Abstract
Cellular response to TBI is a mixture of excitotoxicity, neuroinflammation, and cell death. Biomarkers that can track these lesions and inflammatory processes are being explored for their potential to provide objective measures in the evaluation of TBI, from prehospital care to rehabilitation. By understanding the pathways involved, we could be able to improve diagnostic accuracy, guide management, and prevent long-term disability. We listed some of the recent advances in this translational, intriguing, fast-growing field. Although the knowledge gaps are still significant, some markers are showing promising results and could be helping patients in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
American statistics report 2.8 million medical emergency assessments for traumatic brain injuries (TBI) annually. In middle- and low-income countries, the incidence of TBI is even higher, corresponding to the leading cause of death and disability in young adults. Even mild trauma (mTBI), which accounts for 80 to 90% of all injuries, can be responsible for long-term damage. [16, 28]
Cellular response to TBI is a mixture of excitotoxicity, neuroinflammation, and cell death. Biomarkers that can track these lesions and inflammatory processes are being explored for their potential to provide objective measures in the evaluation of TBI, from prehospital care to rehabilitation. By understanding the pathways involved, we could be able to improve diagnostic accuracy, guide management, and prevent long-term disability. [31] We propose a short review of recent advances on biomarkers for TBI, translating from a physiological point of view to their potential usefulness.
Materials and Methods
We searched PubMed and Google Scholar, using the terms “traumatic brain injury” and “biomarkers.” Additional studies were sought through snowballing. Priority was given to studies in humans and recent publications (last 5 years). Only English language papers were accepted. Articles were analyzed by title and abstract for inclusion in this review.
Results
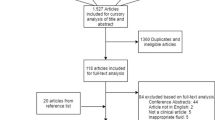
Filtering by the last 5 years, we found 1406 articles in PubMed and 347 in Google Scholar. Among them, a pre-selection screened articles by their titles, considering those that were more appropriate to this review. We analyzed their references, identifying twelve other relevant papers (from 2004 to 2013). The final selection included 40 articles, including 2 randomized trials, 6 observational studies, 10 case-control studies, 6 cohort studies, 1 report, and 15 reviews.
Discussion
Copeptin
The C-terminal part of the vasopressin arginine prohormone, or copeptin, is used to document the existence of a brain concussion, reflecting the pituitary-mediated stress response. The serum levels of copeptin increase proportionally to the severity of TBI, potentially allowing to evaluate the degree of injury in a situation where there are no imaging findings.[15]
GFAP
Glial fibrillary acid protein (GFAP) is a structural astrocyte protein released in the CSF/serum in the acute posttraumatic phase, indicating cellular membrane injury. Levels of GFAP and its breakdown products can also serve as parameters to determine TBI severity. Elevations in CSF GFAP within 3–34 h after trauma and posterior elevation of serum levels correlate with severe TBI (sTBI); conversely, in mTBI, only in serum levels rise, peaking at around 24 h after trauma. Increases in serum GFAP may predict low CPP, elevated ICP and mortality.[8]
GFAP could also help determine patients with mTBI who would benefit from imaging studies. A study comparing the predictive value of multiple biomarkers for TBI-related CT abnormalities identified that GFAP was the best choice. [1, 6, 17, 29, 32]
UCH-L1
Ubiquitin C-terminal hydrolase-L1 (UCH-L1), a deubiquitinating enzyme that corresponds to 10% of all neuronal proteins, is an injury marker expressed not only by the CNS, but also in the peripheral nervous system, endocrine system, tumors, and muscles. While GFAP represents astrocyte damage, UCH-L1 is a surrogate of neuronal damage. [1, 29, 32, 36]
Increased UCH-L1 serum levels can be identified in cases of concussion, mTBI, and sTBI. In severe cases, however, elevations can last longer and can also be detected in CSF. Studies related to UCH-L1 have demonstrated that this marker presents high sensitivity to predict intracranial lesions.
