Abstract
High-grade craniofacial osteosarcoma (CFOS) is an aggressive malignancy with a poor prognosis. Our goals were to evaluate treatment outcomes in those treated at a single referral institution over 35 years and to compare our results to the available literature. A retrospective analysis of all 42 patients treated between 1980 and 2015 at Oslo University Hospital, Norway, identified in a prospectively collected database, was conducted. Mean follow-up was 79.6 months. Overall survival at 2 and 5 years was 70.5 and 44.7%, respectively. The corresponding disease-specific survival rates were 73.0 and 49.8%. Treatment was surgery only in eight cases. Additional therapy was administered in 34 patients: chemotherapy in nine, radiotherapy in seven, and a combination of these in 18 cases. Stratified analysis by resection margins demonstrated significantly better survival at 2 and 5 years after radical surgical treatment. Neoadjuvant chemotherapy and subsequent adequate surgery resulted in better survival than surgery alone. Half of the patients either had a primary or familial cancer predisposition. This is the largest single-center study conducted on high-grade CFOS to date. Our experience indicates that neoadjuvant chemotherapy with complete surgical resection significantly improved survival, compared to surgery alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS) is the most commonly occurring primary malignant bone tumor worldwide [1–3]. The incidence of all OS subtypes is approximately 3.3 per one million persons per year in Norway [4] and is most common during the second decade of life (during natural bone growth) [1]. Approximately 97% of all OSs are high-grade lesions [3].
Craniofacial osteosarcoma (CFOS) represents only 10% of all OS and less than 1% of all head and neck cancers [2, 4, 5]. CFOS is generally diagnosed two decades later than its long-bone counterparts [4, 6] and shows clinical behavior that is different to OS of the trunk and extremity. In this regard, CFOS has less propensity to metastasize, but higher mortality due to the difficulty of obtaining tumor control [4–8].
Radical surgery, with the aim of achieving complete resection with negative histological margins, is the mainstay of curative OS management. The role of multimodal treatment in patients with OS in long bones is well established, and combination chemotherapy (ChT) has been shown to improve survival [9]. In contrast, the role of ChT is less clear in CFOS [4–8].
The goals of this study were to evaluate the management of patients with CFOS with high-grade tumors on histological examination undergoing surgical resection at Oslo University Hospital (OUH) in Norway from 1980 to 2015 and to compare our results with other outcomes reported in the literature.
Materials and method
Patient cohort
We conducted an analysis of all patients with CFOS (defined as OS arising in the mandible, maxilla, or any of the extragnathic bones of the head) treated at OUH from 1980 to 2015. The catchment area of this institution covers approximately 56% of the entire Norwegian population. In addition, our institution accepts referrals involving challenging clinical cases from other health regions in Norway. Patient information was retrieved from a prospective database of all patients treated for OS in our health region.
Inclusion criteria were histologically verified high-grade CFOS [World Health Organization (WHO) grades III–IV] and primary surgical resection at OUH from 1980 to 2015, with or without radiotherapy (XRT) and/or ChT. The medical records of patients were also reviewed retrospectively to identify the study parameters not included in the database records.
Tumor-related variables
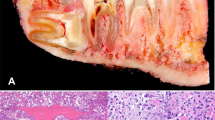
A histopathological diagnosis of OS was made by a consultant pathologist at presentation. All cases were formally re-examined by two dedicated sarcoma pathologists. Tumors were assessed for type, grade, determination of soft tissue infiltration, and chemotherapy response grade [10, 11]. The tumor site was classified according to the region of presumed origin in tumors affecting several craniofacial bones. Tumor size was determined from the surgical specimens and/or radiographical images at diagnosis and categorized depending on the maximum length of the tumor in centimeters. The quality of the surgical margins was also retrospectively scrutinized.
Treatment variables
Surgical treatment was deemed adequate if resection margins were negative according to a surgeon and pathologist joint assessment. ChT was defined adequate if the patient had received at least six chemotherapy courses containing a minimum of two drugs [high-dose methotrexate (at least 8 g/m2), doxorubicin, cisplatin, or ifosfamide], according to previous publications [8, 12]. ChT was defined inadequate if only one drug or less than 6 cycles of therapy was administered.
