Abstract
We investigate the feeding responses of the red fox (Vulpes vulpes) at a regional scale to different densities of European wild rabbit (Oryctolagus cuniculus) in central–southern Spain. Rabbit abundance indices were obtained in 86 localities during summer 2002. The diet of the fox was studied by analysis of 114 scats collected in 47 of these localities. The feeding response of the fox was examined by a representation of the dry weight percent of rabbit in the diet as a function of the abundance of rabbits; this used data only from those localities where at least 3 scats were collected (70 fox scats from 18 localities). We evaluated the relationship between rabbit abundance and the diversity of the diet of the fox. The feeding patterns of red foxes approximated to Holling’s type III functional response, typical of opportunistic predators. There was a negative relationship between the diversity of the fox’s diet and the abundance of rabbits. Therefore, the fox apparently behaves as a facultative predator, feeding on rabbits when they are abundant and shifting to other prey (and hence a more diverse diet) when rabbits are scarce. These findings are the first step towards understanding the potential role of red foxes in regulating rabbit populations in central–southern Spain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European wild rabbit (Oryctolagus cuniculus) is considered a keystone species in Iberian Mediterranean ecosystems (Delibes-Mateos et al. 2007) because it is the most important prey for vertebrate predators (Delibes and Hiraldo 1981) and because it also exerts a strong influence on the habitats of other species through consumption of vegetation, seed dispersal and burrowing (Soriguer 1986; Gómez-Sal et al. 1999; Dellafiore et al. 2006). Over the past 50 years, rabbit populations have suffered a dramatic reduction on the Iberian Peninsula, mainly as a result of the arrival of two viral diseases: myxomatosis in the 1950s (Muñoz 1960) and rabbit haemorrhagic disease (RHD) at the end of the 1980s (Villafuerte et al. 1995).
Rabbit specialist predators such as the Iberian lynx (Lynx pardinus) or the Spanish imperial eagle (Aquila adalberti) have become seriously endangered mainly as a consequence of habitat fragmentation and the decline in rabbit numbers (Ferrer 2001; Palomares et al. 2001). The diminution of abundance of top predators together with habitat fragmentation have been cited as two of the factors that have led to an alarming increase in generalist species (Palomares et al. 1995; Storch et al. 2005; respectively). The red fox (Vulpes vulpes) is the most widespread generalist predator on the Iberian Peninsula (Blanco 1998) where, as in other Mediterranean areas, rabbits are the most consumed prey species when they are available (Amores 1975; Reynolds 1979; Blanco 1988). Despite several studies of the feeding patterns of the red fox in Spain (e.g. Amores 1975; Fedriani 1996; Calzada 2000), little is known of the role of rabbits in the diet of red foxes at a regional scale with varying rabbit abundance.
The overall response of predators to changing prey populations has been divided into two components: the numerical and the functional responses (Solomon 1949). The numerical response describes how the whole predator population responds to changes in prey density, whilst the functional response describes the response of individual predators in terms of the number of prey eaten at different prey densities (Solomon 1949; Pech et al. 1992). Holling (1959) described three basic forms of functional response. Originally, Holling (1959) showed type I to increase linearly with prey density up to a threshold, above which the rate is constant. However, most authors refer to type I as linear without the asymptote (Turchin 2003; Fig. 1a). Type II responses describe prey consumption increasing at a monotonically decreasing rate with prey density (i.e. a convex curve approaching an asymptote; Fig. 1b) and type III responses are sigmoid in shape (Fig. 1c).