Evaluation of this parameter within the first 6 h post-trauma can increase its predictive value for head CT findings, also providing more accurate information about the injury severity. Due to its low specificity, UCH-L1 is more useful as a screening to avoid unneeded CT scans. In mTBI, the association between UCH-L1 and GFAP analysis may aid this decision.[1, 17, 20, 29, 32]
Tau protein
Great attention has been given to the axonal phosphoprotein named Tau, which helps modulating microtubule stability. It is mostly expressed in axons, and also non-neural tissues, such as the liver, kidneys, and testis. One of its isoforms, “Big” tau, is expressed by peripheral nerves and muscles.
Neurodegenerative disorders known as tauopathies are characterized by increased levels of tau inclusions inside neurons and glial cells. Processes of axonal injury, neuronal loss, and cellular toxicity occur in TBI and may stimulate abnormal phosphorylation and tau aggregation to a proportional extent.[1, 22, 23, 29]
Studies with athletes of contact sports show that exposure to repetitive mTBI increases the risk of chronic traumatic encephalopathy (CTE), which is also related to abnormal accumulation of hyperphosphorylated tau protein (P-tau) in neurons and glia.[22, 23, 29]
All severity degrees of TBI have already been linked to higher levels of P-tau, both in serum and CSF samples, particularly in the acute phase. After sTBI, both CSF and serum levels of total tau (T-tau) and P-tau are elevated, decreasing over time until stabilization. Serum P-tau levels tend to remain higher in the long-term, while serum T-tau levels return to normal when compared to controls.[6, 23]
P-tau and P-tau/T-tau ratio are superior to T-tau alone for diagnosing and grading acute TBI. However, one study did not detect increased circulating tau after concussions among soccer players. Such divergent findings highlight the need for a better understanding of how trauma intensity, sample collection and analyses, and other confounders can interfere with the results related to tau levels.[23, 24]
Tau could also be useful to assess the need for imaging after mTBI and to estimate prognosis, particularly the development of CTE or AD similar diseases in the long-term. Higher levels of T-Tau, P-Tau, and P-Tau/T-Tau ratio were identified among TBI patients with positive CT findings and signaled worse prognosis. Notably, P-tau levels and P-tau/T-tau ratio showed a better accuracy to discriminate CT abnormalities.[1, 22, 23, 29]
BDNF
Brain-derived neurotrophic factor (BDNF) is another potential biomarker for TBI assessment. This neurotrophin is secreted from neuronal and glial cells, playing an essential role in neuroplasticity, neurogenesis, and anti-inflammatory responses. Lower serum BDNF concentrations within 36 h after trauma is proportionally related to higher TBI severity.[7, 35]
There is evidence that BDNF may also accurately predict patient recovery, especially in mTBI. During the first hours after trauma, there is a transient overexpression of the mRNA related to BDNF and its receptor. These changes have been detected in specific brain areas, such as the hippocampus, injured cortex, and dentate gyrus. Cognitive decline after TBI in association with reduced expression of these mRNA was identified in adult rats. BDNF may play an important role in preventing secondary injuries after TBI, as well as recovering the primarily injured areas.[7, 35]
The microtubule-associated protein 2 (MAP-2) is abundant in the brain, with high specificity for dendritic injury. Its CSF levels early after TBI could predict 2-week mortality in sTBI. In terms of diagnosis, serum levels of MAP-2 > 0.25 ng/mL demonstrated sensitivity around 89–95% and specificity around 100% to distinguish TBI from other consciousness impairment etiologies (i.e., drug intake), which could aid diagnosis and management of unresponsive patients. MAP-2 elevations can be detected within the first few hours after injury, outperforming other markers in determining TBI severity.[3, 18]
MAP-2 measurements in both serum and CSF could also predict CT findings. CSF concentrations matched the degree of diffuse damage and axonal injury detected on initial CT, mainly within the first 120 h after trauma. However, comparing UCH-L1 and GFAP isolated and in combination, GFAP alone may be the best marker for this purpose.[3, 18]
Coagulation
Serum coagulation biomarkers that predict poor outcomes in severe trauma include D-dimer, thrombospondin-1, and SCUBE1. D-dimer is thought to indicate TBI-induced coagulopathy. The underlying mechanisms comprise tissue factor (TF) release, hyperfibrinolysis, shock, and hypoperfusion, triggering the protein C pathway, disseminated intravascular coagulation, and platelet dysfunction. Thrombospondin-1 is an antiangiogenic factor sensitive to thrombin whose expression is increased after intracerebral hemorrhage. SCUBE1 is released from endothelial cells and platelet alpha granules during platelet activation.[4, 11, 26, 30, 39]
Inflammation
Among the many available inflammation biomarkers (i.e., C-reactive protein, interleukins), a recent study detected raised levels of IL-33 in patients with TBI, identifying this interleukin as an independent prognostic factor. Although not specific for brain insults, they can contribute with prognostic information, helping to characterize strong inflammatory responses to TBI that contribute to secondary brain injury and, ultimately, poor outcomes.[13, 38]
Neuron-specific enolase
Another biomarker correlated to posttraumatic inflammation is the neuron-specific enolase (NSE), also referred to as gamma-enolase or enolase 2. NSE is a glycolytic pathway enzyme expressed in mature neurons and neuroendocrine cells. Increased CSF/serum ratio of this protein indicates neuronal damage, and serum elevations were also documented in mTBI and sTBI.