Statistical analysis
The main endpoints of this study were overall and disease-specific survivals. Follow-up time was calculated from the date of surgery to either death, with or without disease, or last known status. Event time distributions were approximated using the Kaplan-Meier estimator [13], and the log rank test was used to test for any significant differences between the survival curves [14]. Prognostic factors for overall survival were identified using the Cox proportional hazard regression model [15]. Whether or not the observed proportions for a categorical variable differed from the hypothesized proportions was determined using the chi-square test or Fisher’s exact test, as appropriate [16]. The level of statistical significance was set at p value = 0.050. Descriptive statistics were reported as a mean, range, 95% confidence interval (CI), and median, if appropriate. Statistical analysis was conducted using SPSS® version 22 (SPSS Inc., Chicago, USA).
Results
Clinical findings
The medical records of 49 patients were reviewed. Seven patients were excluded due to primary treatment prior to 1980 (three cases), the absence of surgical treatment due to locally advanced disease (two cases), primary treatment at another hospital (one case), and low-grade CFOS (one case), leaving 42 patients eligible for inclusion. Patient characteristics are summarized in Table 1. The sex distribution was nearly equal, comprising 22 male (52%) and 20 female (48%) patients. All of the patients were of Caucasian descent, with 41 of European (98%) and one of Arabic origin (2%). The mean age at diagnosis was 41.6 years (range 6–86 years, 95% CI 35.2–48.1 years). The peak incidence of disease in our cohort occurred from the age of 30–39 years.
Painless swelling was the most common presenting symptom, observed in 50% of all cases. Presenting symptoms were predating primary diagnosis with a mean of 4.7 months (range 0–29 months, 95% CI 2.8–6.5). Painless swelling as presenting symptom has led to significantly longer latency of diagnosis, compared to other, more nocuous symptoms [6.2 months (range 0.0–29.0 months, 95% CI 2.7–9.8) vs. 3.1 months (range 0.0–8.0 months, 95% CI 2.1–4.1), p value = 0.042].
Predisposing factors
A total of 21 (50%) patients in our cohort had either primary or familial cancer predisposition. Six of the patients with a primary predisposition to cancer had at least two first-grade relatives with cancer also (Table 1). Predisposition to cancer was present in 16 patients (38%). Eight patients (19%) had previously been diagnosed with either bilateral retinoblastoma (two patients), squamous cell carcinoma of the nasal and oral cavity (one and two patients, respectively), embryonal rhabdomyosarcoma (one patient), non-Hodgkin’s lymphoma (one patient), and low-grade glioma (one patient). All of them had previously undergone surgical treatment and XRT before developing CFOS. The mean time between XRT and the CFOS diagnosis was 186.7 months (range 70–448 months, 95% CI 50.2–323.3). Eight patients (19%) had fibrous dysplasia, low-grade glioma, malignant melanoma, basal cell carcinoma, von Hippel-Lindau disease, liposarcoma or Li-Fraumeni syndrome prior to the diagnosis of CFOS. In addition, malignant disease was present in the first-degree relatives of 14 of the patients (33%). At least two first-degree relatives of nine of these 14 patients (21%) had cancer.
Tumor characteristics
All of the patients had high-grade CFOS [WHO grade IV in 34 (81.0%) cases]. Histologically, chondroblastic tumors were the most common, observed in 20 cases (48%). The mean tumor size was 5.2 cm (a median of 4.5 cm, 95% CI 4.3–6.0). The jaws were affected in 32 (76%) patients, while the tumor was extragnathic in 10 (24%) patients.
Treatment
Treatment constituted surgery alone for eight cases (19%). Multimodal therapy was given in 34 patients (80%), including ChT in 9 (21%), XRT in 7 (17%), and a combination of ChT and XRT in 18 (43%). Fourteen patients (33%) underwent both neoadjuvant and adjuvant treatments, in addition to surgery. The mean time between diagnosis and primary treatment was 0.5 months (a range of 0.0–4.0 months, 95% CI 0.2–0.7). Treatment characteristics are summarized in Table 1.
Surgical resection
Mandibular resection was the most common procedure (18 cases, 43%). Eleven patients (26%) underwent maxillary resection (combined with craniectomy in four cases). Craniotomy was performed in ten cases (24%). Orbital exenteration had to be undertaken in four cases (10%). One patient had both mandibular and maxillary resections, combined with craniectomy, owing to the presence of a large and highly invasive tumor. Fourteen patients (33%) underwent surgical reconstruction as a part of the primary surgical treatment, using free skin graft in six, fibula graft in five, Vitallium implant in two, and myocutaneous flap in one case.
Adequate surgical resection was achieved in 15 cases (36%). Seven of the 15 patients underwent neoadjuvant therapy, including ChT in six and XRT in one case. Malignant cells were found in, or very close to, the resection margins in 21 cases (50%), while the status of the surgical margins remained unknown in 6 cases (14%).