Functional responses according to Holling (1959). a type I, b type II, c type III
The aim of this paper is to analyse the feeding patterns of red foxes after the RHD outbreak in Spain by means of scat analysis and to use this information to explore the feeding responses of this carnivore to different rabbit densities. We estimate the percentage of rabbits in the diet of red foxes at several localities with different rabbit densities. We hypothesise that feeding patterns of red foxes approximate to Holling’s type III functional response, typical of generalist predators (Keith et al. 1977; Pech et al. 1992). Thus, it is expected that red foxes consume few rabbits at low rabbit densities, that they increase the consumption of rabbits at intermediate densities (density-dependent response) and that this consumption approaches an asymptote at high rabbit densities (Fig. 1). In addition, we evaluate the relationship between the diversity of the red fox diet and rabbit abundance. We expect the diet of the red fox to be more diverse in areas where rabbits are low in number because in these areas no single food is as profitable as rabbits, and foxes should replace them with several food items (Amores 1975; Blanco 1988).
Materials and methods
Rabbit abundances
Rabbit abundance indices were obtained in 86 localities in central–southern Spain (Fig. 2) during a survey performed in the summer of 2002. Fox scats were found in only 47 of these localities (Fig. 2), which were consequently considered for the study of predator–prey interactions. These localities were part of a more comprehensive survey performed in 1993 when over 300 plots were surveyed throughout the entire country (see for details Villafuerte et al. 1995, 1998). At each surveyed locality, 2 observers walked a 4 km transect, counting rabbit pellets within 0.47 m2 circular plots at 100 m intervals, avoiding counts on or near latrines. The densities of pellets counted in the 40 plots along each transect provide a pellet abundance index (pellets/m2). Pellet counts have been widely used to estimate rabbit abundance in Mediterranean environments and are adequate for broad-scale studies (Fa et al. 1999; Palomares 2001).
Diet analysis
Scat analysis was chosen because it is a non-invasive method and also because it is cheaper than stomach and gut analyses. All carnivore scats found along each transect were collected, stored in separate labelled paper bags, oven dried at 60°C for 48 h, weighed to the nearest 0.001 g with a precision scale and kept in plastic containers with naphthalene for later analysis. Of 150 carnivore scats collected during the rabbit abundance transects, 114 from 47 localities (Fig. 2) had been produced by red fox and were included in the analyses. The predator producing each scat was identified by scent, shape, size, thickness, fresh weight and dry weight, and also by identification of predator hairs (Teerink 1991); these usually appear in scats because of grooming.
Scats were analysed according to Reynolds and Aebischer (1991). In summary, scats were soaked in water and detergent for 24 h and then teased apart over a 0.5 mm sieve. The remains from each scat were spread in a Petri dish; this enabled quantification by percentage volume in a comparison of the different sizes of remains. Because of the special importance of rabbits in the study, we used keys to identify mammalian remains by their bones, teeth and hair (Day 1966; Gallego and Alemany 1985; Teerink 1991; Blanco 1998). We also quantified (% v/v, percent volume of un-digested remains for each food class regarding the total scat volume) the remains that could not be separated out. Information from separated and un-separated remains was used for a final quantification (% v/v see above for details) for each food class.
We considered the following diet items for the analysis: rabbit, hare, small mammals, ungulates, other mammals, birds, reptiles, arthropods, fruits and seeds and others. Those diet items that rarely occurred in the scats (for instance eggs, fungi or mollusks) and un-determined remains were included in the ‘others’ category.
The incidence of each food class was expressed in two ways (Table 1 of the Appendix): (1) % dry weight, considering the dry weight of each prey item as the product of the percent volume of each prey item and the scat dry weight and (2) % occurrence, considering the occurrence of each prey item as the proportion of scats that contains this prey item.
Analysis
Feeding patterns of the red fox
The feeding patterns of the red fox were examined by a representation of the percent dry weight of rabbit in the diet as a function of the abundance index of rabbit (Gil-Sánchez et al. 1999), the pellet abundance index (pellets/m2; see “Rabbit abundances” section for details). The feeding patterns of the red fox were fitted to different types of functional response (Holling 1959) by using the TableCurve 2D v5.01 software (AISN Software 1996). Hence, we calculated which of the three types of functional response best fitted the relationship between rabbit in the fox diet and variation in rabbit abundance.