As a drawback, NSE is also expressed in red blood cells, which prompts the need to account for hemolysis. Several studies indicate elevations in NSE levels after TBI, in both serum and CSF. Acutely, serum NSE is a good predictor of the extent of neuronal damage, and persistent elevations were also detected long after mTBI.[6, 32]
In sTBI, it is still controversial whether NSE is associated with contusion volume and clinical outcome. Conversely, increases in its CSF levels are strongly correlated with the extension of brain lesions after sTBI. It might predict fatal outcomes when concentrations are high or if there is a second peak weeks after trauma.[6]
S100B
In addition to increased NSE in acute/subacute phases, higher CSF levels of S100B were detected in the patients who died during hospitalization, compared to those who survived. S100B is a calcium-binding protein expressed in astroglia, adipose tissue, and cardiac and skeletal muscles. Because it is not specific to neural tissues, S100B elevations can also be related to muscle lesions or orthopedic trauma without head injury. However, this astroglial biomarker could aid in the management of TBI patients.[1, 6]
Excessive serum levels of S100B correlate with poor outcomes, including significant mortality and brain death. For S100B serum levels higher than 0.7 ng/mL, studies reported 100% of mortality. Correlations between elevated levels of S100B and chronic complications of TBI, such as cognitive impairment, remain unclear.[1, 6]
Analyzing temporal profiles of S100B may contribute to patient management. As is the case for NSE, a second rise of S100B during the subacute phase indicates ongoing processes of excitotoxicity or inflammation, and thus secondary brain lesions. Oppositely, lower initial levels and the absence of a second peak suggest mTBI and positive outcomes, including recovery, rehabilitation, and safe return to play for athletes. In mTBI patients, S100B could predict CT abnormalities, and in sTBI, it correlates with the extension of brain damage.[1, 6]
Unlike S100B, levels of GFAP and its breakdown products are not increased in the absence of brain injury, which might be helpful in scenarios of polytrauma. Combined analysis of S100B and GFAP may contribute to distinguishing favorable from unfavorable outcomes. In sTBI, combined analyses with UCH-L1 can also contribute to the assessment of severity and clinical outcomes.[1, 6]
Alfa-II-spectrin
Investigation of the alfa-II-spectrin in the context of TBI started to emerge recently. Its breakdown products are split between C-terminal and N-terminal fragments, and their release is associated with necrosis, apoptosis, and neurodegenerative conditions. Although abundantly present in axons of the CNS, alfa-II-spectrin can also be expressed by some organs and peripheral blood mononuclear cells, which could impair interpretations.