Complications relating directly to surgical treatment were registered in seven cases (17%), including cerebrospinal fluid leak and localized infection in two cases and submental fistula, localized hematoma, and acute myocardial infarction in one case each.
Chemotherapy
ChT regimens were not well defined prior to 1990. Thereafter, protocols established by the Scandinavian Sarcoma Group—primarily designed for OS of the extremities—were followed, adjusted for age and toxicity in patients unable to receive all of the cycles according to the predefined protocol [17].
The treatment strategy in our study constituted ChT in 27 patients (64%). Adequate ChT—as defined previously—was administered to 16 of these 27 patients. Fifteen patients (36%) received neoadjuvant ChT prior to surgery, and it was deemed to be adequate in 12. The histological response to neoadjuvant ChT could be evaluated in 13 patients. A response rate of >90% was observed in only three cases.
The number of patients receiving adequate ChT has increased over the last three decades. Forty percent of all patients treated with ChT received adequate treatment between 1980 and 1989, 44% between 1990 and 1999, and 77% between 2000 and 2015.
Radiotherapy
Twenty-five patients (60%) received XRT, mainly administered postoperatively (22 cases). It was used as an adjunct to suboptimal surgical resection (16 cases) or against recurrence and metastasis (six cases) in the majority of cases. XRT was boosted with brachytherapy (192-Iridium) or bone-targeted radionuclide therapy with samarium-153-ethylenediaminetetramethylene phosphonate in five patients. The mean dose of XRT administered postoperatively was 59 Gy (a median of 60 Gy, 95% CI 50.0–68.1).
Outcomes
Fourteen (33%) patients in our cohort were still alive, 13 without evidence of disease on completion of the study. Twenty-three patients (67%) had deceased due to their CFOS, and five deaths were due to other diseases. We obtained 100% follow-up. The mean follow-up time was 79.6 months (range 2–343 months, median 36.5 months, 95% CI 52.6–106.7) as of October 1, 2015 (the final follow-up). The mean follow-up time of patients with no evidence of disease was 141.9 months (range 0–343 months, median 129 months, 95% CI 94.6–189.3). Importantly, none of the patients were lost to follow-up.
The overall survival rate (OS) of the cohort was calculated to be 70.5% at 2 years and 44.7% at 5 years postoperatively (Fig. 1). The corresponding disease-specific survival (DSS) rates were 73 and 49.8% (Fig. 2).
Tumor size at diagnosis of <5 cm correlated significantly with better DSS (88.9 vs. 45.0% at 2 years, 71.8 vs. 15.0% at 5 years, p value = 0.003) (Fig. 3). There was a significant correlation (p value = 0.017) between positive surgical margins and subsequent death from the disease (odds ratio of 2.3). Stratified DSS was 86.7 and 66.7% at 2 and 5 years, respectively, where adequate surgery had been performed, compared to 65.0 and 39.3% for cases of non-radical surgery (p value = 0.042) (Fig. 4).
We found no significant correlations between outcome measures and age, sex, presenting symptoms, histological type, previous XRT, and primary or familial cancer predisposition. Tumor location did not impact survival significantly either. However, it is intuitively convincing that complete resection of a tumor in the mandible should be performed more easily than at other locations of the head. Failure to detect a difference may be the result of a type II statistical error, because of the low number of patients included in the study or a de facto lack of an effect. DSS stratified by tumor location showed best survival for patients with CFOS located to the maxilla in our cohort (83.3 and 58.3% at 2 and 5 years vs. 68.4 and 51.5% with tumor located to the mandibula and 67.5 and 33.8% with tumor located to the extragnathic bones of the head).
Seventeen patients (40.5%) experienced recurrence of disease after a mean time of 19 months postoperatively (median 12 months, range 1–120 months, 95% CI 5.3–33.2). The mean recurrence-free survival (RFS) rate was 62.9% at 2 years and 56.8% at 5 years of follow-up (Fig. 5). Stratified RFS was 78.8% 2 years and 70.9% 5 years postoperatively in cases where adequate surgery was performed, compared to 53.3 and 48.5% at 2 and 5 years of follow-up, respectively, in cases of non-radical surgery (Fig. 6).
Distant metastases affecting the lungs and the skeleton developed in 11 patients (26%), with a mean time of 29 months (range 5–83 months, 95% CI 13.8–44.2).
