate that, under the estimated rabbit abundance, foxes specialize on rabbits (Delibes-Mateos et al. 2008c).
We showed that rabbit population growth was negatively related to the previous month’s rabbit abundance. This suggests that rabbit populations may be limited by intraspecific density dependence (Ruiz-Azpurua et al. 2014). The growth of rabbit populations observed at low rabbit densities (Fig. 6) could be explained by a lower intraspecific competition (Ruiz-Azpurua et al. 2014), which favours exploitation of the resources available in these situations (Sinclair and Krebs 2002; Sinclair et al. 2006). In addition, the interaction between fox predation index and previous month rabbit abundance was included in the models that best explained rabbit population growth. Fox predation at lower rabbit abundance may produce a higher proportional loss in the rabbit population and potentially regulate it, in contrast to what has been observed at higher densities (Pech et al. 1992; Norbury and Jones 2015).
Other factors could also explain the observed rabbit population trends (e.g., diseases, food scarcity or absence of refuges; Moreno et al. 2007; Delibes-Mateos et al. 2014a; Ferreira et al. 2014). For instance, rabbit hunting was carried out in our study site and, apart from the rabbit mortality imposed by hunters and effects on rabbit population trends (Williams et al. 2007; but see Delibes-Mateos et al. 2008b), this practice may also force rabbits to become more nocturnal (J. Fernandez-de-Simon et al., unpublished data). As a consequence, it may also allow higher rabbit availability at night, and thus may attract nocturnal predators like foxes (Reynolds and Tapper 1995), potentially with an increase in fox abundance and pressure. Other studies could further assert our findings for other parts of the rabbit’s range (see e.g., experimental approaches in Pech et al. 1992; Tapper et al. 1996; Allen and Leung 2014; Allen et al. 2014).
In conclusion, we have shown the potential role of fox predation on rabbit abundance and population growth in the Iberian portion of the Mediterranean hotspot. It is essential to increase the understanding of this and other factors driving the abundance of European wild rabbit populations in Iberia, as recovering this keystone species is among the most challenging ecological problems for biodiversity conservation in this region.
References
Allen BL, Leung LKP (2014) The (non)effects of lethal population control on the diet of Australian dingoes. PLoS One 9(9):e108251
Allen BL, Allen LR, Engeman RM, Leung LKP (2014) Sympatric prey responses to lethal top-predator control: predator manipulation experiments. Front Zool 11:56
Banks PB (2000) Can foxes regulate rabbit populations? J Wildl Manag 64:401–406
Blondel J, Aronson J (1999) Biology and wildlife of the Mediterranean region. Oxford University Press, Oxford
Breusch TS (1979) Testing for autocorrelation in dynamic linear models. Aust Econ Pap 17:334–355
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Calzada J (2000) Impacto de la depredación y selección de presa del lince y el zorro sobre el conejo. PhD thesis, Universidad de León, León (in Spanish)
Delibes M, Hiraldo F (1981) The rabbit as prey in the Iberian Mediterranean ecosystem. In: Myers K, MacInnes CD (eds) Proceedings of the World Lagomorph Conference. Univ Guelph, Guelph, pp 614–622
Delibes-Mateos M, Redpath SM, Angulo E, Ferreras P, Villafuerte R (2007) Rabbits as a keystone species in southern Europe. Biol Conserv 137:149–156
Delibes-Mateos M, Delibes M, Ferreras P, Villafuerte R (2008a) Key role of European rabbits in the conservation of the western Mediterranean basin hotspot. Conserv Biol 22:1106–1117
Delibes-Mateos M, Ferreras P, Villafuerte R (2008b) Rabbit populations and game management: the situation after 15 years of rabbit haemorrhagic disease in central-southern Spain. Biodivers Conserv 17:559–574
Delibes-Mateos M, Fernandez de Simon J, Ferreras P, Villafuerte R (2008c) Feeding responses of the red fox (Vulpes vulpes) to different wild rabbit (Oryctolagus cuniculus) densities: a regional approach. Eur J Wildl Res 54:71–78
Delibes-Mateos M, Ferreras P, Villafuerte R (2009) European rabbit population trends and associated factors: a review of the situation in the Iberian Peninsula. Mamm Rev 39:124–140