Increased levels of C-terminal fragments (SBDP150, SBDP145, SBDP120) were detected in CSF after TBI, while higher levels of N-terminal fragments (SNTF) were identified in the blood after concussions. Higher levels of N-terminal fragments in the acute phase correlate with poor prognosis in mTBI, with worse performance on cognitive and sensory motor integration tests. Under normal conditions, those fragments are not detectable in the brain, and their release is possibly provoked by intra-axonal calcium overload and axonal cytoskeletal disruption.[25]
Neurofilament proteins
Neurofilament proteins (NF) are also components of the axonal cytoskeleton, with the advantage of being expressed exclusively in neurons. NF subunits differ in molecular weight—NF-H (heavy), NF-M (medium), and NF-L (light). All of them have already been detected in high concentrations within biofluids after TBI, suggesting a possible correlation with poor outcomes and mortality.
NF continues to be released days after head trauma, which could indicate ongoing processes of proteolysis and impairment of axonal membrane integrity. In contrast, Sandmo et al. found no significantly increased levels of NF-L in soccer players that suffered mild head impacts, which raises questions concerning its reliability.[24, 32]
Anti-pituitary antibodies
Anti-pituitary antibodies were detected in the chronic phase after TBI. Therefore, autoantibodies produced after TBI may be related to the development of certain secondary conditions, like hypopituitarism and growth hormone deficiency.
Other potential biomarkers identified in a recent blast exposure mice model are autoantibodies against the following proteins: fructose-biphosphate aldolase A (ALDOA), phosphorylase b kinase regulatory subunit beta (PHKB), alpha-globin 1 (HBA-A1), dihydropyrimidinase-related protein 2 (DPYSL2), isoform Ib of synapsin-1 (SYN1), and creatine kinase B-type (CKB).[12]
Exosomes
Neuronally derived (NDE) and astrocyte-derived (ADE) exosomes have been linked to the presence and development of AD. Plasma NDE cargo proteins from mTBI samples exhibited toxicity to neuron-like recipients in vitro, suggesting a possible injury mechanism. Winston and collaborators reported differences in measurements of NDE-associated proteins collected 4–6 months after head injuries, supporting the hypothesis that they can identify cellular injury pathways related to TBI.[33]
Nucleic acids
Circulating nucleic acids are possible prognostic tools, although not brain-specific. Total plasma cell–free DNA (cfDNA) correlated with TBI severity, mortality, and functional outcomes after head injuries. Plasma cfDNA was an independent mortality predictor in sTBI patients. Certain microRNAs may also present a relevant prognostic value in mTBI cases, such as miR-425-5p, miR-502, miR-142-3p, and miR-423-3p. Implementation of these biomarkers in the clinical practice should consider the fact that detecting cfDNA is less complicated and costly than RNA.[21, 32]
Cardiac biomarkers
Cardiac function is indirectly disrupted after TBI, particularly in severe cases and among younger patients. Almost a quarter of moderate sTBI patients had developed systolic dysfunction.[9, 10] The cardiac impact may be induced by trauma-related coronary hypoperfusion, sympathetic hyperactivity, release of inflammatory mediators, and impairment of the autonomic nervous system. Elevated CK-MB and troponin I after TBI have been associated with worse outcomes.[5, 9, 28]
Among sTBI patients, studies detected associations between increased brain natriuretic peptide (BNP) concentration, hyponatremia, and higher ICP. Initial BNP values after TBI were found to be 7.3-fold higher compared to controls.[27] Furthermore, progressive levels of BNP are associated with diffuse subarachnoid hemorrhage (SAH) and poor prognosis. [19, 27, 34] Serum NT-proBNP levels correlate with the growth of ischemic or hemorrhagic intra-axial lesion dimensions after mild to moderate TBI. [2] Increased concentrations of NT-proBNP were also detected in CSF samples of sTBI patients without signs of BBB damage. [14]
Although pathophysiological mechanisms of BNP and NT-proBNP elevations after TBI remain unclear, there is great evidence of their usefulness and potential to be included in clinical practice. [2, 19, 27, 34, 37]
Conclusion
Biomarkers could help improve all aspects of patient care in traumatic brain injury, i.e., diagnosis, classification, management, prognosis, and rehabilitation. We listed some of the recent advances in this translational, intriguing, fast-growing field. Although the knowledge gaps are still significant, some markers are demonstrating promising results and could be helping patients in the near future (Table 1).