The use of neoadjuvant ChT in patients who subsequently underwent adequate surgical treatment correlated significantly with better survival than adequate surgery alone (p value ≤0.001). By contrast, a statistically significant correlation between the use of neoadjuvant ChT and negative surgical margins was not observed (p value = 0.666).
Failure to administer adequate ChT correlated significantly with disease recurrence and dismal outcome (relative risk of 1.87, p value = 0.048). Furthermore, DSS in patients who received adequate ChT was superior to that in patients where ChT was inadequate (85.7 vs. 63.6% at 2 years and 62.9 vs. 27.3% at 5 years), but this correlation did not reach statistical significance because of the low cohort size (Fig. 7).
Discussion
The goals of this study were to evaluate the clinical features, management, and outcome of 42 patients with high-grade CFOS undergoing multimodal treatment, including surgical resection, at OUH, Norway, over the last 35 years and to compare our results to the available literature. To our knowledge, our study represents the largest single-institution cohort of high-grade CFOS ever published.
Peak incidence of this disease was observed in the third decade, with no gender predilection. Younger patients usually had a genetic predisposition (i.e., Li-Fraumeni syndrome), had other underlying abnormalities (i.e., fibrous dysplasia), or had undergone previous radiation of the bone due to other malignancies. A remarkably high degree of primary and familial predisposition to cancer was observed in our cohort.
We investigated 57 articles from the surgical, otolaryngological, orthopedic, and oncological literature after a non-systematic review [2, 5–8, 18–70]. Fifty of these were reported on specific treatment methods and survival data for individuals with OS of the head and neck [2, 5, 7, 10, 18, 20, 23, 24, 26, 28–53, 56–70]. Only 21 of these articles were solely based on CFOS including extragnathic bones of the head [5, 18, 20, 24, 26, 29–33, 36, 38, 42–44, 51, 53, 63, 68–70] (Table 2). Three publications were reviews of the relevant literature [21, 27, 54]. Overall survival rates of approximately 60–70% at 5 years, ranging from 9.5 to 74%, have been reported in published studies on CFOS [5, 18, 20, 24, 26, 29–33, 36, 38, 42–44, 51, 53, 63, 68–70]. However, several of these cohorts comprised patients with both low- and high-grade CFOS. Fifty-eight patients were included in the largest reported multicenter study [70], and 38 patients with high-grade CFOS participated in the largest single-institution study [20].
Overall survival rates in our cohort were 70.5 and 44.7% at 2 and 5 years of follow-up, respectively, and corresponding DSS was 73.0 and 49.8%. Interestingly, an improvement in the DSS rate was noted with time (DSS at 5 years was 41.7% when the primary treatment was administered prior to 2000, compared to 63.7% when it was initiated after 2000). The survival rates in our study compare favorably with the relevant international figures, despite the obvious bias in our disfavor when comparing cohorts containing both low- and high-grade CFOS.
Surgical resection of a primary tumor with negative surgical margins is crucial, preferably in combination with adjuvant ChT, when treating OS in the long bones [1, 8, 71–73] CFOS is more challenging to treat as radical resection with negative surgical margins may lead to very severe functional and visible defects. Malignant tumors involving the infratemporal fossa were considered to be unresectable in the past owing to their perceived biological aggressiveness, the difficulty in achieving negative histological margins, and the associated surgical morbidity and mortality [74]. It has also been noted that surgeons are reluctant to perform resections that seriously affect cosmesis and function in younger patients [21]. However, progress within the fields of surgery and radiology has facilitated the use of more aggressive resection and advanced reconstructive techniques, leading to decreased morbidity and improved survival rates in patients with CFOS resection.
The frequency of negative surgical margins in our cohort improved from decade to decade and impacted significantly on survival (p value = 0.042). The effects of adequate surgery on survival are well documented.
ChT changed the prognosis of CFOS dramatically from 1980 onwards, resulting in a significant increase in survival rates. The existing literature on outcomes in patients with CFOS is hard to interpret because patients who were treated over a prolonged period were grouped in an effort to gather sufficient data for statistical analysis. We identified only four articles reporting on single-center experiences, based on selected time periods after 1980 [24, 30, 51, 53].
