Abstract
The incidence of water and electrolyte disturbances following traumatic brain injury (TBI) is considerable and has been attributed to a dysregulation of the hypothalamic peptide arginine-vasopressin (AVP). Copeptin, the C-terminal part of the AVP prohormone, reflects AVP activity.
In 71 TBI patients we measured copeptin in serum by a sandwich immunoassay. Injury severity was assessed by Glasgow Coma Score (GCS) and computed tomography, and recovery by Glasgow Outcome Score (GOS). Neuroendocrine and osmoregulation regulation were examined on day 0, 3 and 7, and 24 months post-injury.
Copeptin was highest on admission (40.0 ± 72.3 pmol/l), stabilized on day 3 and 7 (21.2 ± 18.3 resp. 20.3 ± 17.1 pmol/l), and normalized at follow-up (4.2 ± 1.7 pmol/l). On admission, there was a correlation between serum sodium and urine excretion (p = 0.003), but the correlation got lost on day 3 and 7. Copeptin did not reflect the individual 24 h urine excretion or serum sodium levels indicating an uncoupling of copeptin/AVP release and renal water excretion. High copeptin level on day 3 were correlated with a low GCS (p < 0.001), midline shift (p = 0.019), intracerebral hemorrhage (p = 0.026), SAPS score (p = 0.001), as well as with a low GOS (p = 0.031). Copeptin was significantly decreased following skullbase fracture (p = 0.016).
Our data reveal a loss of hypothalamic osmoregulation following TBI. The measurement of Copeptin/AVP release reveals a significant predictive function for the severity of TBI.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The existence of neuroendocrine dysfunction following traumatic brain injury (TBI) has increasingly attracted attention, and different studies reveal a broad spectrum of post-traumatic neuroendocrine dysfunction (14). Especially in the immediate post-trauma period, the incidence of water and electrolyte disturbances is considerable and contributes to the early morbidity (6). The impaired osmoregulation has been attributed to a dysregulation of the hypothalamic peptide arginine-vasopressin (AVP) subsequently affecting the prognosis of the individual patient. Agha et al. reported 26% of TBI patients to suffer from diabetes insipidus in the acute period (plasma sodium > 145 mmol/l, plasma osmolality > 300 mosmol/kg, ratio osmolality urine/plasma < 2, polyuria > 3.5 l/24 h), and 14% demonstrated symptoms suggestive for an inappropriate antidiuretic hormone (syn. AVP) secretion (SIADH, plasma sodium < 130 mmol/l, plasma osmolality < 270 mosmol/kg, urine osmolality > 100 mosmol/kg, urinesodium > 40 mM) (1). The diabetes insipidus persisted in 6% of patients while all patients recovered from SIADH.
Because of methodological problems, the hypothalamic-posterior pituitary function has been poorly investigated, yet. In the study mentioned above, both diabetes insipidus and SIADH have been diagnosed on the basis of water and electrolyte dysbalances alone but were not confirmed by AVP measurements (1). Copeptin, the C-terminal part of the AVP prohormone, is concomitantly secreted together with mature AVP (5). Due to an ex vivo stability lasting several days, copeptin can be readily assayed in serum or plasma. In the present study, we investigated the copeptin release in the acute period following TBI, and after more than 24 months combined with a repetitive neurological examination. In parallel, we performed a comprehensive assessment of different osmoregulatory parameter.
2 Methods
Seventy-one consecutive patients (57 male, 14 female, mean age 53 years, range 18–87years), who were admitted to our neurosurgical unit, were studied. According to the Glasgow Coma Score (GCS, 15), 24 patients suffered from mild (GCS 13–15), 32 from moderate (GCS 9–12), and 15 from severe (GCS 3–8) TBI. All patients underwent routinely an initial computerized tomography (CT) scan. The CT demonstrated an open TBI in 19, a skull fracture in 36, a cranio-facial fracture in 6, a skull base fracture in 17, a subdural hematoma in 14, an epidural hematoma in 8, an intracerebral hemorrhagic contusion in 20, a subarachnoid hemorrhage in 17, a midline shift in 2, and findings suggestive of a diffuse axonal injury in 2 patients. The study protocol was approved by the local Ethical Committee. Informed written consent was given by the patient or the next-of-kin, in each case. Exclusion criteria were an age below 18 years, pregnancy, an extremely poor prognosis, a pre-existing endocrine dysfunction, and whenever the patient’s or their relatives’ consent could not be obtained.