To obtain the fit of the different types of the functional response, we used only those localities where at least 3 scats were collected (3 scats were collected in 9 localities, 4 scats in 5 localities and ≥5 scats in 4 localities). Hence, we used 70 scats of red foxes from 18 localities (Fig. 2). In each locality, we calculated the percent dry weight of rabbit remains in fox scats.
Diet diversity
We calculated the diet diversity of red foxes within 4 different situations of rabbit density: (1) rabbits virtually absent (0–2 rabbit pellets/m2, 31 fox scats); (2) low rabbit density (2–15 rabbit pellets/m2, 35 fox scats); (3) intermediate rabbit density (15–50 rabbit pellets/m2, 24 fox scats) and (4) high rabbit density (>50 rabbit pellets/m2, 25 fox scats). To calculate diet diversity, we used the Shannon diversity index (SDI; Weaver and Shannon 1949)
where p i is the proportion by dry weight of each prey item in the diet as the total dry weight of each prey item in the scats collected in one class of rabbit density/total dry weight of the scats collected at this rabbit density. To control for un-equal sample sizes and to provide a measure of precision of the estimates, average values and 95% confidence limits were derived from 1,000 random sub-samples of 30 scats with replacement for each rabbit density grouping (Reynolds and Aebischer 1991; Webbon et al. 2006).
Finally, we used Spearman’s rank correlation to test for a correlation between the SDI and the abundance of rabbits (see above).
Results
In total, 114 red fox scats were collected within the 86 rabbit abundance transects (average 1.32 scats/transect; range 0–8; SE = 0.17). The average index of rabbit abundance was 25.32 pellets/m2 (range 0–259.41; SE = 4.87).
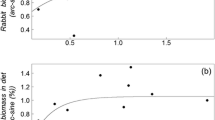
The feeding patterns of the red foxes best fitted to a sigmoid curve of Holling’s type III (see Fig. 3; type I: R 2 = 0.38, adjusted R 2 = 0.30, SE = 29.88; type II: R 2 = 0.71, adjusted R 2 = 0.67, SE = 29.88; type III: R 2 = 0.79, adjusted R 2 = 0.75, SE = 17.79). In other words, the feeding patterns approximated to a functional response of Holling’s type III, consuming very few rabbits at low rabbit density, increasing the consumption of rabbits at intermediate rabbit densities (density-dependent response) and approaching an asymptote at high rabbit densities (Fig. 3c).
Functional response of the red fox to changes in rabbit density (% dry weight of rabbits in diet). a type I functional response \( Y = 21.4 + 0.35x \); b type II functional response \( Y = \frac{{5.26x}} {{1 + 0.05x}} \); c type III functional response \( Y = \frac{{74.61}} {{1 + \exp {\left( {\frac{{ - {\left( {x - 9.53} \right)}}} {{0.52}}} \right)}}} \)
Regarding the diversity of the fox diet, we have observed a negative relationship between the SDI and the abundance of rabbits (Spearman’s R = −1; p < 0.001). Thus, the lowest value of the SDI was obtained in areas of high rabbit density, whereas the highest diversity was observed in areas of low rabbit density (Fig. 4).
Diet diversity of red foxes within 4 densities of rabbit: rabbits virtually absent (0–2 rabbit pellets/m2), low rabbit density (2–15 rabbit pellets/m2), intermediate rabbit density (15–50 rabbit pellets/m2) and high rabbit density (>50 rabbit pellets/m2). The average value and 95% confidence limits derived from 1,000 random sub-samples of 30 scats with replacement (Reynolds and Aebischer 1991; Webbon et al. 2006) are represented for each rabbit density
Discussion
According to our results, the fox responded as an opportunistic predator to changing densities of rabbits. In other words, foxes feed upon rabbits according to their availability with a strong tendency to feed on rabbits where the species is more abundant.