References
Agoston DV, Shutes-David A, Peskind ER (2017) Biofluid biomarkers of traumatic brain injury. Brain Inj 31:1195–1203. https://doi.org/10.1080/02699052.2017.1357836
Akgun B, Erol FS, Yildirim H, Ilhan N, Kaplan M (2013) The correlation of serum NT-proBNP levels of hemorrhagic and ischemic lesions detected with diffusion MRI in head traumas. Turk Neurosurg 23:336–343. https://doi.org/10.5137/1019-5149.JTN.6982-12.1
Anderson T, Hwang J, Munar M, Papa L, Hinson HE, Vaughan A, Rowell S (2020) Blood-based biomarkers for prediction of intracranial hemorrhage and outcome in patients with moderate or severe traumatic brain injury. J Trauma Acute Care Surg Publish Ah 89:80–86. https://doi.org/10.1097/TA.0000000000002706
Di Battista AP, Rizoli SB, Lejnieks B, Min A, Shiu MY, Peng HT, Baker AJ, Hutchison MG, Churchill N, Inaba K, Nascimento BB, De Oliveira Manoel AL, Beckett A, Rhind SG (2016) Sympathoadrenal activation is associated with acute traumatic coagulopathy and endotheliopathy in isolated brain injury. Shock. 46:96–103. https://doi.org/10.1097/SHK.0000000000000642
Bender M, Stein M, Schoof B, Kolodziej MA, Uhl E, Schöller K (2020) Troponin I as an early biomarker of cardiopulmonary parameters during the first 24 h of intensive care unit treatment in isolated traumatic brain injury patients. Injury 51:1189–1195. https://doi.org/10.1016/j.injury.2020.01.002
Böhmer AE, Oses JP, Schmidt AP, Perón CS, Krebs CL, Oppitz PP, D’Avila TT, Souza DO, Portela LV, Stefani MA (2011) Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery. 68:1624–1631. https://doi.org/10.1227/NEU.0b013e318214a81f
Corrigan F, Arulsamy A, Teng J, Collins-Praino LE (2017) Pumping the brakes: neurotrophic factors for the prevention of cognitive impairment and dementia after traumatic brain injury. J Neurotrauma 34:971–986. https://doi.org/10.1089/neu.2016.4589
Dash PK, Zhao J, Hergenroeder G, Moore AN (2010) Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurother 7(1):100–14. https://doi.org/10.1016/j.nurt.2009.10.019
El-Menyar A, Goyal A, Latifi R, Al-Thani H, Frishman W (2017) Brain-heart interactions in traumatic brain injury. Cardiol Rev 25:279–288. https://doi.org/10.1097/CRD.0000000000000167
et al. C, (2016) 乳鼠心肌提取 HHS Public Access. Physiol Behav 176:139–148. https://doi.org/10.1016/j.physbeh.2017.03.040
Harhangi BS, Kompanje EJO, Leebeek FWG, Maas AIR (2008) Coagulation disorders after traumatic brain injury. Acta Neurochir 150:165–175
Harper MM, Rudd D, Meyer KJ, Kanthasamy AG, Anantharam V, Pieper AA, Vázquez-Rosa E, Shin M-K, Chaubey K, Koh Y, Evans LP, Bassuk AG, Anderson MG, Dutca L, Kudva IT, John M (2020) Identification of chronic brain protein changes and protein targets of serum auto-antibodies after blast-mediated traumatic brain injury. Heliyon 6:e03374. https://doi.org/10.1016/j.heliyon.2020.e03374
Hinson HE, Rowell S, Schreiber M (2015) Clinical evidence of inflammation driving secondary brain injury: a systematic review. J Trauma Acute Care Surg 78(1):184–191. https://doi.org/10.1097/TA.0000000000000468
Kirchhoff C, Stegmaier J, Bogner V, Buhmann S, Mussack T, Kreimeier U, Mutschler W, Biberthaler P (2006) Intrathecal and systemic concentration of NT-proBNP in patients with severe traumatic brain injury. J Neurotrauma 23:943–949. https://doi.org/10.1089/neu.2006.23.943
