Abstract
The European rabbit (Oryctolagus cuniculus) is a key prey species in Mediterranean ecosystems. However, it is also considered a pest on many oceanic islands, even though its true abundance and ecological effects on different island habitats are poorly understood. We present data on rabbit abundance for the best-preserved habitats of the Canary Islands, Spain (National Parks), including ecosystems differing in climate, topography, plant species richness and composition. Three methods of assessing rabbit abundance from faecal pellet density are compared to ascertain the best method to compare highly distinct habitats. The Cleaning method was used during spring, summer, autumn and winter to check whether there were differences in pellet degradation among habitats that could prevent comparisons between them. Rabbit abundance is determined by complex interactions among abiotic and biotic factor. Despite differences in climate conditions, the results obtained for rabbit density with fast methods correlated well with the slow Cleaning method. The Circular method was the most useful to work with for extensive sampling in different habitats. The best models for explaining rabbit density for all habitat types combined included tree cover, abiotic and topographic and climatic variables. Thus, factors influencing rabbit density vary depending on habitat type with Macaronesian laurel forests being the ecosystem least likely to be invaded by rabbits. The present study highlights that rabbits reach damaging densities for plant conservation in most areas on the Canary Islands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Islands make up approximately 5% of land area worldwide, yet they harbour a relatively high percentage of biodiversity, including many endemic and endangered species (Kreft et al., 2008; Myers et al., 2000). Endemic plant species on many oceanic islands have evolved without mammalian herbivores (Nogales et al., 2006), making them highly vulnerable when these invasive mammals are subsequently introduced (Courchamp et al., 2003). A significant portion of plant species richness, especially on oceanic islands, has been lost predominately due to the impacts of invasive mammals (Courchamp et al., 2003), and among these, a limited subset of feral mammals is responsible for the majority of insular diversity declines (Atkinson, 1996; Bonnaud et al., 2011; Canale et al., 2019; Capizzi et al., 2016).
The European rabbit (Oryctolagus cuniculus L.) is a native species to southern Europe and northern Africa (Smith & Boyer, 2008). It has been effectively introduced into more than 800 islands and is one of the most widely distributed animal species worldwide (Flux & Fullagar, 1992), with a paradoxical ecological role when comparing its presence on island and mainland areas (Kontsiotis et al., 2017; Lees & Bell, 2008). The ecological effect of this species on oceanic islands has been one of the worse known, directly affecting numerous endemic plants (Cubas et al., 2019). Although a vulnerable keystone species was considered in their regions of origin (Villafuerte and Delibes-Mateos, 2008), rabbits are often seen as pests on oceanic islands (Lowe et al., 2004; Courchamp et al., 2003). Rabbit density and distribution are often determined by complex interactions among abiotic and biotic factors, such as human activities, predator pressure and in some cases viral diseases that can cause significant reductions in population density (Delibes-Mateos et al., 2009; Kontsiotis et al., 2017; Penteriani et al., 2013; Rocha et al., 2017).
Factors influencing rabbit abundance and distribution have been carefully analysed where rabbits are a native species (Carvalho & Gomes, 2004; Delibes-Mateos et al., 2008; Lombardi et al., 2003; Saldaña et al., 2007). However, variations in rabbit density on islands have been poorly studied. Most studies concern rabbits’ effects on endemic plant species richness and composition (Cubas et al., 2019; Donlan et al., 2002; Nogales et al., 2005), the recovery of vegetation after rabbit eradication (Hess & Jacobi, 2011) or using rabbit exclusion plots (e.g., Cubas et al., 2018; Garzón-Machado et al., 2010; Irl et al., 2012). However, ecological responses to rabbit abundance in different habitats are still little understood on oceanic islands.
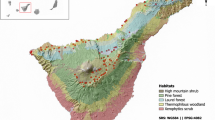
The Canary Islands show great habitat diversity with high levels of endemics in both flora and fauna (Sundseth, 2005). In this island, various and contrasting habitats are present, such as succulent Euphorbia scrub, Laurel and pine forest and summit scrubland (del Arco and Rodríguez-Delgado, 2018). However, they were significantly affected by rabbit introduction during the fifteenth century (de Abreu Galindo, 1977). On the islands, there is a good representation of arid succulent, shrublands at lower elevations, thermos-sclerophyllous woodland at mid-elevations, laurel forests in northern cloud areas, endemic pine forests above 1500 m altitude and a summit legume shrubland vegetation above 2000 m (del Arco and Rodríguez-Delgado 2018). These highly diverse habitats provide a useful opportunity to investigate whether rabbit density depends on habitat type and the main factors influencing this relationship.
Estimating rabbit density to assess their environmental effects is a challenging task. Although different census methods have been applied to lagomorph populations in their areas of origin, a quantitative comparison of census methods is still lacking for different habitats on oceanic island ecosystems. The Cleaning method (using fresh pellets) provides one of the best estimates of rabbit density (Fernández-de-Simón et al., 2011a); however, it is time-consuming and cannot reliably estimate low rabbit abundance (Palomares, 2001). Higher sampling intensity is needed to overcome these shortcomings, and rapid sampling methods allow many more sites to be assessed for the same effort. The use of faecal pellet counts is often the only viable approach (Fernández-de-Simón et al., 2011a; Sugimura et al., 2000). The persistence of dung is a valuable tool in measuring the average population density of rabbits and their impact on native vegetation (Cubas et al., 2019; González-Mancebo et al., 2019), since dung persistence provides an integrated measure of longer-term population levels (Mutze et al., 2014). Rabbit pellets may last for more than six months in Mediterranean environments (Fernández-de-Simón et al., 2011a), but we have observed persistence up to eight months as reported by Fernandez-de-Simón et al. (2011a) or even more than one year (Wood, 1988). Dung persistence depends on the season, habitat and the overall climatic conditions (Iborra & Lumaret, 1997; Putman, 1984), so pellet counts obtained in the same months, but different years may not be comparable. The question that arises is whether it is possible to use the same method, counting faeces in different habitats, where there may be differences in degradation rates.