There are several potential biases associated with scat analysis that could affect functional response results: sampling biases, the amount of diagnostic material a prey species contributes to a scat (differential detectability) and equating more than one prey item in a scat (Bartel and Knowlton 2005 and references cited therein). Although we acknowledge such biases, this study did not specifically address most of them. One of the main limitations of our work was that we did not know the numbers of prey consumed and therefore could not fully evaluate functional response. However, changes in diet composition in conjunction with changes in prey availability provide an accurate measure of the shape of the functional response (Bartel and Knowlton 2005). Another limitation of our study is the low number of scats collected within some localities. Collecting too few scats increases the likelihood of not finding a species in scat that is consumed in low numbers (Trites and Joy 2005). From this perspective, we could under-estimate the percentage of rabbits consumed by foxes in areas where this prey is scarce. However, if this has occurred in some areas, the percentage of rabbits in the fox’s diet would not be very large because it is widely known that foxes rarely consume rabbits under this scenario (e.g. Amores 1975). On the other hand, although the sample size within each locality was small, we sampled extensively (although not with an intensive sampling effort) and simultaneously within the whole range of rabbit densities, from very high to virtual absence; this is not common in studies of functional response.
Opportunistic carnivores generally show a type III functional response sensu Holling because they can capture alternative prey (Keith et al. 1977; Akre and Johnson 1979; Markstrom et al. 1988; Pech et al. 1992; Revilla and Palomares 2002; Bartel and Knowlton 2005). According to our results, the best fit of feeding patterns to Holling’s type III functional response (Fig. 3c) confirms the red fox as an opportunistic predator. For instance, the Iberian lynx and other selective carnivores (e.g. the predation of Lynx lynx on the snowshoe hare Lepus americanus, Keith et al. 1977) show a type II functional response because its diet is based mainly on only one prey, even when prey abundance is low (Calzada 2000). Therefore, it is not surprising that our results show that the feeding pattern of the red fox is closer to a type III response than to a convex curve characterising a type II response (Figs. 3b,c). In addition, our findings suggest that the feeding pattern of the fox is far from a type I linear response (Fig. 3a), probably because this type of linear response rarely occurs in nature and it has even been suggested as being exclusive to filter feeders (Jeschke et al. 2004).
A type III functional response can be caused by prey switching at low prey densities (Taylor 1984). In other words, opportunistic predators are able to find alternative prey when the primary resource becomes scarce (Akre and Johnson 1979; Markstrom et al. 1988; Pech et al. 1992; Revilla and Palomares 2002; Bartel and Knowlton 2005). In this study, we have observed that even when rabbits were at high densities, they were the main prey; foxes rarely consumed rabbits in areas where this prey was present at low densities (Fig. 3). In those areas where rabbits are scarce, red foxes replace rabbits by several other food items such as small mammals, birds, invertebrates, fruits or carrion (Table 1; see also Amores 1975). As optimal foraging theory predicts, the main reason for this switching behaviour of predators is that they prefer those prey that provide the greatest return on investment of handling time (Rapport 1971). Similar findings regarding prey switching have previously been reported for other opportunistic predators, either invertebrates (Akre and Johnson 1979) or vertebrates (Malo et al. 2004).
The type III curve is sigmoid, which means that the slope increases over low prey densities; this implies that a larger proportion of the population is taken per predator over low to intermediate prey densities (Boutin 1995). Thus, prey species are rarely consumed until their abundance reaches a certain threshold. In the present study, the predation of foxes on rabbits increased when rabbits reached a threshold at moderate densities (Fig. 3). After the increasing slope at intermediate prey densities, the curve approaches an asymptote at high prey densities because both satiation and handling time limit the number of prey eaten per predator per unit time (Sinclair and Pech 1996). Thus, although the food spectrum of the fox is very wide, its predation is centred fundamentally on the rabbit when this species is abundant (Fig. 3). Our results agree with previous studies (Amores 1975; Newsome et al. 1989). In these localities, foxes find an ideal prey in rabbits because of their abundance and size, in contrast to other less abundant prey with a lower energetic yield due to their smaller size or the difficulty of their capture (Amores 1975).