Kleindienst A, Brabant G, Morgenthaler NG, Dixit KC, Parsch H, Buchfelder M (2009) Following brain trauma, copeptin, a stable peptide derived from the AVP precusor, does not reflect osmoregulation but correlates with injury severity. Acta Neurochir Suppl 106:221–4. https://doi.org/10.1007/978-3-211-98811-4_41
Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime A-C, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang J, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Söderberg J, Stanworth SJ, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K, Adams H, Agnoletti V, Allanson J, Amrein K, Andaluz N, Anke A, Antoni A, van As AB, Audibert G, Azaševac A, Azouvi P, Azzolini ML, Baciu C, Badenes R, Barlow KM, Bartels R, Bauerfeind U, Beauchamp M, Beer D, Beer R, Belda FJ, Bellander B-M, Bellier R, Benali H, Benard T, Beqiri V, Beretta L, Bernard F, Bertolini G, Bilotta F, Blaabjerg M, den Boogert H, Boutis K, Bouzat P, Brooks B, Brorsson C, Bullinger M, Burns E, Calappi E, Cameron P, Carise E, Castaño-León AM, Causin F, Chevallard G, Chieregato A, Christie B, Cnossen M, Coles J, Collett J, Della Corte F, Craig W, Csato G, Csomos A, Curry N, Dahyot-Fizelier C, Dawes H, DeMatteo C, Depreitere B, Dewey D, van Dijck J, Đilvesi Đ, Dippel D, Dizdarevic K, Donoghue E, Duek O, Dulière G-L, Dzeko A, Eapen G, Emery CA, English S, Esser P, Ezer E, Fabricius M, Feng J, Fergusson D, Figaji A, Fleming J, Foks K, Francony G, Freedman S, Freo U, Frisvold SK, Gagnon I, Galanaud D, Gantner D, Giraud B, Glocker B, Golubovic J, Gómez López PA, Gordon WA, Gradisek P, Gravel J, Griesdale D, Grossi F, Haagsma JA, Håberg AK, Haitsma I, Van Hecke W, Helbok R, Helseth E, van Heugten C, Hoedemaekers C, Höfer S, Horton L, Hui J, Huijben JA, Hutchinson PJ, Jacobs B, van der Jagt M, Jankowski S, Janssens K, Jelaca B, Jones KM, Kamnitsas K, Kaps R, Karan M, Katila A, Kaukonen K-M, De Keyser V, Kivisaari R, Kolias AG, Kolumbán B, Kolundžija K, Kondziella D, Koskinen L-O, Kovács N, Kramer A, Kutsogiannis D, Kyprianou T, Lagares A, Lamontagne F, Latini R, Lauzier F, Lazar I, Ledig C, Lefering R, Legrand V, Levi L, Lightfoot R, Lozano A, MacDonald S, Major S, Manara A, Manhes P, Maréchal H, Martino C, Masala A, Masson S, Mattern J, McFadyen B, McMahon C, Meade M, Melegh B, Menovsky T, Moore L, Morgado Correia M, Morganti-Kossmann MC, Muehlan H, Mukherjee P, Murray L, van der Naalt J, Negru A, Nelson D, Nieboer D, Noirhomme Q, Nyirádi J, Oddo M, Okonkwo DO, Oldenbeuving AW, Ortolano F, Osmond M, Payen J-F, Perlbarg V, Persona P, Pichon N, Piippo-Karjalainen A, Pili-Floury S, Pirinen M, Ple H, Poca MA, Posti J, Van Praag D, Ptito A, Radoi A, Ragauskas A, Raj R, Real RGL, Reed N, Rhodes J, Robertson C, Rocka S, Røe C, Røise O, Roks G, Rosand J, Rosenfeld JV, Rosenlund C, Rosenthal G, Rossi S, Rueckert D, de Ruiter GCW, Sacchi M, Sahakian BJ, Sahuquillo J, Sakowitz O, Salvato G, Sánchez-Porras R, Sándor J, Sangha G, Schäfer N, Schmidt S, Schneider KJ, Schnyer D, Schöhl H, Schoonman GG, Schou RF, Sir Ö, Skandsen T, Smeets D, Sorinola A, Stamatakis E, Stevanovic A, Stevens RD, Sundström N, Taccone FS, Takala R, Tanskanen P, Taylor