Sundaresan et al. and Salvati et al. were the first to address the effects of ChT on survival and demonstrated dramatically improved prognosis in patients receiving chemotherapy as adjuvant treatment [44, 68]. Smith et al. also reported on improved survival rates in the last two decades of the last century [33]. Kassir et al. assessed the reported effect of adjuvant therapy on the outcome of OS, based on a meta-analysis of the literature between 1980 and 1994 [21]. They did not find any survival benefit from the addition of ChT or XRT to surgery, but this study included unvalidated data in terms of surgical adequacy, while the outcome variables of patients were pooled, irrespective of the margin status. Smeele et al. documented the effect of ChT on survival with respect to CFOS in the same year, only including data from studies that reported on the surgical margins [55]. They found that patients with free margins and who had received chemotherapy treatment had the best survival rates, followed by those with free margins and no chemotherapy.
Patel et al. reported on a noticeable improvement in the ability to exert local disease control over a 5-year period, the distant metastases, and overall survival, when comparing the authors’ own historical cohort and a new one treated in the ChT era. However, they found that the negative surgical margins were the only significant predictor of overall and DSS [24].
Oda et al. also documented the possible benefits of neoadjuvant ChT in 2013 [30]. Boon et al. have recently (in 2016) documented than in patients younger than 75 years of age with surgically resected high- and intermediate-grade OS of the head and neck, treatment with (neo-)adjuvant ChT resulted in a significantly smaller risk of local recurrence [70].
We documented that the administration of neoadjuvant ChT correlated significantly better with survival than surgery alone in patients undergoing adequate surgical resection (p value <0.001). The management strategy consisting of a combination of surgery and ChT was associated with better DSS than surgery and XRT or surgery with both ChT and XRT (p value = 0.015).
High-dose methotrexate (at least 8 g/m2), doxorubicin, cisplatin, and also ifosfamide are the most commonly used ChT drugs worldwide, but there is still no international consensus on their optimal combination [9, 75]. Cases of head and neck OS—including CFOS—should be evaluated in multidisciplinary teams with surgeons and sarcoma specialists. This kind of cooperation is crucial to identify the best multimodal treatment option for each patient. We have intended to follow treatment protocols for classical—extremity localized—OS, adjusted for age and kidney function [76, 77].
Adjuvant XRT might be indicated postoperatively in cases of non-radical surgery [20–23, 27, 51]. Proton beam therapy may offer some benefit to patients with skull base lesions, as it limits the dose of radiation to the visual apparatus, cranial nerves, and central nervous system, thereby reducing the risk of long-term complications [27]. Hyperfractionated and intensity-modulated XRT should also be considered with regard to reducing the risk of optic neuropathy in selected patients [78].
We propose continuous follow-up for at least 10 years, partly divided between a head and neck surgeon and an oncologist, ensuring the discovery of both local recurrences, distant metastases, and delayed complications related to ChT. We recommend four times yearly during the first year, three times yearly between years 2 to 5, and once per year between years 5 to 10. Chest X-ray should be obtained at each consultation in addition to full blood count in patients who underwent ChT. Magnetic resonance imaging is indicated yearly during the first 3 years or at any time if deemed necessary after clinical evaluation.
Study limitations and strengths
A weakness of this study is that it is based on observational data. CFOS is so rare that it is unlikely that the impact of multimodal treatment would ever be analyzed in a randomized prospective fashion, even within the framework of a multi-institutional study. Our cohort included patients who had been treated for more than three decades. Thus, it was subject to the impact of improvements in radiological, surgical, radiotherapy, and chemotherapy techniques.
Study strengths were the setting, sample size, design, and follow-up duration (long term). The data were restricted to one health center only, reducing the possible confounding effect of differences in access to the healthcare service. Thus, the selection bias that is inherently present in a larger multicentered study was seemingly avoided.
The study reflects the largest single-institution cohort of high-grade CFOS among published series, involving patients solely treated in the ChT era with surgery performed for histologically verified high-grade CFOS. Only endpoints that were verifiable were used with respect to the data quality. Lastly, 100% follow-up was obtained.