According to previous considerations, we hypothesize that rabbit abundance depends on habitat type. To test this, we examined rabbit abundance in the best-preserved areas on the Canary Islands National Parks and tested whether the same method could be applied successfully in highly distinct habitats (alpine habitats, laurel forests, pine forest and coastal vegetation). We compared three different methods for estimating rabbit abundances based on counts of rabbit faeces (e.g., Fernández-de-Simón et al. 2011b; Sugimura et al., 2000) and related these to presumed topographic (elevation, rocks and soil cover) and climatic variables (temperature and humidity related variables), vegetation cover and species composition.
Material and methods
Study area
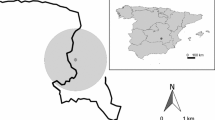
The Canary Islands are located between 27°37′–29°25′N and 13°20′–18°10′W (Fig. 1). This study was conducted on four islands (Lanzarote, La Gomera, La Palma and Tenerife) in the best-preserved habitats (National Parks, N.P.): Timanfaya N.P. (Lanzarote), Garajonay N.P. (La Gomera), Caldera de Taburiente N.P. (La Palma) and El Teide N.P. (Tenerife) (Table 1).
These National Parks include the four main ecosystems of these islands: Timanfaya N.P. includes hyper-arid to arid infra-Mediterranean bioclimatic belts and a coastal Euphorbia scrub dominated by Odontospermo (Asterisco) intermedii-Euphorbietum balsamiferae, although 70% of the park is covered by lava flows from the 1730–36 eruption and has very low vegetation cover (mostly lichens) (del Arco and Rodríguez-Delgado, 2018). Garajonay N.P. mostly includes laurel forests, Pruno hixae-Lauretea novocanariensis, linked to the cloudy area of NE trade winds within dry to humid infra-thermo-Mediterranean belts (del Arco and Rodríguez-Delgado, 2018). The Caldera de Taburiente N.P. largely comprises local Canary endemic Pine forest, Loto hillebrandii-Pinetum canariensis developed within dry to sub humid thermo- and meso-Mediterranean belts beyond the influence of trade wind clouds, changing at higher elevations to a supra Mediterranean shrubland vegetation dominated by legumes, Genisto benehoavensis-Adenocarpetum spartioidis (del Arco et al., 2006; del Arco and Rodríguez-Delgado, 2018). Finally, El Teide N.P. mostly includes the legume shrubland Spartocytisetum supranubii, representative of the alpine ecosystem above the pine tree line (> 2000 m.), which forms a dry to sub-humid supra- and oro-Mediterranean zone on the island’s summit (del Arco et al., 2006; del Arco and Rodríguez-Delgado, 2018) (Table 1). The threshold values for thermotypes, bioclimates and ombrotypes were taken from Rivas-Martínez (1997) (see S1 for details).
Estimating rabbit abundance
Dung-based survey methods provide an approximately linear index of rabbit density (Ballinger & Morgan, 2002) and have been widely used to census rabbit abundance in Australia and Europe (e.g., Bird et al., 2012; Calvete et al., 2006; Fernández-de-Simón et al., 2011b; Mutze et al., 2002, 2014; Rouco et al., 2016; Wood, 1988), because they provide an acceptable approximation to relative rabbit abundance that is adequate for large-scale sampling (Palomares, 2001).
The census methods vary in amount of labour required. There are rapid field methods that allow more data to be collected for subsequent ecological analyses, but with the drawback of presumably less accuracy than labour-intensive methods (Fernández-de-Simón et al., 2011a; Mutze et al., 2014; Wood, 1988). To balance effectiveness with accuracy and to test whether the same method can be applied to different habitats, we calculated rabbit density using three different methods with rabbit faecal pellets as the base measure. In the following section, they are termed Cleaning (Fernández-de-Simón et al., ), Cooke (Cooke et al., 2008) and Circular methods (Mutze et al., 2014; Rouco et al., 2016) respectively.
Randomly, a total of 192 sampling sites were established across the different type of habitats (Table 1, Fig. 1). Within all sample sites, areas with slopes > 30° and flat ground immediately below them were avoided because faeces deposited roll downhill and accumulate on flatter areas, distorting apparent rabbit abundance. This is also justified because rabbits prefer a flat or just moderately undulating terrain (Delibes-Mateos et al., 2009; Villafuerte and Delibes-Mateos, 2008), and rabbit abundance is negatively correlated with slope (Cubas et al., 2019). While Cooke and Circular methods were applied to all sample sites, we applied the Cleaning method to only a small proportion of the sampling sites (27) due to its high labour demand: 3 in coastal spurge scrub (in the ancient substrates of the National Park), 4 in the laurel forest, 3 in pine forest and 17 in the alpine ecosystem (Table 1).
The labour-intensive Cleaning method was carried out in all four seasons between spring 2017 and winter 2017, and during each season, a total of 27 days was required to count pellets in the experimental plots. Comparisons with the results obtained by the quicker Cooke and Circular methods were made only in summer 2017, since this is the season with highest rabbit abundance during the year (Cabrera-Rodríguez, 2008; Rouco et al., 2016).
1. Cleaning method Regular pellet removal from permanently marked quadrats allows rabbit densities to be estimated at regular intervals (e.g., seasons) following a sampling protocol suggested by Fernández-de-Simón et al. (2011a). In contrast to the Cooke and Circular methods described below, the Cleaning method requires regular counting and removal of rabbit excrement to be able to assess the number of pellets deposited in each interval (spring, summer, autumn and winter). This method is the most accurate due to estimate rabbit density using fresh pellets (Fernández-de-Simón et al., 2011a).