Regarding the diversity of the diet of the fox, we observed a negative relationship between the SDI and the abundance of rabbits (Fig. 4). This negative relationship between diet diversity and prey group has been interpreted as a good indicator of trophic specialisation (Fedriani et al. 1998; but see Revilla and Palomares 2002). According to this interpretation, foxes should be considered as rabbit specialists, but optimal foraging principles suggest that if foxes are rabbit specialists they should feed on rabbits irrespective of the availability of alternative prey (Glasser 1982). The observed shift in our study from rabbits to other prey when the former were scarce does not support the rabbit specialisation hypothesis. Thus, foxes apparently behave as a facultative predator in their consumption of rabbits with a specialisation in rabbits when they are abundant and a shift to other prey (and hence a more diverse diet) when rabbits are scarce (Fig. 3c). A similar pattern has also been reported in other Mediterranean predators such as the Eurasian badger (Meles meles, Revilla and Palomares 2002) and the wildcat (Felis silvestris; Malo et al. 2004).
The role of predators in regulating prey populations has been debated over many years (Erlinge et al. 1984). As regards rabbits, predator pressure can be a potential regulating factor, especially at low rabbit densities (Trout and Tittensor 1989; Banks 2000). After the outbreak of RHD in central–southern Spain, some populations dropped dramatically and even some cases of local extinction occurred (Blanco and Villafuerte 1993). Therefore, under certain levels of rabbit numbers, predator pressure could be preventing rabbit population recovery—the “predator pit” hypothesis (Trout and Tittensor 1989; Pech et al. 1992). In a recent research, Williams et al. (2007) found that rabbit population trends in north-eastern Spain were not correlated with fox density. Therefore, they speculated that foxes are probably not regulating rabbit populations in this region. However, to understand to what extent predators regulate their prey, it is necessary to study the total response (numerical and functional) of predators to changing prey populations. Consequently, exploring the functional response of red foxes through the study of the relationship between their diet and the abundance of rabbits is the first step towards understanding the potential role of red foxes in regulating rabbit populations. In this study, predation by foxes on rabbit populations was density-dependent over low prey densities (Fig. 3c). Therefore, predation could be regulating the prey around a lower equilibrium at moderate prey densities. However, further work should be encouraged to study in depth the effects of predation by foxes on rabbit populations. Experimental studies are required to investigate the potential regulation that foxes could exert on rabbit recovery after the RHD outbreak in Spain. The numerical response of foxes to different rabbit densities should also be studied to estimate the total response and hence the likely role of red foxes in the regulation of rabbit populations on the Iberian Peninsula.
References
Akre BG, Johnson DM (1979) Switching and sigmoid functional response curves by damselfly naiads with alternative prey available. J Anim Ecol 48:703–720
Amores F (1975) Diet of the red fox (Vulpes vulpes) in the western Sierra Morena (South Spain). Doñana Acta Vertebr 2:221–240
Banks PB (2000) Can foxes regulate rabbit populations? J Wildl Manage 64:401–406
Bartel RA, Knowlton FF (2005) Functional feeding responses of coyotes, Canis latrans, to fluctuating prey abundance in the Curlew Valley, Utah, 1977–1993. Can J Zool 83:568–578
Blanco JC (1988) Estudio ecológico del zorro, Vulpes vulpes (L., 1758), en la sierra de Guadarrama. Ph.D. thesis, University of Oviedo