References
Hogendoorn PC, Group EEW, Athanasou N, Bielack S, De Alava E, Dei Tos AP et al (2010) Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v204–v213
Mirabello L, Troisi RJ, Savage SA (2009) International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 125(1):229–234
Fletcher C, Bridge J, Hogendoorn PC, Mertens F (2013) WHO classification of tumours of soft tissue and bone, 4th edn. International Agency for Research on Cancer, Lyon
Berner K, Johannesen TB, Berner A, Haugland HK, Bjerkehagen B, Bohler PJ et al (2015) Time-trends on incidence and survival in a nationwide and unselected cohort of patients with skeletal osteosarcoma. Acta Oncol 54(1):25–33
Tran LM, Mark R, Meier R, Calcaterra TC, Parker RG (1992) Sarcomas of the head and neck. Prognostic factors and treatment strategies. Cancer 70(1):169–177
Saeter G, Bruland OS, Folleras G, Boysen M, Hoie J (1996) Extremity and non-extremity high-grade osteosarcoma—the Norwegian Radium Hospital experience during the modern chemotherapy era. Acta Oncol 35(Suppl 8):129–134
Thiele OC, Freier K, Bacon C, Egerer G, Hofele CM (2008) Interdisciplinary combined treatment of craniofacial osteosarcoma with neoadjuvant and adjuvant chemotherapy and excision of the tumour: a retrospective study. Br J Oral Maxillofac Surg 46(7):533–536
Berner K, Hall KS, Monge OR, Weedon-Fekjaer H, Zaikova O, Bruland OS (2015) Prognostic factors and treatment results of high-grade osteosarcoma in Norway: a scope beyond the “classical” patient. Sarcoma 2015:516843
Luetke A, Meyers PA, Lewis I, Juergens H (2014) Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev 40(4):523–532
Huvos AG, Rosen G, Marcove RC (1977) Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 101(1):14–18
Fletcher C, Unni K (2002) Pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon
Saeter G, Bruland O (1998) High-grade osteosarcoma: the scope beyond the classical patient. Towards the Eradication of Osteosarcoma Metastasis-An Odyssey 21-3
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50(3):163–170
Cox D (1972) Regression models and life tables. J R Stat Soc B 34:187–220
Fisher RA (1922) On the interpretation of Χ2 from contingency tables, and the calculation of P. J R Stat Soc 85(1):87–94
Bruland OS, Bauer H, Alvegaard T, Smeland S (2009) Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res 152:309–318
Huber GF, Dziegielewski P, Wayne Matthews T, Dort JC (2008) Head and neck osteosarcoma in adults: the province of Alberta experience over 26 years. J Otolaryngol Head Neck Surg 37(5):738–743
Sturgis EM, Potter BO (2003) Sarcomas of the head and neck region. Curr Opin Oncol 15(3):239–252
Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM (2009) Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer 115(14):3262–3270
Kassir RR, Rassekh CH, Kinsella JB, Segas J, Carrau RL, Hokanson JA (1997) Osteosarcoma of the head and neck: meta-analysis of nonrandomized studies. Laryngoscope 107(1):56–61
Schwarz R, Bruland O, Cassoni A, Schomberg P, Bielack S (2009) The role of radiotherapy in osteosarcoma. Cancer Treat Res 152:147–164
Kammerer PW, Shabazfar N, Vorkhshori Makoie N, Moergel M, Al-Nawas B (2012) Clinical, therapeutic and prognostic features of osteosarcoma of the jaws—experience of 36 cases. J Craniomaxillofac Surg 40(6):541–548
Patel SG, Meyers P, Huvos AG, Wolden S, Singh B, Shaha AR et al (2002) Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer 95(7):1495–1503
Konig M, Mork J, Hall KS, Osnes T, Meling TR (2010) Multimodal treatment of osteogenic sarcoma of the jaw. Skull Base 20(3):207–212
Jasnau S, Meyer U, Potratz J, Jundt G, Kevric M, Joos UK et al (2008) Craniofacial osteosarcoma experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral Oncol 44(3):286–294
Mendenhall WM, Fernandes R, Werning JW, Vaysberg M, Malyapa RS, Mendenhall NP (2011) Head and neck osteosarcoma. Am J Otolaryngol 32(6):597–600
Ferrari D, Codeca C, Battisti N, Broggio F, Crepaldi F, Violati M et al (2014) Multimodality treatment of osteosarcoma of the jaw: a single institution experience. Med Oncol 31(9):171