Depending on the area of each National Park, we established a minimum of three sampling sites, each consisting of 24 permanent plots of 1 × 1 m2, regularly arranged every 20 m along two parallel lines 220 m length and 20 m apart (12 plots per line). In each of these 24 permanent plots, we counted fresh pellets (avoiding latrines, where droppings are not randomly distributed). All faecal pellets were then removed, and plots were revisited in the next season to repeat the process (Cubas et al., 2018; Fernández-de-Simón et al., 2011a). Rabbit density (D) was calculated following the equation proposed by Eberhardt and Van Etten (1956): D = d/r*t, where d is the mean number of recent pellets per square metre, r is the estimated mean number of pellets produced per rabbit per day (i.e., 350 pellets = 1 rabbit/day), as assessed for Mediterranean environments by Fernández-de-Simón et al., (2011a), similar to that found in other countries (Wood, 1988), and t is the number of days since excrement was last removed.
2. Cooke method This method was originally designed to help land managers with little experience in rabbit management to quickly assess whether rabbits were abundant enough to cause problems (e.g., prevent regeneration of plants, Cabrera-Rodríguez, 2008). Observations of rabbit dung pellets are scored according to a descriptive table which relates distribution and frequency of sightings of rabbit dung to rabbit density (see S2 for details). In our case, two persons walked for 20 min continuously at each site and assessed the amount of faeces, scoring each site on an ordinal scale between 0 and 5 (Cooke et al., 2008).
3 Circular method At each site, following a circular transect of 500 m, rabbit density was estimated by counting pellets in 150 randomly distributed circular 0.1 m2 plots at each site as recommended by Mutze et al. (2014) and Rouco et al. (2016). These counts were assumed to correlate with the average rabbit density across a longer period of up to two years because of the persistence of old dung pellets (Mutze et al., 2014).
Predictor variables
At each site, vegetation structure and plant species diversity were evaluated using four variables in the field: total species richness (α-diversity), total herbaceous, shrub (< 7 m) and tree (> 7 m.) cover, distinguishing endemic and non-endemic species (Acebes et al., 2010; Borges et al., 2008) on three randomly distributed plots of 50 × 50 m (2500 m2) per site. In regard to climatic variables, we obtained data from meteorological stations of the Spanish Meteorological Agency (AEMET) focusing on mean annual temperature and annual precipitation. For those two variables, we performed generalized additive models (GAM) in R Studio using coordinates and topographic variables (elevation and aspect) as explanatory variables. With the best-fitted models (D-squared values above 0.8), we predicted the values for the whole archipelago and our localities. We applied Spearman’s rank correlations for all pairs of variables to check for multicollinearity (R > 0.7), which may disrupt multiple regression models, and excluded all maximum and minimum values, and we left just mean temperature and mean precipitation for model building. Additionally, we included the variables elevation, slope, rock cover and bare soil (indicating the proportion of open areas) recorded in the field.
In this study, soil hardness and the ability of rabbits to dig warrens, which are vital in other areas (Delibes-Mateos et al., 2009), have not been considered because warrens are rare due to the high availability of rock holes and shrubby vegetation as refuges.
Data analysis
We compared the densities or scores of rabbit faecal pellets as estimated from all three survey methods using Spearman’s rank correlations, due to non-normality even after transformation. To evaluate whether there were differences of estimated rabbit densities between different habitats, we performed a Kruskal–Wallis test and a Dunn post hoc test (Oksanen et al., 2018).
We applied non-metric multidimensional scaling (NMDS, Clarke, 1993) to assess the structure of floristic composition and its relation to abiotic and biotic variables. We decide left out two plots in the NMDS because they are extreme outliers masking the configuration of the other NMDS plots. We exclude those plots where we had not species richness.
Independently, we analysed relationships between rabbit density in each habitat and the chosen environmental variables using ordinary least-square regression models including all explanatory variables and subsequently with all possible explanatory variable combinations. There is good reason to note that the model with the highest explanatory power (measured as R2) is not necessarily the only possible interpretation of relationships between the data. In order to include information from all informative models, we ranked all possible models by their AIC (Akaike’s information criterion, Turkheimer et al., 2003). The AIC balances explanatory power (in terms of R2) and the number of variables within the model (simplicity), penalizing models with high R2, albeit with a high number of variables. We generally chose the model with the lowest AIC as the 'best' model; however, in some cases of low performance, models with the lowest AIC still contained insignificant variables. In these cases, we dropped these insignificant variables which were permissible according to the criteria of Burnham and Anderson (2002). The information of all models with ΔAIC < 2 (‘best’ models) were then combined into one final model by applying the model averaging procedure (Burnham et al., 2002; Grueber et al., 2011). Prior to modelling, we standardized all variables (zero mean, unit variance) to obtain meaningful model estimates for assessing the weight of variables within models. For detailed information, see Supporting Information S3-4.
We applied linear mixed effects models to assess the relation between the density of rabbit (dependent variable) and the above-mentioned biotic and abiotic variables (explanatory factors), considering type of habitat as a random factor. The best model was assessed by iteratively selecting explanatory variable combinations resulting in the lowest cAIC (conditional Akaike information criterion; Saefken et al., 2014). We assessed the statistical significance of differences between the null model (rabbit ~ 1 + (1|Habitat)) and this best model (rabbit ~ variables + (1|Habitat)) using a likelihood ratio test with ANOVA function. We also report the predictor variable coefficients and their significance in the final models. All analyses were carried out with the statistical software R (R Core Team, 2017) and the packages vegan (Oksanen et al., 2018), MuMin (Barton, 2013; Burnham et al., 2002) and lme4 (Bates et al., 2014).
Results
Comparison of sampling methods and seasonal patterns of rabbit density
The Cleaning method showed the same seasonal trend of rabbit faeces across all habitats, with the highest densities in summer (Fig. 2), highest seasonal differences in coastal spurge scrub and the lowest ones in the pine forest. Maximum density of rabbits (rabbit/ha) was observed at the end of summer in all habitats (coastal spurge scrub: 2.73 ± 3.47, alpine shrub: 1.62 ± 1.57, laurel forest: 0.60 ± 0.42 and pine forest: 0.07 ± 0.11). However, the time of lowest rabbit density differed among habitats: laurel forest at the end of winter (0.48 ± 0.43), whereas in coastal spurge scrub at the end of autumn (0.43 ± 0.42) and in pine forest in spring (0.03 ± 0.05). In the alpine ecosystem, the time of lowest rabbit density was at the end of winter (0.30 ± 0.10) (Fig. 2).