MS, Telgmann R, Temkin N, Teodorani G, Thomas M, Tolias CM, Trapani T, Turgeon A, Vajkoczy P, Valadka AB, Valeinis E, Vallance S, Vámos Z, Vargiolu A, Vega E, Verheyden J, Vik A, Vilcinis R, Vleggeert-Lankamp C, Vogt L, Volovici V, Voormolen DC, Vulekovic P, Vande Vyvere T, Van Waesberghe J, Wessels L, Wildschut E, Williams G, Winkler MKL, Wolf S, Wood G, Xirouchaki N, Younsi A, Zaaroor M, Zelinkova V, Zemek R, Zumbo F (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16:987–1048. https://doi.org/10.1016/S1474-4422(17)30371-X
Mahan MY, Thorpe M, Ahmadi A, Abdallah T, Casey H, Sturtevant D, Judge-Yoakam S, Hoover C, Rafter D, Miner J, Richardson C, Samadani U (2019) Glial fibrillary acidic protein (GFAP) outperforms S100 calcium-binding protein B (S100B) and ubiquitin C-terminal hydrolase L1 (UCH-L1) as predictor for positive computed tomography of the head in trauma subjects. World Neurosurg 128:e434–e444. https://doi.org/10.1016/j.wneu.2019.04.170
Papa L, Robicsek SA, Brophy GM, Wang KKW, Hannay HJ, Heaton S, Schmalfuss I, Gabrielli A, Hayes RL, Robertson CS (2018) Temporal profile of microtubule-associated protein 2: a novel indicator of diffuse brain injury severity and early mortality after brain trauma. J Neurotrauma 35:32–40. https://doi.org/10.1089/neu.2017.4994
Rahmani F (2016) Diagnostic value of brain natriuretic peptide in head trauma. J Anal Res Clin Med 4:75–76. https://doi.org/10.15171/jarcm.2016.012
Ramezani F, Bahrami-Amiri A, Babahajian A, Shahsavari Nia K, Yousefifard M (2018) Ubiquitin C-terminal hydrolase-L1 (UCH-L1) in prediction of computed tomography findings in traumatic brain injury; a meta-analysis. Emerg (Tehran) 6:e62
Regner A, Meirelles L d S, Ikuta N, Cecchini A, Simon D (2018) Prognostic utility of circulating nucleic acids in acute brain injuries. Expert Rev Mol Diagn 18:925–938. https://doi.org/10.1080/14737159.2018.1535904
Rubenstein R, Chang B, Davies P, Wagner AK, Robertson CS, Wang KKW (2015) A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids. J Neurotrauma 32:342–352. https://doi.org/10.1089/neu.2014.3548
Rubenstein R, Chang B, Yue JK, Chiu A, Winkler EA, Puccio AM, Diaz-Arrastia R, Yuh EL, Mukherjee P, Valadka AB, Gordon WA, Okonkwo DO, Davies P, Agarwal S, Lin F, Sarkis G, Yadikar H, Yang Z, Manley GT, Wang KKW, Cooper SR, Dams-O’Connor K, Borrasso AJ, Inoue T, Maas AIR, Menon DK, Schnyer DM, Vassar MJ (2017) Comparing plasma phospho tau, total tau, and phospho tau–total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol 74:1063–1072. https://doi.org/10.1001/jamaneurol.2017.0655
Sandmo SB, Filipcik P, Cente M, Hanes J, Andersen TE, Straume-Naesheim TM, Bahr R (2020) Neurofilament light and tau in serum after head-impact exposure in soccer. Brain Inj 34:602–609. https://doi.org/10.1080/02699052.2020.1725129
Siman R, Cui H, Wewerka SS, Hamel L, Smith DH, Zwank MD (2020) Serum SNTF, a Surrogate marker of axonal injury, is prognostic for lasting brain dysfunction in mild TBI treated in the emergency department. Front Neurol 11. https://doi.org/10.3389/fneur.2020.00249
Stein SC, Smith DH (2004) Coagulopathy in traumatic brain injury. Neurocrit Care 1:479–488