Mark RJ, Sercarz JA, Tran L, Dodd LG, Selch M, Calcaterra TC (1991) Osteogenic sarcoma of the head and neck. The UCLA experience. Arch Otolaryngol Head Neck Surg 117(7):761–766
Oda D, Bavisotto LM, Schmidt RA, McNutt M, Bruckner JD, Conrad EU 3rd et al (1997) Head and neck osteosarcoma at the University of Washington. Head Neck 19(6):513–523
Ha PK, Eisele DW, Frassica FJ, Zahurak ML, McCarthy EF (1999) Osteosarcoma of the head and neck: a review of the Johns Hopkins experience. Laryngoscope 109(6):964–969
Gorsky M, Epstein JB (2000) Craniofacial osseous and chondromatous sarcomas in British Columbia—a review of 34 cases. Oral Oncol 36(1):27–31
Smith RB, Apostolakis LW, Karnell LH, Koch BB, Robinson RA, Zhen W et al (2003) National Cancer Data Base report on osteosarcoma of the head and neck. Cancer 98(8):1670–1680
Garrington GE, Scofield HH, Cornyn J, Hooker SP (1967) Osteosarcoma of the jaws. Analysis of 56 cases. Cancer 20(3):377–391
Roca AN, Smith JL Jr, Jing BS (1970) Osteosarcoma and parosteal osteogenic sarcoma of the maxilla and mandible: study of 20 cases. Am J Clin Pathol 54(4):625–636
Caron AS, Hajdu SI, Strong EW (1971) Osteogenic sarcoma of the facial and cranial bones. A review of forty-three cases. Am J Surg 122(6):719–725
Russ JE, Jesse RH (1980) Management of osteosarcoma of the maxilla and mandible. Am J Surg 140(4):572–576
Nora FE, Unni KK, Pritchard DJ, Dahlin DC (1983) Osteosarcoma of extragnathic craniofacial bones. Mayo Clin Proc 58(4):268–272
Clark JL, Unni KK, Dahlin DC, Devine KD (1983) Osteosarcoma of the jaw. Cancer 51(12):2311–2316
Forteza G, Colmenero B, Lopez-Barea F (1986) Osteogenic sarcoma of the maxilla and mandible. Oral Surg Oral Med Oral Pathol 62(2):179–184
Bertoni F, Dallera P, Bacchini P, Marchetti C, Campobassi A (1991) The Istituto Rizzoli-Beretta experience with osteosarcoma of the jaw. Cancer 68(7):1555–1563
Vege DS, Borges AM, Aggrawal K, Balasubramaniam G, Parikh DM, Bhaser B (1991) Osteosarcoma of the craniofacial bones. A clinico-pathological study. J Craniomaxillofac Surg 19(2):90–93
Wanebo HJ, Koness RJ, MacFarlane JK, Eilber FR, Byers RM, Elias EG et al (1992) Head and neck sarcoma: report of the Head and Neck Sarcoma Registry. Society of Head and Neck Surgeons Committee on Research. Head Neck 14(1):1–7
Salvati M, Ciappetta P, Raco A (1993) Osteosarcomas of the skull. Clinical remarks on 19 cases. Cancer 71(7):2210–2216
Delgado R, Maafs E, Alfeiran A, Mohar A, Barrera JL, Zinser J et al (1994) Osteosarcoma of the jaw. Head Neck 16(3):246–252
Koka V, Vericel R, Lartigau E, Lusinchi A, Schwaab G (1994) Sarcomas of nasal cavity and paranasal sinuses: chondrosarcoma, osteosarcoma and fibrosarcoma. J Laryngol Otol 108(11):947–953
August M, Magennis P, Dewitt D (1997) Osteogenic sarcoma of the jaws: factors influencing prognosis. Int J Oral Maxillofac Surg 26(3):198–204
van Es RJ, Keus RB, van der Waal I, Koole R, Vermey A (1997) Osteosarcoma of the jaw bones. Long-term follow up of 48 cases. Int J Oral Maxillofac Surg 26(3):191–197
Canadian Society of O-H, Neck Surgery Oncology Study G (2004) Osteogenic sarcoma of the mandible and maxilla: a Canadian review (1980-2000). J Otolaryngol 33(3):139–144
Fernandes R, Nikitakis NG, Pazoki A, Ord RA (2007) Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg 65(7):1286–1291
Laskar S, Basu A, Muckaden MA, D’Cruz A, Pai S, Jambhekar N et al (2008) Osteosarcoma of the head and neck region: lessons learned from a single-institution experience of 50 patients. Head Neck 30(8):1020–1026
Paparella ML, Olvi LG, Brandizzi D, Keszler A, Santini-Araujo E, Cabrini RL (2013) Osteosarcoma of the jaw: an analysis of a series of 74 cases. Histopathology 63(4):551–557
Lim S, Lee S, Rha SY, Rho JK (2013) Craniofacial osteosarcoma: single institutional experience in Korea. Asia Pac J Clin Oncol
Smeele LE, Kostense PJ, van der Waal I, Snow GB (1997) Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol 15(1):363–367
Smeele LE, Snow GB, van der Waal I (1998) Osteosarcoma of the head and neck: meta-analysis of the nonrandomized studies. Laryngoscope 108(6):946