Density values measured with the Cleaning method were significantly correlated with the Cooke method (ρ = 0.50; p < 0.01; n = 27, Fig. 3a) and the Circular method (ρ = 0.61; p < 0.001; n = 27, Fig. 3b). However, estimates were more closely related for the Circular method than for the Cooke method for all habitats. Moreover, density values measured with the Cooke and Circular methods were highly correlated (ρ = 0.91; p < 0.001; n = 192, Fig. 3c).
Regression analysis between different methods for estimated rabbit density: Cleaning method (Fernandez-de-Simón et al., ) with a Cooke method (Cooke et al., 2008) and b Circular method (Mutze et al., 2014). Also, we compared with c Cooke method (Cooke et al., 2008) and Circular method (Mutze et al., 2014)
Environmental factors and rabbit abundance
Species richness varied between plots in each habitat. The lowest number of species was found in the coastal spurge scrub with 5.76 ± 6.40 species (17.2% endemic species, total 57 species) followed by the alpine shrub (5.83 ± 4.07 species per sampling plot) with 80.8% endemic species (total 52 species) and the pine forest (9.21 ± 5.32, 37.5% endemic species, total 72 species). The laurel forests showed the highest species richness per plot (14.19 ± 5.73) which 48.5% endemics (total 138 species). According to NMDS, the floristic composition of habitats was well separated along the first two axes (NMDS: R2 = 0.87; p < 0.0001) (Fig. 4). As expected, the habitats are well separated, just alpine shrub and pine forest share species assemblages.
Rabbit density also differed between habitats (Kruskal–Wallis test, χ23 = 79.99; p < 0.001; n = 192). The highest rabbit density was found significant differences in alpine shrub, followed by pine forest, coastal spurge scrub and laurel forest (Fig. 5).
Density of rabbits in each habitat type (following Mutze et al., 2014) during summer 2017. Lowercase letters indicate significant groups (p < 0.05) using Dunn test
Linear mixed models revealed significant differences between habitats (χ23 = 25.22; p < 0.0001). The model including rabbit density is negatively correlated with mean annual temperature (β = − 0.17; p < 0.0001), rock cover (β = − 0.02; p < 0.001), slope (β = − 0.08; p < 0.001) and canopy cover (TREE) (β = -0.03; p < 0.0001), although not with very high explanatory power (Marginal R2 = 0.28, p < 0.001; Conditional R2 = 0.28, p < 0.001).
Rabbit density in each habitat followed different patterns and only a low proportion of variance was explained, albeit significant, with an R2 between 0.09 and 0.38 (Table 2). The best rabbit density model was negatively related to mean annual temperature (β = − 0.34; p < 0.05), canopy cover (TREE) (β = 0.43; p < 0.05, the most important variable), slope and scrub cover and positively to plant species richness in laurel forest. In the pine forest, rabbit density was positively correlated with precipitation (β = 0.89; p < 0.05) and scrub cover. In coastal spurge scrub, the best model only contained a positive correlation with plant species richness (β = 0.84; p < 0.001). Finally, in alpine shrub, rabbit density was positively correlated with mean annual temperature (β = 1.94; p < 0.001, the most important variable) and herbaceous cover, and negatively with precipitation and plant species richness.
Discussion
The worldwide distribution of rabbits demonstrates their capacity for colonizing many different habitats (Thompson & King, 1994), and it has been evidenced in our study. The presence of rabbits in areas with recent lava flows, low plant species richness and vegetation cover highlights the ecological plasticity of the species and its ability to change behaviour to increase its fitness (Gibb, 1993). The relationship between rabbit density and environmental and biotic factors varied strongly depending on the ecosystem considered, though it seems that rabbit density does not depend on habitat type. The fact that such different habitats like coastal spurge scrub, pine forest and laurel forests showed similar rabbit densities indicates that it is not possible to relate control effort to habitat type. All measured variables were identified as significant in the models in at least one of the habitat types analysed, indicating a complex relationship between rabbit density, vegetation structure, climate and other abiotic variables (Calvete et al., 2004; Delibes-Mateos et al., 2008) as sources of shelter and food as well as environmental variables (Villafuerte et al., 1993).
Environmental variables and rabbit abundance
We identified tree cover as the most important factor influencing the population density of rabbits. The laurel forest, with highest tree cover, was the least favourable habitat for rabbits, despite their high vegetation diversity, indicating that vegetation structure may be even more important for rabbits than diversity of food resources. In addition, temperature and rock covering were also highly but negatively correlated with rabbit abundance, although both factors were correlated with other variables that need to be analysed separately in each ecosystem. Rabbit density was also negatively correlated with slope, as has been found in other Mediterranean ecosystems, where rabbits prefer flat or moderately undulated terrain (Saldaña et al., 2007; Villafuerte and Delibes-Mateos, 2008). In this study, soil hardness, an important factor that determines the ability of rabbits to dig warrens, which are vital in other areas (Delibes-Mateos et al., 2009), has not been considered, since here, probably due to the high refuge availability (rock holes and shrubby vegetation), warrens are rare.
The results show that limiting factors for rabbits vary between ecosystems. In the laurel forest, tree cover was the limiting factor, showing that rabbits prefer open habitats as in Mediterranean areas (Villafuerte and Delibes-Mateos, 2008; Villafuerte & Moreno, 1997). However, rabbit density in the pine forest was dependent on the shrub cover and precipitation, and in the coastal spurge scrub, it widely depended on plant species richness. Resource availability may underlie the relationship outlined in these two drier habitats. Food and vegetation cover may affect habitat selection by individuals (Rangeley & Kramer, 1998). However, rabbit density in the alpine shrub is best explained by mean annual temperature and negatively correlated with precipitation and plant species richness. Above 2000 m, the strong influence of temperature and rainfall may be explained by the high elevation gradient considered (up to 3718 m). Climate is a key factor influencing the broad-scale geographical distribution of rabbits in the Iberian Peninsula (Villafuerte and Delibes-Mateos, 2008). The alpine habitats in the Canary Islands provide a favourable combination of food and refuge for this invasive species, which explain the highest rabbit densities there. Although climate conditions (temperature) were presumably limiting in the past, these days, global warming increases the survival rate of rabbits above 2000 m (Bello-Rodríguez et al., 2020). This is a habitat with a high proportion of endemic species (Steinbauer et al., 2016), which represent high-quality food sources for rabbits (Cubas et al., 2019).