The patients were neurologically assessed daily, and the GCS was documented. On admission to the intensive care unit (ICU), the GCS was 12.09 ± 3.94, and improved to 14.27 ± 2.04 on day 7. The daily fluid balance was recorded, an output above 3.5 l was considered suspicious of diabetes insipidus. The simplified acute physiology (SAPS) II score (estimation of hospital mortality ∼21% at 32 [9]) was 40.43 ± 20.64. Blood samples for serum sodium, osmolality and copeptin were obtained on day 0 (n = 66), on day 3 (n = 69) and on day 7 (n = 52). Venous blood samples were drawn in the morning between 7:00 and 9:00 a.m. into pre-cooled tubes. Clotted samples were promptly centrifuged at 3,000Xg for 15 min at 4°C, and then the plasma was frozen at −80°C until analysis. Sodium (mmol/l) was measured by indirect potentiometry (predilution 1:20) with an ion-sensitive electrode on an AU2700 analyzer (sodium, potassium and chloride-unit with a reference-electrode) manufactured by Olympus Diagnostics Coop., Japan. Precision is less than 1.3%. Osmolality (mosmol/kg) measurements (reproducibility < ± 1.0%) were done by freezing-point depression with an autosampler (Osmomat Auto, Gonotec Inc.). Copeptin (CT-proAVP) measurements were performed in a blinded fashion in a single batch with a commercial sandwich immunoluminometric assay (B.R.A.H.M.S LUMItest CT-proAVP, B.R.A.H.M.S AG, Hennigsdorf/Berlin, Germany), as described in detail elsewhere (11). The Glasgow Outcome Score (GOS) assessed outcome at 6 months (1 = death, 2 = vegetative, 3 = severly disabled, 4 = moderately disabled, 5 = good recovery, [4]). Twenty-three patients consented to undergo a follow-up examination 24 to 36 months after the injury. The results are expressed as the mean ± SD. The correlation between variables was sought calculating the Pearson coefficient with the commercially available SPSS software.
3 Results
The incidence of polyuria (>3.5 l/day) was initially 21% and increased to 65% on day 7. Serum sodium was 135 ± 7.9 mmol/l on admission, 135 ± 9.1 mmol/l on day 3, and 133 ± 7.9 mmol/l on day 7. Copeptin was highest on admission (40.0 ± 72.3 pmol/l), stabilized on days 3 and 7 (21.2 ± 18.3 and 20.3 ± 17.1 pmol/l, respectively) and was normalized at follow-up in all but one patient (4.2 ± 1.7 pmol/l, Fig. 1). On the day of admission, a high urine output was correlated with high serum sodium levels (r = 0.536, p = 0.003, Fig. 2a). However, while polyuria resulted in a lowered urine osmolality (r = −0.556, p = 0.009), the positive correlation between urine volume, serum sodium and serum osmolality was lost on day 7 (r = −0.041, r = 0.571 and r = −0.132, p = 0.512). The correlation of serum sodium and serum osmolality was re-established at the follow-up examination (r = 0.894, p < 0.001).
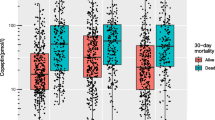
Interestingly, copeptin level did not correlate with urine volume, serum and urine sodium or osmolality within the acute period after TBI, and on follow-up. However, copeptin level correlated with a poorer neurological performance when they were persistently high on day 3 and 7 post-injury, i.e. with a low GCS (r = −0.396, r = 0.020 and r = −0.520, p = 0.011, Fig. 3). Copeptin level reflected the morphological severity of injury as assessed by the CT, i.e. intracerebral hemorrhage (r = 0.334, p = 0.043) and midline shift (r = −0.350, p = 0.034), as well as an unfavorable intensive care score SAPS II (r = 0.530, p = 0.002) and a low GOS score (r = −0.302, p = 0.031), when not declined by day 7. Notably, skull base fractures were associated with an increased serum osmolality on day 0 and 3 (r = 0.517, r < 0.001 and r = 0.320, p = 0.012) and with decreased copeptin levels on day 7 (r = −0.343, p = 0.038).
4 Discussion
With respect to the mechanism of brain injury, the primary trauma or direct damage to the pituitary or its stalk has to be differentiated from a secondary insult due to the TBI induced hypoxia, hypotension, brain edema or other intracranial pathology with subsequent effects on the hypothalamic-pituitary complex. The bony encasement of the pituitary within the sella turcica and its blood supply from different sources are anatomic features protecting the delicate organ from injury. Fractures of the sella turcica and the petrous bone are a possible cause of direct damage to the pituitary and have been reported to occur with variable frequency (2,7,13). In those patients presenting with sellar fractures, the frequency of endocrine deficiencies increases dramatically (3,17). A case series from our group (12) described the endocrinological findings of 11 head injured patients with skull base fractures involving the sella turcica. Diabetes insipidus was present in 8 patients in the acute phase, but was transient in 6 of them. In the present study, we found a skull base fracture to result significantly more often in a high serum osmolality and low copetin levels on day 7 post-injury, thereby suggesting a damage of the hypothalamo-posterior pituitary complex with a subsequent AVP deficiency.