Delibes-Mateos M, Díaz-Fernández S, Ferreras P, Viñuela J, Arroyo B (2013) The role of economic and social factors driving predator control in small game estates in central Spain. Ecol Soc 18(2):28
Delibes-Mateos M, Ferreira C, Carro F, Escudero MA, Gortázar C (2014a) Ecosystem effects of variant rabbit hemorrhagic disease virus, Iberian Peninsula. Emerg Infect Dis 20:2166–2168
Delibes-Mateos M, Ferreira C, Rouco C, Villafuerte R, Barrio IC (2014b) Conservationists, hunters and farmers: the European rabbit Oryctolagus cuniculus management conflict in the Iberian peninsula. Mamm Rev 44:190–203
Development Core Team R (2013) R: A language and environment for statistical computing. Austria R Foundation for Statistical Computing, Vienna
Díaz-Ruiz F, Ferreras P (2013) Conocimiento científico sobre la gestión de depredadores generalistas en España: el caso del zorro (Vulpes vulpes) y la urraca (Pica pica). Ecosistemas 22:40–47 (in Spanish with English abstract)
Díaz-Ruiz F, Delibes-Mateos M, García-Moreno JL, López–Martín JM, Ferreira C, Ferreras P (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian peninsula. Mamm Rev 43:59–70
Fernandez-de-Simon J (2013) Predation and the recovery of European rabbit populations in central–southern Iberian peninsula. An assessment of the predator pit hypothesis. PhD thesis, Universidad de Castilla–La Mancha, Ciudad Real
Fernandez-de-Simon J, Díaz-Ruiz F, Villafuerte R, Delibes–Mateos M, Ferreras P (2011a) Assessing predictors of pellet persistence in European rabbits Oryctolagus cuniculus: towards reliable population estimates from pellet counts. Wildl Biol 17:317–325
Fernandez-de-Simon J, Díaz-Ruiz F, Cirilli F, Tortosa FS, Villafuerte R, Delibes–Mateos M, Ferreras P (2011b) Towards a standardized index of European rabbit abundance in Iberian Mediterranean habitats. Eur J Wildl Res 57:1091–1100
Ferreira C, Touza J, Rouco C, Díaz-Ruiz F, Fernandez-de-Simon J, Ríos-Saldaña CA, Ferreras P, Villafuerte R, Delibes-Mateos M (2014) Habitat management as a generalized tool to boost European rabbit Oryctolagus cuniculus populations in the Iberian peninsula: a cost-effectiveness analysis. Mamm Rev 44:30–43
Ferrer M, Negro JJ (2004) The near extinction of two large European predators: super specialists pay a price. Conserv Biol 18:344–349
Gálvez L, Belliure J, Rebollo S (2009) European rabbits as ecosystem engineers: warrens increase lizard density and diversity. Biodivers Conserv 18:869–885
Godfrey LG (1978) Testing against general autoregressive and moving average error models when the regressors include lagged dependent variables. Econometrica 46:1293–1302
Gómez-Sal A, Rey-Benayas JM, López-Pintor A, Rebollo S (1999) Role of disturbance in maintaining a savanna-like pattern in Mediterranean Retama sphaerocarpa shrubland. J Veg Sci 10:365–370
Korpimäki E, Norrdahl K, Rinta-Jaskari T (1991) Responses of stoats and least weasels to fluctuating food abundances: is the low phase of the vole cycle due to mustelid predation? Oecologia 88:552–561
Lombardi L, Fernández N, Moreno S, Villafuerte R (2003) Habitat–related differences in rabbit (Oryctolagus cuniculus) abundance, distribution, and activity. J Mammal 84:26–36
Macdonald DW, Reynolds JC (2004) Red fox Vulpes vulpes Linnaeus, 1758. In: Sillero-Zubiri C, Hoffmann M, Macdonald DW (eds) Canids: foxes, wolves, jackals and dogs. Status survey and conservation action plan, IUCN/SSC Canid Specialist Group, Gland and Cambridge, pp 129–136
Moreno S, Beltrán JF, Cotilla I, Kuffner B, Laffite R, Jordán G, Ayala J, Quintero C, Jiménez A, Castro F, Cabezas S, Villafuerte R (2007) Long-term decline of the European wild rabbit (Oryctolagus cuniculus) in south-western Spain. Wildl Res 34:652–658
Norbury G, Jones C (2015) Pests controlling pests: does predator control lead to greater European rabbit abundance in Australasia? Mamm Rev 45:79–87
Pech RP, Sinclair ARE, Newsome AE, Catling PC (1992) Limits to predator regulation of rabbits in Australia: evidence from predator-removal experiments. Oecologia 89:102–112
Reynolds J, Aebischer N (1991) Comparison and quantification of carnivore diet by faecal analysis: a critique with recommendations based on a study of the fox Vulpes vulpes. Mamm Rev 21:97–122
Reynolds JC, Tapper SC (1995) The ecology of the red fox Vulpes vulpes in relation to small game in rural southern England. Wildl Biol 1:105–119