Counting methods and annual patterns of rabbit densities at each type of habitats
In this study, we show that summertime rabbit density in different habitat types in the Canary Islands averages between 0.5 and 4 adult rabbits per hectare, although it is much higher in small localized areas (Fig. 5). The higher densities of rabbits recorded here are higher than those in semi-arid Australia, where rabbits cause significant changes in the flora at densities of between 1 and 2 rabbits per hectare (Mutze et al., 2016). In the Canary Islands, densities even below one rabbit per hectare may be dangerous for the endemic flora in the poorest ecosystems (Cubas et al., 2018).
Despite differences in climate conditions (temperature, but especially precipitation), the results obtained for rabbit densities were perfectly comparable, as was evidenced by the correlations found with the Cleaning method. This method greatly reduces the problem of differences in the degradation rate of the faecal pellets, since counting scattered pellets is, therefore, the most accurate alternative (Guerrero-Casado et al., 2020). On the other hand, the correlations between methods, especially with the Circular method, show that fast methods are adequate for estimating minimum population size. This highlights the possibility of analysing rabbit densities in larger areas and being able to obtain abundance data regularly for better management of rabbit control in protected areas. The three methods we used to estimate rabbit abundance (Cooke et al., 2008; Fernández-de-Simón et al., 2011a; Mutze et al., 2014) were mostly well-correlated and showed which habitats had the greatest and least rabbit abundance.
The Circular method showed that in our study area, the maximum density of rabbits was always detected at the beginning of summer, confirming that the application of the other two methods in this season was an appropriate choice. This is because the rainy season in the Canary Islands usually occurs from October/November to March/April, and the initiation of pasture growth triggers rabbit reproduction as has been observed in the Mediterranean ecosystem (Beltran, 1991; Villafuerte et al., 1997) and in places where rabbits have been introduced (e.g., Cabrera-Rodríguez, 2008). The annual rabbit population increase due to breeding occurs between the end of winter and at the beginning of summer (Villafuerte and Delibes-Mateos 2008) although there is a lag between the onset of breeding and the increase in pellet abundance explained by the duration of pregnancy (4 weeks) and the two–three months that the young rabbits stay close to the maternal nest (Villafuerte and Delibes-Mateos 2008). In other places, inter-annual variation in rabbit density has been found to depend mainly on the length of drought periods and the level of rainfall (Angulo & Villafuerte, 2003), with dry years reducing food availability for rabbits (Villafuerte et al., 1997).
The lowest rabbit densities were variable over the year between habitats. Temperature is the factor limiting rabbit density in winter in the laurel forest, while in drier habitats, food availability and temperature explain differences in rabbit density. Summer drought limits food availability in autumn in coastal spurge scrub and alpine ecosystems. In alpine habitats, frost during winter has traditionally been the most important factor limiting rabbits in the past (Bello-Rodríguez et al., 2020; Martín et al., 2012). However, during the last 30 years, global warming is responsible for their current survival in the winter period (Martín et al., 2015). In pine forests, the lowest values in spring may be explained by a delay in the time of maximum productivity with elevation.
Conclusion
Although estimating rabbit abundance is dependent on pellet counts, we show that the Circular method best combines the accuracy and the rapidity needed to collect sufficient data for statistical analysis, working in wide areas and comparing different habitats. Therefore, it was justified to proceed with the rabbit density measurements from the Circular method. It is preferable to simplified methods such as the Cooke method and less time-consuming than regular collection of rabbit dung from set quadrats as in the Cleaning method. The Circular method is also useful to compare different habitats, even with presumable differences in faecal pellet degradation, due to differences in rain conditions (Fernandez-de-Simón et al., 2011a).
Variation of rabbit density depends on a mix of environmental conditions and vegetation diversity variables. We found that rabbits were present in all studied ecosystems, albeit at low density in laurel forests and areas with lowest productivity. Highly differentiated ecosystems in vegetation structure, plant species richness and climate can exhibit similar rabbit densities, at least in summer, when the highest rabbit density was recorded in all the ecosystems studied.
This study provides useful data to argue that rabbit management is required in all habitats, although areas such as laurel forests where rabbit density is low are possible exceptions. Additional studies are necessary to better understand rabbit distribution factors in wettest areas of the laurel forests. Although rabbit damage to specific vegetation types or individual threatened endemic plant species will determine where rabbit control is most needed (Cubas et al., 2019; González-Mancebo et al., 2019), the knowledge of the rabbit density distribution seems necessary to prioritize management actions (Brown et al., 2020). From a conservation perspective, considering that complete rabbit eradication on large islands is technically and socially very difficult, we urgently advocate for decisive measures for the control of rabbit density, at least in all three arid ecosystems.
References
Acebes, J. R., León, M. C., Rodríguez, L., Del Arco, M., García, A., Pérez-de-Paz, P. L., Rodríguez, O., Martín, V. E., & Wildpret, W. (2010). Pteridophyta, Spermatophyta In Arechavaleta, M., Rodríguez, S., Zurita, N., & García, A. (Eds.). Lista de especies silvestres de Canarias Hongos, plantas y animales terrestres 2009. Gobierno de Canarias (pp 119–172).
Angulo, E., & Villafuerte, R. (2003). Modeling hunting strategies for the conservation of wild rabbit populations. Biological Conservation, 115, 291–301.
Atkinson, I. A. E. (1996). Introductions of wildlife as a cause of species extinctions. Wildlife Biology, 2(3), 135–141.
Ballinger, A., & Morgan, D. G. (2002). Validating two methods for monitoring population size of European rabbit (Oryctolagus cuniculus). Wildlife Research, 29, 431–437.