Sviri GE, Soustiel JF, Zaaroor M (2006) Alteration in brain natriuretic peptide (BNP) plasma concentration following severe traumatic brain injury. Acta Neurochir 148:529–533. https://doi.org/10.1007/s00701-005-0666-4
Taylor CA, Bell JM, Breiding MJ, Xu L (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. https://doi.org/10.15585/mmwr.ss6609a1
Thelin E, Al Nimer F, Frostell A, Zetterberg H, Blennow K, Nyström H, Svensson M, Bellander BM, Piehl F, Nelson DW (2019) A Serum protein biomarker panel improves outcome prediction in human traumatic brain injury. J Neurotrauma 36:2850–2862. https://doi.org/10.1089/neu.2019.6375
Tu CF, Su YH, Huang YN, Tsai MT, Li LT, Chen YL, Cheng CJ, Dai DF, Yang RB (2006) Localization and characterization of a novel secreted protein SCUBE1 in human platelets. Cardiovasc Res 71:486–495. https://doi.org/10.1016/j.cardiores.2006.04.010
Wagner AK, Zitelli KT (2013) A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 20:39–48. https://doi.org/10.1016/j.pathophys.2012.02.007
Wang KK, Yang Z, Zhu T, Shi Y, Rubenstein R, Tyndall JA, Manley GT (2018) An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn 18:165–180
Winston CN, Romero HK, Ellisman M, Nauss S, Julovich DA, Conger T, Hall JR, Campana W, O’Bryant SE, Nievergelt CM, Baker DG, Risbrough VB, Rissman RA (2019) Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front Neurosci 13. https://doi.org/10.3389/fnins.2019.01005
Wu X, Sha H, Sun Y, Gao L, Liu H, Yuan Q, Zhang T, Zhu J, Zhou L, Hu J (2011) N-terminal pro-B-type natriuretic peptide in patients with isolated traumatic brain injury: a prospective cohort study. J Trauma 71:820–825. https://doi.org/10.1097/TA.0b013e3182277b69
Wurzelmann M, Romeika J, Sun D (2017) Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen Res 12:7. https://doi.org/10.4103/1673-5374.198964
Yue JK, Upadhyayula PS, Avalos LN, Deng H, Wang KKW (2020) The role of blood biomarkers for magnetic resonance imaging diagnosis of traumatic brain injury. Medicina (Kaunas) 56:87. https://doi.org/10.3390/medicina56020087
Zhang Y, Feng Z, Bao Y, Zhou L, Qiu B (2017) Could B-type natriuretic peptides be a biomarker for trauma brain injury? A systematic review and meta-analysis. Am J Emerg Med 35:1695–1701. https://doi.org/10.1016/j.ajem.2017.05.051
Zhang Z-Y, Li J, Ye Q, Dong Y, Bao G-M, Shen Y-K, Weng J-F, Luo L-F, Cen M (2019) Usefulness of serum interleukin-33 as a prognostic marker of severe traumatic brain injury. Clin Chim Acta 497:6–12. https://doi.org/10.1016/j.cca.2019.07.008
Zhou HJ, Zhang HN, Tang T, Zhong JH, Qi Y, Luo JK, Lin Y, Yang QD, Li XQ (2010) Alteration of thrombospondin-1 and -2 in rat brains following experimental intracerebral hemorrhage: Laboratory investigation. J Neurosurg 113:820–825. https://doi.org/10.3171/2010.1.JNS09637
Funding
This work had support from the CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico- Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The local IRB waived the need for ethical approval due to the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gutierre, M.U., Telles, J.P.M., Welling, L.C. et al. Biomarkers for traumatic brain injury: a short review. Neurosurg Rev 44, 2091–2097 (2021). https://doi.org/10.1007/s10143-020-01421-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01421-0