Blanco JC (1998) Mamíferos de España. Editorial Planeta, Barcelona
Blanco JC, Villafuerte R (1993) Factores ecológicos que influyen sobre las poblaciones de conejos. Incidencia de la enfermedad hemorrágica. Technical report, Empresa de Transformación Agraria, S.A., Madrid
Boutin S (1995) Testing predator–prey theory by studying fluctuating populations of small mammals. Wildl Res 22:89–100
Calzada J (2000) Impacto de la depredación y selección de presa del lince y el zorro sobre el conejo. Ph.D. thesis, University of León
Day MG (1966) Identification of hair and feather remains in the gut and faeces of stoats and weasels. J Zool 148:201–217
Delibes M, Hiraldo F (1981) The rabbit as prey in the Iberian Mediterranean ecosystem. In: Myers K, MacInnes CD (eds) Proceedings of the World Lagomorph Conference 1979. University of Guelph, Ontario, Canada, pp 614–622
Delibes-Mateos M, Redpath, SM, Angulo E, Ferreras P, Villafuerte R (2007) Rabbits as a keystone species in southern Europe. Biol Conserv 137:149–156
Dellafiore CM, Muñoz Vallés S, Gallego Fernández JB (2006) Rabbits (Oryctolagus cuniculus) as dispersers of Retama monosperma seeds in a coastal dune system. Ecoscience 13:5–10
Erlinge S, Göransson G, Högstedt G, Jansson G, Liberg O, Loman J, Nilsson IN, von Schantz T, Sylven M (1984) Can vertebrate predators regulate their prey? Am Nat 123:125–133
Fa JE, Sharples CM, Bell DJ (1999) Habitat correlates of European rabbit (Oryctolagus cuniculus) distribution after the spread of RVHD in Cadiz Province, Spain. J Zool 249:83–96
Fedriani JM (1996) Dieta anual del zorro, Vulpes vulpes, en dos hábitats del Parque Nacional de Doñana. Doñana Acta Vertebr 23:143–152
Fedriani JM, Ferreras P, Delibes M (1998) Dietary response of the Eurasian badger, Meles meles, to a decline of its main prey in the Doñana National Park. J Zool 245:214–218
Ferrer M (2001) The Spanish imperial eagle. Lynx Editions, Barcelona
Gallego L, Alemany A (1985) Vertebrados Ibéricos, 6. Roedores y Lagomorfos. Antiga Imprenta Soler, Palma de Mallorca
Gil-Sánchez JM, Valenzuela G, Sánchez JF (1999) Iberian wild cat Felis silvestris tartessia predation on rabbit Oryctolagus cuniculus: functional response and age selection. Acta Theriol 44:421–428
Glasser JW (1982) A theory of trophic strategies: the evolution of facultative species. Am Nat 119:250–262
Gómez-Sal A, Rey-Benayas JM, López-Pintor A, Rebollo S (1999) Role of disturbance in maintaining a savanna-like pattern in Mediterranean Retama sphaerocarpa shrubland. J Veg Sci 10:365–370
Holling CS (1959) The functional components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Jeschke JM, Kopp M, Tollrian R (2004) Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol Rev 79:337–349
Keith LB, Todd AW, Brand CJ, Adamcik RS, Rusch DH (1977) An analysis of predation during a cyclic fluctuation of snowshoe hares. Proceedings of the International Congress of Game Biologists 13:151–175
Malo AF, Lozano J, Huertas DL, Virgós E (2004) A change of diet from rodents to rabbits (Oryctolagus cuniculus): is the wildcat (Felis silvestris) a specialist predator? J Zool 263:401–407
Markstrom V, Kenward RE, Engren E (1988) The impact of predation on boreal tetraonids during vole cycles: an experimental study. J Anim Ecol 57:859–872
Muñoz G (1960) Anverso y reverso de la mixomatosis. Dirección General de Montes, Caza y Pesca Fluvial, Madrid
Newsome AE, Parer, Catling PC (1989) Prolonged prey suppression by carnivores—predator-removal experiments. Oecologia 78:458–467
Palomares F (2001) Comparison of 3 methods to estimate rabbit abundance in Mediterranean environment. Wildl Soc Bull 29:578–585
Palomares F, Gaona P, Ferreras P, Delibes M (1995) Positive effects on game species of top predators by controlling smaller predator populations: an example with lynx, mongooses and rabbits. Conserv Biol 9:295–305