Barton, K. (2013). Package 'MuMin' (Multi-model inference). Model selection and model averaging based on information criteria (AICc and alike) R package version 1.9.0.
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2014). lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7. http://CRAN.Rproject.org/package=lme4.
Bello-Rodríguez, V., Mateo, R. G., Pellissier, L., Cubas, J., Cooke, B., & González-Mancebo, J. M. (2020). Forecast increase in invasive rabbit spread into ecosystems of an oceanic island (Tenerife) under climate change. Ecological Applications, 00, e02206. https://doi.org/10.1002/eap.2206
Beltran, J. F. (1991). Temporal abundance pattern of the wild rabbit in Doñana, SW Spain. Mammalia, 55, 591–599.
Bird, P., Mutze, G., Peacock, D., & Jennings, S. (2012). Damage caused by low-density exotic herbivore populations: the impact of introduced European rabbits on marsupial herbivores and Allocasuarina and Bursaria seedling survival in Australian coastal shrubland. Biological Invasions, 14, 743–755.
Bonnaud, E., Medina, F. M., Vidal, E., Nogales, M., Tershy, B., Zavaleta, E., Donlan, C. J., Keitt, B., Le Corre, M., & Horwath, S. V. (2011). The diet of feral cats on islands: a review and a call for more studies. Biological Invasions, 13, 581–603.
Borges, P. A. V., Abreu, C., Aguiar, A. M. F., Carvalho, P., Jardim, R., Melo, I., Oliveira, P., Sérgio, C., Serrano, A. R. M., & Vieira, P. (2008). A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. Funchal and Angra do Heroísmo (pp. 440).
Brown, S. C., Wells, K., Roy-Dufresne, E., Campbell, S., Cooke, B., Cox, T., & Fordham, D. A. (2020). Models of spatiotemporal variation in rabbit abundance reveal management hot spots for an invasive species. Ecological Applications, 30(4), e02084.
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach. Springer.
Cabrera-Rodríguez, F. (2008). Seasonal Abundance and Management Implications for Wild rabbits (Oryctolagus cuniculus) on La Palma, Canary Islands, Spain. Wildlife Biology in Practise, 4(2), 39–47.
Calvete, C., Estrada, R., Angulo, E., & Cabezas-Ruiz, S. (2004). Habitat factors related to wild rabbit conservation in an agricultural landscape. Landscape Ecology, 19, 531–542.
Calvete, C., Pelayo, E., & Sampietro, J. (2006). Habitat factors related to wild rabbit population trends after the initial impact of rabbit haemorrhagic disease. Wildlife Research, 33, 467–474.
Canale, D. E., Di Dio, V., Massa, B., & Mori, E. (2019). First successful eradication of Norway rats Rattus norvegicus from a small Mediterranean island (Isola delle Femmine, Italy). Folia Zoologica, 68, 21–26.
Capizzi, D., Baccetti, N., & Sposimo, P. (2016). Fifteen years of rat eradication on Italian islands. In F. M. Angelici (Ed.), Problematic wildlife A cross-disciplinary approach (pp. 205–227). Springer.
Carvalho, J. C., & Gomes, P. (2004). Influence of herbaceous cover, shelter and land cover structure on wild rabbit abundance in NW Portugal. Acta Theriologica, 49(1), 63–74.
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18, 117–143.
Cooke, B., McPhee, S., & Quentin, H. (2008). Rabbits: A threat to conservation and natural resource management. How to rapidly assess a rabbit problem and take action (p. 16).
Courchamp, F., Chapuis, J. L., & Pascal, M. (2003). Mammal invaders on islands: Impact, control and control impacts. Biological Review, 78, 347–383.
Cubas, J., Irl, S. D. H., Villafuerte, R., Bello-Rodríguez, V., Rodríguez-Luengo, J. L., del Arco, M., Martín-Esquivel, J. L., & González-Mancebo, J. M. (2019). Endemic plant species are more palatable to introduced herbivores than non-endemics. Proceedings of the Royal Society B-Biological Sciences , 286, 20190136.
Cubas, J., Martín-Esquivel, J. L., Nogales, M., Irl, S. D. H., Hernández-Hernández, R., López-Darias, M., Marrero-Gómez, M., del Arco, M. J., & González-Mancebo, J. M. (2018). Contrasting effects of invasive rabbits on endemic plants driving vegetation change in a subtropical alpine insular environment. Biological Invasions, 20(3), 793–807.
De Abreu, G. J. (1977). Historia de la conquista de las siete islas de Canarias. Santa Cruz de Tenerife.
Del Arco Aguilar, M. J., & Rodríguez-Delgado, O. (2018). Vegetation of the Canary Islands. In: Vegetation of the Canary Islands. Plant and Vegetation 16:83–319 Springer, Cham. Switzerland.
Del Arco, M., Wildpret, W., Pérez De Paz, P. L., Rodríguez, O., Acebes, J. R., García-Gallo, A., Martín, V. E., Reyes-Betancort, J. A., Salas, M., Bermejo, J. A., González, R., Cabrera, M. V., & García, S. (2006). Mapa de Vegetación de Canarias (p. 550). GRAFCAN.
Delibes-Mateos, M., Delibes, M., Ferreras, P., & Villafuerte, R. (2008). Key Role of European Rabbits in the conservation of the western mediterranean basin hotspot. Conservation Biology, 22, 1106–1117.
Delibes-Mateos, M., Ferreras, P., & Villafuerte, R. (2009). European rabbit population trends and associated factors: A review of the situation in the Iberian Peninsula. Mammal Review, 39(2), 124–140.
Donlan, C. J., Tershy, B. R., & Croll, D. A. (2002). Islands and introduced herbivores: Conservation action as ecosystem experimentation. Journal of Applied Ecology, 39, 235–246.
Eberhardt, L., & Van Etten, R. (1956). Evaluation of de pellet group count as a deer census method. Journal of Wildlife Management, 20, 70–74.