Smeele LE, van der Wal JE, van Diest PJ, van der Waal I, Snow GB (1994) Radical surgical treatment in craniofacial osteosarcoma gives excellent survival. A retrospective cohort study of 14 patients. Eur J Cancer B Oral Oncol 30B(6):374–376
Akbiyik N, Alexander LL (1981) Osteosarcoma of the maxilla treated with radiation therapy and surgery. J Natl Med Assoc 73(4):355–356
Briant TD, Bird R (1981) Osteogenic sarcoma of the mandible. J Otolaryngol 10(2):149–161
deFries HO, Perlin E, Leibel SA (1979) Treatment of osteogenic sarcoma of the mandible. Arch Otolaryngol 105(6):358–359
Goepfert H, Ballantyne AJ, Matalon V, McCarthy E (1979) Osteogenic sarcoma of the mandible: surgical resection and prosthetic rehabilitation. Otolaryngol Head Neck Surg (1979) 87(4):417–419
Gomez AC, Youmans RD, Chambers RG (1960) Osteogenic sarcoma of the mandible. A method of treatment. Am J Surg 100:613–616
Hadley C, Gressot LV, Patel AJ, Wang LL, Flores RJ, Whitehead WE et al (2014) Osteosarcoma of the cranial vault and skull base in pediatric patients. J Neurosurg Pediatr 13(4):380–387
Kragh LV, Dahlin DC, Erich JB (1958) Osteogenic sarcoma of the jaws and facial bones. Am J Surg 96(4):496–505
LiVolsi VA (1977) Osteogenic sarcoma of the maxilla. Arch Otolaryngol 103(8):485–488
Mardinger O, Givol N, Talmi YP, Taicher S (2001) Osteosarcoma of the jaw. The Chaim Sheba Medical Center experience. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91(4):445–451
Schwartz DT, Alpert M (1963) The clinical course of mandibular osteogenic sarcoma. Oral Surg Oral Med Oral Pathol 16:769–776
van den Berg H, Schreuder WH, de Lange J (2013) Osteosarcoma: a comparison of jaw versus nonjaw localizations and review of the literature. Sarcoma 2013:316123
Sundaresan N, Huvos AG, Rosen G, Galicich JH (1985) Combined-modality treatment of osteogenic sarcoma of the skull. J Neurosurg 63(4):562–567
Huvos AG, Sundaresan N, Bretsky SS, Butler A (1985) Osteogenic sarcoma of the skull. A clinicopathologic study of 19 patients. Cancer 56(5):1214–1221
Boon E, van der Graaf WT, Gelderblom H, Tesselaar ME, van Es RJ, Oosting SF, et al. (2016) Impact of chemotherapy on the outcome of osteosarcoma of the head and neck in adults. Head Neck
Carrle D, Bielack SS (2006) Current strategies of chemotherapy in osteosarcoma. Int Orthop 30(6):445–451
Isakoff MS, Bielack SS, Meltzer P, Gorlick R (2015) Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol 33(27):3029–3035
Bielack SS, Wulff B, Delling G, Gobel U, Kotz R, Ritter J et al (1995) Osteosarcoma of the trunk treated by multimodal therapy: experience of the Cooperative Osteosarcoma study group (COSS). Med Pediatr Oncol 24(1):6–12
Bilsky MH, Bentz B, Vitaz T, Shah J, Kraus D (2005) Craniofacial resection for cranial base malignancies involving the infratemporal fossa. Neurosurgery 57(4 Suppl):339–347 discussion -47
Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC et al (2011) Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer 47(16):2431–2445
Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM et al (2015) Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 33(20):2279–2287
Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P et al (2005) Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol 23(34):8845–8852
Bhandare N, Monroe AT, Morris CG, Bhatti MT, Mendenhall WM (2005) Does altered fractionation influence the risk of radiation-induced optic neuropathy? Int J Radiat Oncol Biol Phys 62(4):1070–1077
Acknowledgements
The authors would like to thank Dr. Kjetil Berner and Dr. Olga Zaikova for their valuable feedback. They would also like to extend their gratitude to Trine Thoresen for maintaining the sarcoma database at the Norwegian Radium Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was approved by the data protection official at OUH. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
König, M., Osnes, T.A., Lobmaier, I. et al. Multimodal treatment of craniofacial osteosarcoma with high-grade histology. A single-center experience over 35 years. Neurosurg Rev 40, 449–460 (2017). https://doi.org/10.1007/s10143-016-0802-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-016-0802-z