Palomares F, Delibes M, Revilla E, Calzada J, Fedriani JM (2001) Spatial ecology of the Iberian lynx and abundance of European rabbits in southwestern Spain. Wildl Monogr 148:1–36
Pech RP, Sinclair ARE, Newsome AE, Catling PC (1992) Limits to predator regulation of rabbits in Australia: evidence from predator removal experiments. Oecologia 89:102–112
Rapport DJ (1971) An optimization model for food selection. Am Nat 105:575–587
Revilla E, Palomares F (2002) Does local feeding specialization exist in Eurasian badgers? Can J Zool 80:83–93
Reynolds P (1979) Preliminary observation on the food of the fox in the Camargue, with special reference to rabbit predation. Mammalia 43:295–307
Reynolds J, Aebischer N (1991) Comparison and quantification of carnivore diet by faecal analysis: a critique with recommendations based on a study of the Fox Vulpes vulpes. Mamm Rev 21:97–122
Sinclair AER, Pech RP (1996) Density dependence, stochasticity, compensation and predator regulation. Oikos 75:164–173
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35
Soriguer RC (1986) The rabbit as a plant seed disperser. Mamm Rev 16:197–200
Storch I, Woitke E, Krieger S (2005) Landscape-scale edge effect in predation risk in forest–farmland mosaics of central Europe. Landsc Ecol 20:927–940
Taylor RJ (1984) Predation. Chapman and Hall, London
Teerink BJ (1991) Hair of west-European mammals. Cambridge University Press, Cambridge
Trites AW, Joy R (2005) Dietary analysis from fecal samples: how many scats are enough? J Mammal 86:704–712
Trout RC, Tittensor AM (1989) Can predators regulate wild rabbit Oryctolagus cuniculus population density in England and Wales? Mamm Rev 19:153–173
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, Princeton, New York
Villafuerte R, Calvete C, Blanco JC, Lucientes J (1995) Incidence of viral hemorrhagic disease in wild rabbit populations in Spain. Mammalia 59:651–659
Villafuerte R, Viñuela J, Blanco JC (1998) Extensive predator persecution caused by population crash in a game species: the case of red kites and rabbits in Spain. Biol Conserv 84:181–188
Weaver W, Shannon CE (1949) The mathematical theory of communication. Illinois University Press, Urbana, Illinois
Webbon CC, Baker PJ, Cole NC, Harris S (2006) Macroscopic prey remains in the winter diet of foxes Vulpes vulpes in rural Britain. Mamm Rev 36:85–97
Williams D, Acevedo P, Gortazar C, Escudero MA, Labarta JL, Marco J, Villafuerte R (2007) Hunting for answers: rabbit (Oryctolagus cuniculus) population trends in northeastern Spain. Eur J Wildl Res 53:19–28
Acknowledgments
MDM was supported by a I3P grant funded by the European Social Fund through the “Consejo Superior de Investigaciones Científicas” (CSIC). JFS was supported by a FPI grant from the Spanish Ministry of Education and Science. Funding was provided by the projects CGL 2005-02340/BOS by the Spanish Ministry of Education and Science and PAI06-170 by the regional government of Castilla-La Mancha. We are indebted to Dr. J.L. Yela and three anonymous referees for the helpful comments on previous drafts of the manuscript. The authors would also like to thank “International Science Editing” for improving the English version. Special thanks go to G. Calabuig, J. Castillo, P. Castro, A. Finque, A. Linares, S. Luna, J. Martínez, L. Mínguez, M. Reglero, O. Rodríguez, C. Rouco, J. Viñuela and E. Virgós for the field assistance during rabbit surveys.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Lutz
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Delibes-Mateos, M., Fernandez de Simon, J., Villafuerte, R. et al. Feeding responses of the red fox (Vulpes vulpes) to different wild rabbit (Oryctolagus cuniculus) densities: a regional approach. Eur J Wildl Res 54, 71–78 (2008). https://doi.org/10.1007/s10344-007-0111-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-007-0111-5