Fernandez-de-Simon, J., Díaz-Ruiz, F., Cirilli, F., Sánchez Tortosa, F., Villafuerte, R., Delibes-Mateos, M., & Ferreras, P. (2011). Towards a standardized index of European rabbit abundance in Iberian Mediterranean habitats. European Journal of Wildlife Research, 57, 1091–1100.
Fernández-de-Simón, J., Díaz-Ruiz, F., Villafuerte, R., Delibes-Mateos, M., & Ferreras, P. (2011). Assessing predictors of pellet persistence in European rabbits Oryctolagus cuniculus: Towards reliable population estimates from pellet counts. Wildlife Biology, 17, 317–325.
Flux, J. C., & Fullagar, P. (1992). World distribution of the rabbit Oryctolagus cuniculus on islands. Mammal Review, 22(3–4), 151–205.
Garzón-Machado, V., González-Mancebo, J. M., Palomares, A., Acevedo, A., Fernández-Palacios, J. M., Del Arco, M., & Pérez-de-Paz, P. L. (2010). Strong negative effect of alien herbivores on endemic legumes of the Canary pine forest. Biological Conservation, 143, 2685–2694.
Gibb, J. A. (1993). Sociality, time and space in a sparse population of rabbits (Oryctolagus cuniculus). Journal of Zoology, 229, 581–607.
González-Mancebo, J. M., Cubas, J., Bello-Rodríguez, V., & del Arco, M. (2019). Cinco siglos de invasión del conejo europeo (Oryctolagus cuniculus L.) en ecosistemas áridos y secos de Tenerife. Vieraea, 46, 597–624.
Grueber, C. E., Nakagawa, S., Laws, R. J., & Jamieson, I. G. (2011). Multimodel inference in ecology and evolution: challenges and solutions. Journal of Evolutionary Biology, 24, 699–711.
Guerrero-Casado, J., Ström, H., Hilström, L., Prada, L., Carpio, A. J., & Tortosa, F. S. (2020). Assessment of the suitability of latrine counts as an indirect method by which to estimate the abundance of European rabbit populations at high and low abundance. European Journal of Wildlife Research, 66, 10. https://doi.org/10.1007/s10344-019-1349-4
Hess, S. C., & Jacobi, J. C. (2011). The history of mammal eradications in Hawai’I and the United States associated islands of the Central Pacific. In C. R. Veitch, M. N. Clout, & D. R. Towns (Eds.), Island invasives: Eradication and management (pp. 67–73). IUCN.
Iborra, O. L., & Lumaret, J. P. (1997). Validity limits of the pellet group counts in wild rabbit (Oryctolagus cuniculus). Mammalia, 61, 205–218.
Irl, S. D. H., Steinbauer, M. J., Babel, W., Beierkuhnlein, C., Blume-Werry, G., Messinger, J., Palomares Martínez, A., Strohmeier, S., & Jentsch, A. (2012). An 11-yr exclosure experiment in a high-elevation island ecosystem: Introduced herbivore impact on shrub species richness, seedling recruitment and population dynamics. Journal of Vegetation Science, 23, 1114–1125.
Kontsiotis, J., Bakaloudis, E., & Liordos, V. (2017). Impact of European wild rabbits foraging in different habitat and vegetation types in an insular environment. Mammalia, 82(2), 193–196.
Kreft, H., Jetz, W., Mutke, J., Kier, G., & Barthlott, W. (2008). Global diversity of island floras from a macroecological perspective. Ecology Letters, 11, 116–127.
Lees, A., & Bell, D. J. (2008). A conservation paradox for the 21st century: The European wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal Review, 38, 304–320.
Lombardi, L., Fernández, N., Moreno, A., & Villafuerte, R. (2003). Habitat-related differences in rabbit (Oryctolagus cuniculus) abundance, distribution, and activity. Journal of Mammalogy, 84(1), 26–36.
Lowe, S., Browne, M., Boudjelas, S., & De Poorter, M. (2004). 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database. In: The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN) New Zealand (p. 12).
Martín, J., Bethencourt, J., & Cuevas-Agulló, E. (2012). Assessment of global warming on the island of Tenerife, Canary Islands (Spain). Trends in minimum, maximum and mean temperatures since 1944. Climatic Change, 114, 343–355.
Martín, J., Marrero, M., del Arco, M., & Garzón-Machado, V. (2015). Aspectos clave para un plan de adaptación de la biodiversidad terrestre de Canarias al cambio climático. In A. Herero-Méndez & M. de Zavala-Gironés (Eds.), Los bosques y la biodiversidad frente al cambio climático: Impactos, vulnerabilidad y adaptación en España (pp. 573–580). Ministerio de Agricultura.
Mutze, G., Bird, P., Peacock, D., Jennings, S., & Cooke, B. (2002). Emerging epidemiological patterns in rabbit haemorrhagic disease and myxomatosis and their effects on rabbit populations in South Australia. Wildlife Research, 29, 577–590.
Mutze, G., Cooke, B., & Jennings, S. (2016). Density-dependent grazing impacts of introduced European rabbits and sympatric kangaroos on Australian native pastures. Biological Invasions, 18, 2365–2376.
Mutze, G., Cooke, B., Lethbridge, M., & Jennings, S. (2014). A rapid survey method for estimating population density of European rabbits living in native vegetation. Rangeland Journal, 36, 239–247.
Myers, N., Mittermeier, R., Mittermeier, C., da Fonseca, G., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858.
Nogales, M., Nieves, C., Illera, J. C., Padilla, D. P., & Traveset, A. (2005). Effect of native and alien vertebrate frugivores on seed viability and germination patterns of Rubia fruticosa (Rubiaceae) in the eastern Canary Islands. Functional Ecology, 19, 429–436.
Nogales, M., Rodríguez, J. L., & Marrero, P. (2006). Ecological effects and distribution of invasive non-native mammals on the Canary Islands. Mammal Review, 36(1), 49–65.
Oksanen, J., Guillaume-Blanchet, F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P. R., O'Hara, R. B., Simpson, G. L., Solymos, P., Stevens, M. H. H., Szoecs, E., & Wagner, H. (2018). vegan: Community Ecology Package. R package version 2.5-1.