Beside the direct trauma to the pituitary-hypothalamic system, both TBI in general and consecutive critical illness may induce an adaptive endocrine response (16). We demonstrated that the hypothalamic osmoregulation is still present immediately after injury, but gets lost during the further course of illness. Instead, our data reveal that the enhanced AVP activity in the early phase following TBI as assessed by the copeptin release reflects the seriousness of the condition. The homoestatic correction to cope with the catastrophic event of serious brain injury may engage a shift of AVP’s osmoregulatory responsibilities towards its vasopressor effects (10). This has been shown in life threatening conditions such as cardiopulmonary resuscitation (8). Thus, copeptin may be used as a surrogate parameter for the severity of brain injury, and even more importantly for the outcome of the patients.
Conflict of interest statement NGM is employed by BRAHMS AG, a company developing, marketing and selling in vitro diagnostic products, including a Copeptin assay.
References
Agha A, Thornton E, O’Kelly P, Tormey W, Phillips J, Thompson CJ (2004) Posterior pituitary dysfunction after traumatic brain injury. J Clin Endocrinol Metab 89:5987–5992
Dublin AB, Poirier VC (1976) Fracture of the sella turcica. AJR Am J Roentgenol 127:969–972
Edwards OM, Clark JD (1986) Post-traumatic hypopituitarism. Six cases and a review of the literature. Medicine (Baltimore) 65:281–290
Jennett B (1975) Outcome of severe damage to the central nervous system. Scale, scope and philosophy of the clinical problem. Ciba Found Symp 34:3–21
Katan M, Muller B, Christ-Crain M (2008) Copeptin: a new and promising diagnostic and prognostic marker. Crit Care 12:117
Kaufman HH, Timberlake G, Voelker J, Pait TG (1993) Medical complications of head injury. Med Clin North Am 77:43–60
Kojima T, Waga S, Furuno M (1985) Fracture of the sella turcica. Neurosurgery 16:225–229
Krismer AC, Wenzel V, Stadlbauer KH, Mayr VD, Lienhart HG, Arntz HR, Lindner KH (2004) Vasopressin during cardiopul-monary resuscitation: a progress report. Crit Care Med 32:S432–S435
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Lindner KH, Prengel AW, Pfenninger EG, Lindner IM, Strohmenger HU, Georgieff M, Lurie KG (1995) Vasopressin improves vital organ blood flow during closed-chest cardiopulmonary resuscitation in pigs. Circulation 91:215–221
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112–119
Nomikos P, Buchfelder M, Honegger J, Fahlbusch R (1997) Head injuries with fractures of the sella turcica. Ann Endocrinol 58(Suppl):1S130
Ortega FJ, Longridge NS (1975) Fracture of the sella turcica. Injury 6:335–337
Schneider M, Schneider HJ, Yassouridis A, Saller B, von Rosen F, Stalla GK (2008) Predictors of anterior pituitary insufficiency after traumatic brain injury. Clin Endocrinol (Oxf) 68:206–212
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84
Vanhorebeek I, Van den Berghe G (2006) The neuroendocrine response to critical illness is a dynamic process. Crit Care Clin 22:1–15
Young HA, Olin MS, Schmidek HH (1980) Fractures of the sella turcica. Neurosurgery 7:23–29
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag/Wien
About this paper
Cite this paper
Kleindienst, A., Brabant, G., Morgenthaler, N.G., Dixit, K.C., Parsch, H., Buchfelder, M. (2010). Following Brain Trauma, Copeptin, a Stable Peptide Derived from the AVP Precusor, Does Not Reflect Osmoregulation but Correlates with Injury Severity. In: Czernicki, Z., Baethmann, A., Ito, U., Katayama, Y., Kuroiwa, T., Mendelow, D. (eds) Brain Edema XIV. Acta Neurochirurgica Supplementum, vol 106. Springer, Vienna. https://doi.org/10.1007/978-3-211-98811-4_41
Download citation
DOI: https://doi.org/10.1007/978-3-211-98811-4_41
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-211-98758-2
Online ISBN: 978-3-211-98811-4
eBook Packages: MedicineMedicine (R0)