Ruiz-Azpurua L, Guerrero-Casado J, Carpio AJ, Tortosa FS (2014) High rabbit abundance proves detrimental to the population growth rate in European rabbit (Oryctolagus cuniculus L.) extensive breeding enclosures. World Rabbit Sci 22:179–186
Sarmento P (1996) Feeding ecology of the European wildcat Felis silvestris in Portugal. Acta Theriol 41:409–414
Saunders G, Gentle MN, Dickman CR (2010) The impacts and management of foxes Vulpes vulpes in Australia. Mamm Rev 40:181–211
Sinclair ARE, Krebs CJ (2002) Complex numerical responses to top–down and bottom–up processes in vertebrate populations. Philos Trans R Soc B Biol Sci 357:1221–1231
Sinclair ARE, Pech RP, Dickman CR, Hik D, Mahon P, Newsome AE (1998) Predicting effects of predation on conservation of endangered prey. Conserv Biol 12:564–575
Sinclair ARE, Fryxell JM, Caughley G (2006) Wildlife ecology, conservation, and management, 2nd edn. Blackwell, Oxford
Sobrino R, Acevedo P, Escudero MA, Marco J, Gortázar C (2009) Carnivore population trends in Spanish agrosystems after the reduction in food availability due to rabbit decline by rabbit haemorrhagic disease and improved waste management. Eur J Wildl Res 55:161–165
Tapper SC, Potts GR, Brockless MH (1996) The effect of an experimental reduction in predation pressure on the breeding success and population density of grey partridges Perdix perdix. J Appl Ecol 33:965–978
Valkama J, Korpimäki E, Arroyo B, Beja P, Bretagnolle V, Bro E, Kenward R, Mañosa S, Redpath SM, Thirgood S, Viñuela J (2005) Birds of prey as limiting factors of gamebird populations in Europe: a review. Biol Rev 80:171–203
Verdú JR, Galante E (2004) Behavioural and morphological adaptations for a low-quality resource in semi-arid environments: dung beetles (Coleoptera, Scarabaeoidea) associated with the European rabbit (Oryctolagus cuniculus L.). J Nat Hist 38:705–715
Villafuerte R, Delibes-Mateos M (2007) Oryctolagus cuniculus (Linnaeus, 1758). In: Palomo LJ, Gisbert J, Blanco JC (eds) Atlas y libro rojo de los mamíferos terrestres de España. Dirección General para la Biodiversidad–SECEM–SECEMU, Madrid, pp 490–491 (in Spanish)
Villafuerte R, Luco DF, Gortázar C, Blanco JC (1996) Effect on red fox litter size and diet after rabbit haemorrhagic disease in north-eastern Spain. J Zool 240:764–767
Wagner SC (2012) Keystone species. Nature Education Knowledge 3(10):51
Webbon C, Baker P, Harris S (2004) Faecal density counts for monitoring changes in red fox numbers in rural Britain. J Appl Ecol 41:768–779
Williams D, Acevedo P, Gortázar C, Escudero MA, Labarta JL, Marco J, Villafuerte R (2007) Hunting for answers: rabbit (Oryctolagus cuniculus) population trends in northeastern Spain. Eur J Wildl Res 53:19–28
Willot SJ, Miller AJ, Incoll LD, Compton SG (2000) The contribution of rabbits (Oryctolagus cuniculus L.) to soil fertility in semi-arid Spain. Biol Fertil Soils 31:379–384
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York
Acknowledgments
This is a partial result from the I+D National Plan Projects CGL2005-02340, CGL2009-10741 and CGL2013-43197, funded by the Spanish Ministry of Science and Innovation and EU FEDER funds. JFdS benefitted from a FPI PhD scholarship (BES-2006-12562) funded by the Spanish Ministry of Science and Innovation and the European Social Fund. J. Fernandez-de-Simon and F. Díaz-Ruiz are currently holding a postdoctoral fellowship from Junta de Castilla-La Mancha and the European Social Fund (Operational Programme FSE 2007/2013). M. Delibes-Mateos is currently funded by Junta de Andalucía and the EU FP7, under grant agreement 267226. The authors had the necessary licences and permits (dated 1 February 2004) for the activities described in the paper and took into account the welfare of animals. We are indebted to landowners and personnel of the study area. We thank many people who kindly collaborated with us. Prof. J. E. Fa carefully revised the English text. In addition, F.S. Tortosa, F. Cirilli, B. Arroyo, M. Delibes, P.C. Alves, C. Johnson, B. Allen, T. Saitoh, and three anonymous reviewers provided helpful suggestions in earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernandez-de-Simon, J., Díaz-Ruiz, F., Rodríguez-de la Cruz, M. et al. Can widespread generalist predators affect keystone prey? A case study with red foxes and European rabbits in their native range. Popul Ecol 57, 591–599 (2015). https://doi.org/10.1007/s10144-015-0510-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-015-0510-5