Palomares, F. (2001). Comparison of 3 methods to estimate rabbit abundance in a mediterranean environment. Wildlife Soc B, 29, 578–585.
Penteriani, V., Kuparinen, A., del Mar, D. M., Palomares, F., López-Bao, J. V., Fedriani, J. M., & Lourenço, R. (2013). Responses of a top and a meso predator and their prey to moon phases. Oecologia, 173, 753–766.
Putman, R. J. (1984). Facts from faeces. Mammal Review, 14, 79–97.
R Core Team. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rangeley, R. W., & Kramer, D. L. (1998). Density dependent antipredator tactics and habitat selection in juvenile Pollock. Ecology, 79, 943–952.
Rivas-Martínez, S. (1997). Syntaxonomical synopsis of the North American natural potential vegetation communities I. Itinera Geobot. 10: 5–148 (Bioclimatic classification system of the World: 10–11).
Rocha, R., Sequeira, M. M., Douglas, L. R., Gouveia, M., Jardim, R., Jesus, J., Jones, P., & Russo, D. (2017). Extinctions of introduced game species on oceanic islands: Curse for hunters or conservation opportunities? Biodiversity and Conservation, 26(10), 2517–2520.
Rouco, C., Santoro, S., Delibes-Mateos, M., & Villafuerte, R. (2016). Optimization and accuracy of faecal pellet count estimates of population size: The case of European rabbits in extensive breeding nuclei. Ecological Indicators, 64, 212–216.
Saefken, B., Kneib, T., van Waveren, C. S., & Greven, S. (2014). A unifying approach to the estimation of the conditional Akaike information in generalized linear mixed models. Electronic Journal of Statistics, 8(1), 201–225.
Saldaña, A., García-Salgado, G., & Rebollo, S. (2007). European rabbit (Oryctolagus cuniculus L.) abundance at a regional scale: controlling factors. In Bunce, R. G. H., Jongman, R. H. G., & Hojas, L., & Wee, S. (Eds.) 25 Years of Landscape Ecology: Scientific Principles in Practice (l), Proceedings of the 7th IALE World Congress. Wageningen, The Netherlands (pp. 375–376).
Smith, A. T., & Boyer, A. F. (2008). Oryctolagus cuniculus. The IUCN Red List of Threatened Species 2008:e.T41291A10415170.
Steinbauer, M. J., Field, R., Grytnes, J. A., Trigas, P., Ah-Peng, C., Attorre, F., Birks, H. J. B., Borges, P. A. V., Cardoso, P., Chou, C. H., De Sanctis, M., de Sequeira, M. M., Duarte, M. C., Elias, R. B., Fernández-Palacios, J. M., Gabriel, R., Gereau, R. E., Gillespie, R. G., Greimler, J., … Beierkuhnlein, C. (2016). Topography-driven isolation, speciation and a global increase of endemism with elevation. Global Ecology and Biogeography, 25(9), 1097–1107.
Sugimura, K., Sato, S., Yamada, F., Abe, S., Hirakawa, H., & Handa, Y. (2000). Distribution and abundance of the Amami rabbit Pentalagus furnessi in the Amami and Tokuno Islands, Japan. Oryx, 34, 198–206.
Sundseth, K. (2005). Natura 2000 in the Macaronesian region. Luxembourg (p. 12).
Thompson, H. V., & King, D. C. M. (1994). The European rabbit: The history and biology of a successful colonizer. Oxford University Press.
Turkheimer, F. E., Hinz, R., & Cunningham, V. J. (2003). On the undecidability among kinetic models: From model selection to model averaging. Journal of Cerebral Blood Flow & Metabolism, 23, 490–498.
Villafuerte, R., & Delibes-Mateos, M., et al. (2008). El Conejo. In L. Palomo & J. Gisbert (Eds.), Atlas y Libro Rojo de los Mamíferos Terrestres de España (pp. 490–491). Spain Dirección General para la Biodiversidad-SECEM-SECEMU.
Villafuerte, R., Kufner, M., Delibes, M., & Moreno, S. (1993). Environmental factors influencing the seasonal daily activity of the European rabbit (Oryctolagus cuniculus) in a Mediterranean area. Mammalia, 57(3), 341–348.
Villafuerte, R., Lazo, A., & Moreno, S. (1997). Influence of food abundance and quality on rabbit fluctuations: Conservation and management implications in Doñana National Park (SW Spain). Revue d’écologie, 52, 345–356.
Villafuerte, R., & Moreno, S. (1997). Predation risk, cover type, and group size in European rabbits in Doñana (SW Spain). Acta Theriologica, 42, 225–230.
Wood, D. H. (1988). Estimating rabbit density by counting dung pellets. Australian Wildlife Research, 15, 665–667.
Acknowledgements
We thank Julio Leal Pérez, Raquel Hernández-Hernández and Jesús Parada Díaz for their great help in obtaining data in the field. In addition, we thank Ángel Palomares Martínez (Caldera de Taburiente N.P.), Ángel Fernández López (Garajonay N.P.), José Luís Martín Esquivel (El Teide N.P.) and Luís Pascual González (Timanfaya N.P.) for their help during this work and providing us with all kinds of facilities for the development of this study. Moreover, we thank TRAGSATEC for helping during this work in Caldera de Taburiente N.P. We also thank the ‘Ministerio para la Transición Ecológica’ (REF 1621/2015) for financial support. Jonay Cubas has received a PhD scholarship from La Laguna University. We also thank Clive Tyrell for the English revision of original manuscript.
Author information
Authors and Affiliations
Contributions
J.M.G.M. designed the study; all authors collected the data; J.C. and J.K. analysed the data; J.C., J.K, B.C. and J.M.G.M. lead the writing with all co-authors contributing. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cubas, J., Kluge, J., Bello-Rodríguez, V. et al. Can habitat type predict the abundance of the European rabbits on oceanic islands?. COMMUNITY ECOLOGY 22, 93–105 (2021). https://doi.org/10.1007/s42974-021-00039-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-021-00039-6